Abstract
A 67-year-old man was evaluated for haematuria, with a rising creatinine level from 88 to 906 µmol/L and positive c-anti-neutrophil cytoplasm antibody (ANCA)/anti-proteinase 3 (anti-PR3). A kidney biopsy revealed necrotizing glomerulonephritis with a ‘full-house’ pattern on immunofluorescence microscopy. Echocardiography and blood cultures growing Gemella sanguinis diagnosed endocarditis. Dialysis was required for a month. Three months later, following valve replacement, glucocorticoids and 2 months of antibiotic therapy, the creatinine level decreased to 62 µmol/L and c-ANCA/anti-PR3 disappeared. This first case of c-ANCA/anti-PR3 positive glomerulonephritis with a ‘full-house’ immunofluorescence pattern due to bacterial endocarditis underlines the importance of ruling out infection with ANCA positivity or kidney biopsy suggestive of lupus nephritis.
Keywords: ANCA, endocarditis, Gemella spp., glomerulonephritis
Background
Glomerulonephritis is a common cause of acute kidney injury in patients with endocarditis [1] and renal failure is a strong predictor of mortality in these patients [2]. Cytoplasmic type anti-neutrophil cytoplasm antibody (c-ANCA) with anti-proteinase 3 (anti-PR3) specificity are usually associated with small-vessel vasculitis and pauci-immune glomerulonephritis [3], but cases of c-ANCA-positivity have also been described with bacterial endocarditis with frequently associated glomerulonephritis [4,5]. Several bacterial species have been reported in cases of endocarditis associated with positive c-ANCA [6], including Gemella morbillorum [7]. Gemella sanguinis is a rare cause of endocarditis that has only been previously reported in three cases [8–10]. We report the first case of G. sanguinis endocarditis with c-ANCA/anti-PR3 positivity and its association with a focal necrotizing glomerulonephritis with full-house immune complex deposition (IgA, IgG, IgM, C3 and C1q positive).
Case report
A 67-year-old man was referred for a rising creatinine level from 88 to 906 µmol/L (1.0–10.3 mg/dL) over a 4-month period. He had a history of upper and lower back pain of 4 months duration and was treated by pregabaline, oxycodone and naproxen. He had a weight loss of 13 kg and complained of nycturia, gross haematuria, fatigue, nausea and occasional vomiting. Physical examination revealed normal vital signs and temperature. The patient appeared euvolemic, presented asterixis without focal neurological deficit, poor dental hygiene with multiple cavities and severe parodontis, an apical holosystolic murmur radiating to the axillary region and an enlarged spleen.
On admission, his serum creatinine level was 906 µmol/L (10.3 mg/dL) and urea nitrogen 33 mmol/L (92 mg/dL). His urine sediment showed 0.3–0.8 g/L proteins with more than 100 erythrocytes/high power field. C-ANCA using an immunofluorescence technique was positive at a titre of 1/80. Anti-PR3 were positive at 85 U/mL (<20 U/mL) and anti-myeloperoxidase were negative using enzyme-linked immunosorbent assay (QUANTA Lite® PR3 and QUANTA Lite® MPO, Inova Diagnostics®, San Diego). C3 was low (0.66 g/l [N:0.90–1.80]) and C4 was normal, but these results were available only 8 days after admission. Table 1 presents the initial laboratory data.
Table 1.
Initial laboratory data
| Laboratory data | Value |
|---|---|
| Creatinine (gmol/L) | 906 |
| Urea Nitrogen (mmol/L) | 33 |
| Potassium (mmol/L) | 5.0 |
| Haemoglobin (g/L) | 79 |
| WBC count | 7.8 × 109/L |
| Platelet count | 122 × 109/L |
| Albumin (g/L) | 23 |
| Protein (urinalysis) (g/L) | 0.3–0.8 |
| RBC count (urine microscopy) | >100/HPF |
| c-ANCA | 1/80 |
| Anti-PR3 (U/mL) | 85 (<20) |
| C3 (g/L) | 0.66 (0.90–1.80) |
| C4 (g/L) | 0.29 (0.10–0.40) |
| Hepatitis B serology | Negative |
| Hepatitis C serology | Negative |
| Anti-ENA | Negative |
| Anti-ds-DNA | Negative |
| ANA | Negative |
| Anti-GBM | Negative |
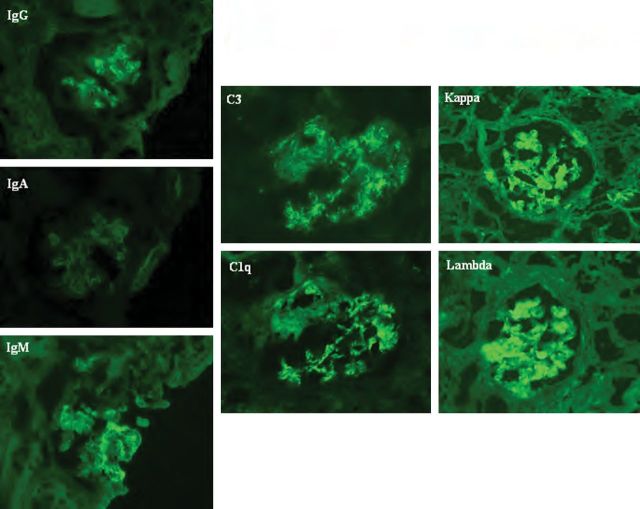
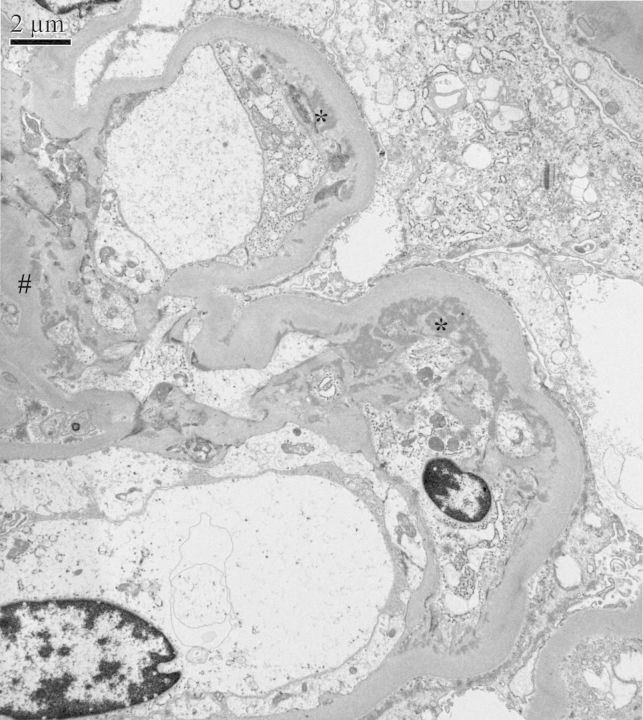
Haemodialysis was required on the second day of admission. Transthoracic echocardiography showed severe mitral regurgitation with a 9 × 27 mm pannus and a 4-mm vegetation attached to the anterior mitral leaflet, with multiple microperforations. The transoesophageal echocardiography confirmed severe mitral regurgitation with an 11 × 13 mm vegetation on the anterior leaflet, and moderate aortic and tricuspid regurgitation. A kidney biopsy revealed focal necrotizing glomerulonephritis without significant endocapillary or mesangial proliferation (Figure 1). The glomeruli showed cellular crescents (4/21) and/or fibrinoid necrosis (3/21) with mildly increased mesangial matrix. The tubulointerstitial compartment revealed mild tubular atrophy and moderate-to-severe interstitial oedema. Severe acute tubular injury was also present. Vessels showed nothing but focally severe arteriosclerosis. Immunofluorescence microscopy revealed primarily mesangial immune complex deposits with focal capillary loop involvement in a full-house pattern with strongly positive C3, IgM and C1q, moderately positive IgG and mildly positive IgA (Figure 2). Electron microscopy showed subendothelial (Figure 3) and mesangial electron dense deposits with focal mesangial interposition. No tubuloreticular inclusions were found. In the context of a probable endocarditis, ceftriaxone and gentamicin were started, and methylprednisolone 500 mg IV ID was given for 3 days followed by daily prednisone (50 mg) for 2 weeks in an attempt to treat the necrotizing glomerulonephritis.
Fig. 1.
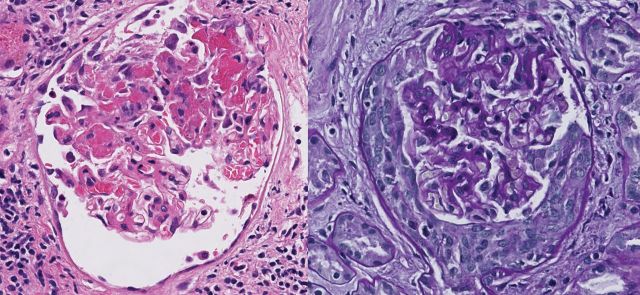
Kidney biopsy. Glomerulus showing fibrinoid necrosis (H&E) and cellular crescent (PAS stain). Magnification 40×.
Fig. 2.
Kidney biopsy immunofluorescence. Immunofluorescence positive for IgG, IgM, IgA, C3, C1q, kappa and lambda light chains mainly in the mesangium and focally in capillary loops.
Fig. 3.
Electron microscopy of glomerulus. Mesangial (#) and subendothelial immune complex deposits (*) with mesangial cell interposition (electron microscopy).
On Day 3, several blood cultures grew slow-growing streptococcus-like gram-positive bacteria that yielded Gemella haemolysans by conventional biochemical identification methods. The bacterial strain was sent to the reference laboratory for identification by sequencing of the 16S ribosomal RNA gene using a previously described method [11] and was identified as G. sanguinis. A positron emission tomography–computed tomography (CT) scan revealed signs of spondylodisciitis at C6-7 and L3-4 levels. A CT-scan showed splenomegaly (14.8 cm cephalocaudal length) with an infarct of 4.2 × 2.4 cm. No pulmonary abnormalities were noted on the CT-scan. Vancomycin and gentamicin were given from Days 4–6, and subsequently vancomycin was replaced by penicillin G upon receipt of in vitro susceptibility analysis. The patient underwent mitral and aortic valve replacement on Day 17. Valve cultures were negative, but histological examination showed devitalized gram-positive cocci. The patient received penicillin and gentamicin until Day 69.
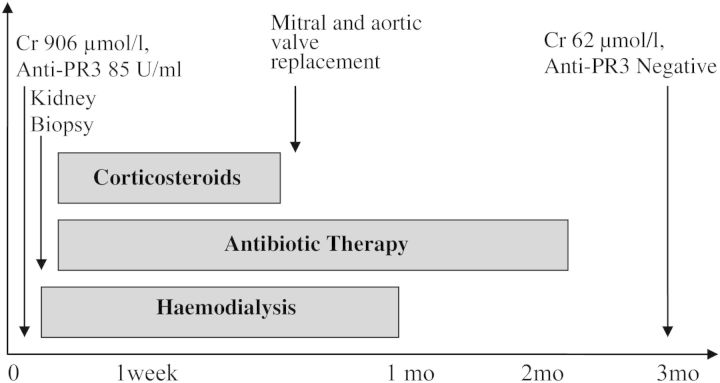
One month after admission, dialysis was stopped, and by the third month, the creatinine level decreased to 62 µmol/L (0.7 mg/dL), c-ANCA level decreased to 1/40, anti-PR3 and blood cultures were negative. Figure 4 presents the case history.
Figure 4.
Case history.
Discussion
Bacterial endocarditis is associated with several types of kidney injuries, the commonest being septic emboli and glomerulonephritis [1,12]. Glomerulonephritis was found in 26% of specimens in a study combining biopsy and necropsy cases [1].
False-positive results for c-ANCA/anti-PR3 are rare (98% specificity) [3]. However, in the context of bacterial endocarditis, 22 cases of positive c-ANCA/anti-PR3 and 1 case of P-ANCA/anti-PR3 have been reported [4–6,13–16]. Causal bacteria include Staphylococcus aureus, Enterococcus spp., Streptococcus spp., Bartonella spp., Propionibacterium acnes and Gemella morbillorum. Bacterial endocarditis-related ANCA associated with glomerulonephritis have been described in 12 cases [4–6,14–16]. The previously reported immunofluorescence studies (n = 6) are variable ranging from pauci-immune (n = 2) [5,16], to positivity for at least a complement fraction and an immunoglobulin subtype (n = 4) [4,6,14,16]. Table 2 lists the known causes of false-positive c-ANCA, which should guide clinicians in their differential diagnosis while considering a c-ANCA-associated vasculitis.
Table 2.
Causes of c-ANCA false positivity
| Connective tissue disease |
| Cocaine-induced midline destructive lesions |
| Tuberculosis |
| Chronic hepatitis B infection |
| Chronic hepatitis C infection |
| Amoebic liver abscess |
| Phlegmon |
| Cystic fibrosis |
| Ventriculoperitoneal shunt nephritis |
| Parvovirus B19 infection |
| Endocarditis |
| Endovascular prosthetic material infection |
The mechanisms by which c-ANCA/anti-PR3 develop in bacterial endocarditis and the role of ANCA in the pathogenesis of immune complex glomerulonephritis are presently uncertain. Some authors have proposed that the bacterial-induced activation of neutrophils via chromatin fibre webs called Neutrophil Extracellular Traps (NETs) may be involved in the development of c-ANCA/anti-PR3 antibodies [17].
Gemella sanguinis is a gram-positive, catalase-negative, streptococcus-like bacterium first described in 1998 [10], which is part of the normal human oropharynx, urogenital and gastrointestinal flora [18]. To date, three cases of G. sanguinis endocarditis have been described and all were associated with poor oral hygiene [8,9]. However, G. sanguinis has never been reported as a cause of positive c-ANCA/anti-PR-3. The species is difficult to identify with conventional microbiological diagnostic methods, and 16S rRNA gene sequencing is often necessary [10].
The diagnosis of bacterial endocarditis can easily be overlooked when glomerulonephritis is associated with c-ANCA/anti-PR3 or when a kidney biopsy reveals immune complex glomerulonephritis with a full-house immunofluorescence pattern. Indeed, marantic endocarditis associated with ANCA vasculitis can mimick subacute bacterial endocarditis [19] and the full-house immunofluorescence pattern raises a high degree of suspicion for lupus nephritis [20–22] and Libman-Sacks endocarditis. To our knowledge, only one case of proliferative glomerulonephritis with a full-house immunofluorescence pattern has previously been described in a patient with Streptococcus viridans endocarditis [23]. In the context of ANCA positivity, the presence of splenomegaly, hypocomplementemia, immune complex deposits on kidney biopsy and the absence of other autoantibodies are highly in favour of a bacterial endocarditis associated c-ANCA/anti-PR3 rather than idiopathic ANCA-associated vasculitis [6,16,24]. Endocarditis, as a manifestation of idiopathic ANCA-associated vasculitis, is quite unusual [19]. However, infective endocarditis should be ruled out before attributing it to an underlying vasculitis or lupus. Therefore, repeated search for a bacterial pathogen in blood cultures is the cornerstone of the appropriate diagnosis.
Treatment of glomerulonephritis associated with endocarditis and c-ANCA/anti-PR3 is controversial as some authors advise the use of immunosuppressive therapy combined with antibiotics [25], while others suggest the use of antibiotics alone as the treatment of choice [6]. Due to the severity of the immune reaction on kidney biopsy, our patient was treated simultaneously with antibiotics and corticosteroids. The outcome was favourable as he recovered normal renal function. In the literature, a few cases of glomerulonephritis associated with bacterial endocarditis and c-ANCA/anti-PR3 have shown a dramatic improvement in renal function following treatment [7,15]. One patient required dialysis and recovered adequate renal function following antibiotic treatment alone [7]. In our case, we used both antibiotics and corticosteroids while waiting for definitive results, as leaving the patient without treatment for an infectious endocarditis or auto-immune disease could have been devastating. However, due to the paucity of reports, addition of corticosteroids to IV antibiotics should be individualized in the presence of suspected bacterial endocarditis.
In conclusion, this first case of G. sanguinis endocarditis with anti-PR3/c-ANCA-associated immune complex necrotizing glomerulonephritis with a full-house pattern on immunofluorescence studies underlines the importance of considering subacute bacterial endocarditis in the differential diagnosis of ANCA vasculitis or lupus nephritis.
Conflict of interest statement
None declared.
Acknowledgements
The authors thank Marc-Christian Domingo, LSPQ; François Gagnon, Centre hospitalier du Grand-Portage, and Patrick Béliveau, CHUQ-HDQ.
References
- 1.Majumdar A, Chowdhary S, Ferreira MA, et al. Renal pathological findings in infective endocarditis. Nephrol Dial Transplant. 2000;15:1782–1787. doi: 10.1093/ndt/15.11.1782. [DOI] [PubMed] [Google Scholar]
- 2.Conlon PJ, Jefferies F, Krigman HR, et al. Predictors of prognosis and risk of acute renal failure in bacterial endocarditis. Clin Nephrol. 1998;49:96–101. [PubMed] [Google Scholar]
- 3.Kallenberg CG, Brouwer E, Weening JJ, et al. Anti-neutrophil cytoplasmic antibodies: current diagnostic and pathophysiological potential. Kidney Int. 1994;46:1–15. doi: 10.1038/ki.1994.239. [DOI] [PubMed] [Google Scholar]
- 4.Fukuda M, Motokawa M, Usami T, et al. PR3-ANCA-positive crescentic necrotizing glomerulonephritis accompanied by isolated pulmonic valve infective endocarditis, with reference to previous reports of renal pathology. Clin Nephrol. 2006;66:202–209. doi: 10.5414/cnp66202. [DOI] [PubMed] [Google Scholar]
- 5.Kishimoto N, Mori Y, Yamahara H, et al. Cytoplasmic antineutrophil cytoplasmic antibody positive pauci-immune glomerulonephritis associated with infectious endocarditis. Clin Nephrol. 2006;66:447–454. doi: 10.5414/cnp66447. [DOI] [PubMed] [Google Scholar]
- 6.Uh M, McCormick IA, Kelsall JT. Positive cytoplasmic antineutrophil cytoplasmic antigen with PR3 specificity glomerulonephritis in a patient with subacute bacterial endocarditis. J Rheumatol. 2011;38:1527–1528. doi: 10.3899/jrheum.101322. [DOI] [PubMed] [Google Scholar]
- 7.Satake K, Ohsawa I, Kobayashi N, et al. Three cases of PR3-ANCA positive subacute endocarditis caused by attenuated bacteria (Propionibacterium, Gemella, and Bartonella) complicated with kidney injury. Modern Rheumatol. 2011;21:536–541. doi: 10.1007/s10165-011-0434-7. [DOI] [PubMed] [Google Scholar]
- 8.Gundre P, Pascal W, Abrol S, et al. Prosthetic valve endocarditis caused by Gemella sanguinis: a consequence of persistent dental infection. Am J Med Sci. 2011;341:512–513. doi: 10.1097/MAJ.0b013e31821389f0. [DOI] [PubMed] [Google Scholar]
- 9.Shukla SK, Tak T, Haselby RC, et al. Second case of infective endocarditis caused by Gemella sanguinis. WMJ. 2002;101:37–39. [PubMed] [Google Scholar]
- 10.Collins MD, Hutson RA, Falsen E, et al. Description of Gemella sanguinis sp. nov., isolated from human clinical specimens. J Clin Microbiol. 1998;36:3090–3093. doi: 10.1128/jcm.36.10.3090-3093.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bekal S, Gaudreau C, Laurence RA, et al. Streptococcus pseudoporcinus sp. nov., a novel species isolated from the genitourinary tract of women. J Clin Microbiol. 2006;44:2584–2586. doi: 10.1128/JCM.02707-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornér A, Kaartinen K, Aaltonen S, et al. Membranoproliferative glomerulonephritis complicating Propionibacterium acnes infection. Clin Kidney J. 2013;6:35–39. doi: 10.1093/ckj/sfs165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bauer A, Jabs WJ, Sufke S, et al. Vasculitic purpura with antineutrophil cytoplasmic antibody-positive acute renal failure in a patient with Streptococcus bovis case and Neisseria subflava bacteremia and subacute endocarditis. Clin Nephrol. 2004;62:144–148. doi: 10.5414/cnp62144. [DOI] [PubMed] [Google Scholar]
- 14.Subra JF, Michelet C, Laporte J, et al. The presence of cytoplasmic antineutrophil cytoplasmic antibodies (C-ANCA) in the course of subacute bacterial endocarditis with glomerular involvement, coincidence or association? Clin Nephrol. 1998;49:15–18. [PubMed] [Google Scholar]
- 15.Hanf W, Serre JE, Salmon JH, et al. Glomerulonephrite rapidement progressive a ANCA revelant une endocardite infectieuse subaigue [Rapidly progressive ANCA positive glomerulonephritis as the presenting feature of infectious endocarditis] La Revue de Medecine Interne. 2011;32:e116–118. doi: 10.1016/j.revmed.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 16.Bonaci-Nikolic B, Andrejevic S, Pavlovic M, et al. Prolonged infections associated with antineutrophil cytoplasmic antibodies specific to proteinase 3 and myeloperoxidase: diagnostic and therapeutic challenge. Clin Rheumatol. 2010;29:893–904. doi: 10.1007/s10067-010-1424-4. [DOI] [PubMed] [Google Scholar]
- 17.Park SJ, Pai KS, Kim JH, et al. Glomérulonéphrite à ANCA chez un patient ayant une endocardite infectieuse: rôle du piège neutrophilique extracellulaire? [ANCA-associated glomerulonephritis in a patient with infectious endocarditis: the role of neutrophil extracellular traps?] La Revue de Medecine Interne. 2012;33:57. doi: 10.1016/j.revmed.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Kilpper-Balz R, Schleifer KH. Transfer of Streptococcus morbillorum to the genus Gemella as Gemella morbillorum comb.nov. Int J Syst Bacteriol. 1988;38:442–443. [Google Scholar]
- 19.Knockaert DC. Cardiac involvement in systemic inflammatory diseases. Eur Heart J. 2007;28:1797–1804. doi: 10.1093/eurheartj/ehm193. [DOI] [PubMed] [Google Scholar]
- 20.Baskin E, Agras PI, Menekse N, et al. Full-house nephropathy in a patient with negative serology for lupus. Rheumatol Int. 2007;27:281–284. doi: 10.1007/s00296-006-0198-0. [DOI] [PubMed] [Google Scholar]
- 21.Gianviti A, Barsotti P, Barbera V, et al. Delayed onset of systemic lupus erythematosus in patients with ‘full-house’ nephropathy. Pediatr Nephrol. 1999;13:683–687. doi: 10.1007/s004670050681. [DOI] [PubMed] [Google Scholar]
- 22.Caltik A, Demircin G, Bulbul M, et al. An unusual case of ANA negative systemic lupus erythematosus presented with vasculitis, long-standing serositis and full-house nephropathy. Rheumatol Int. 2013;33:219–222. doi: 10.1007/s00296-010-1540-0. [DOI] [PubMed] [Google Scholar]
- 23.Lee LC, Lam KK, Lee CT, et al. ‘Full house’ proliferative glomerulonephritis: an unreported presentation of subacute infective endocarditis. J Nephrol. 2007;20:745–749. [PubMed] [Google Scholar]
- 24.Chirinos JA, Corrales-Medina VF, Garcia S, et al. Endocarditis associated with antineutrophil cytoplasmic antibodies: a case report and review of the literature. Clin Rheumatol. 2007;26:590–595. doi: 10.1007/s10067-005-0176-z. [DOI] [PubMed] [Google Scholar]
- 25.Haseyama T, Imai H, Komatsuda A, et al. Proteinase-3-antineutrophil cytoplasmic antibody (PR3-ANCA) positive crescentic glomerulonephritis in a patient with Down's syndrome and infectious endocarditis. Nephrol Dial Transplant. 1998;13:2142–2146. doi: 10.1093/ndt/13.8.2142. [DOI] [PubMed] [Google Scholar]