Abstract
Although peritoneal dialysis (PD) is recognized as an effective renal replacement therapy (RRT) alternative to haemodialysis (HD), its prevalence is around 15% in most of the industrialized countries. In the French-speaking part of Belgium, PD is clearly underused with a prevalence of 8.7% in 2009. The main objectives of this work were to evaluate the nephrologists' perceived obstacles to PD implementation and reflect on possible actions towards PD development. A computer-based 33-item questionnaire was sent by e-mail to all nephrologists affiliated to the French-speaking association. Among 120 adult nephrologists targeted by this inquiry, 97 completed the online questionnaire (response rate 80.8%). Among them, 29% had little experience with PD (treating less than five patients) and 39% reported no specific training with this modality of RRT. However, 88% of responders claimed PD prevalence should be around 20–25%. Half of the responders would choose PD as a first RRT option if they required RRT for themselves. The three main reasons given to the low prevalence of PD were an easy access to HD, patient refusal and lack of nephrologist motivation. Almost all the nephrologists insisted on the need for a dedicated nursing team delivering an effective educational programme and PD management and care. They believe that PD could and should be implemented in Belgium. Enhanced nephrologist motivation and training in PD were identified as predominant factors to be upgraded, as well as patient education programmes.
Keywords: barriers, educational nephrology, peritoneal dialysis, pre-dialysis care, renal replacement therapy
Introduction
Peritoneal dialysis (PD) is a well-recognized technique of renal replacement therapy (RRT), exhibiting an efficacy similar to that of haemodialysis (HD) [1, 2]. Despite numerous advantages (prolonged preservation of residual renal function, patient autonomy, relative simplicity, lower cost) and also significant advances provided by automated techniques and commercialization of more biocompatible solutions, the overall prevalence of patients treated with PD is still only ∼15% in most industrialized countries with exceptions such as Hong Kong or New Zealand where the prevalence rates reach 80 and 36%, respectively [3]. In Europe, the countries with high prevalence (over 20%) are Great Britain, the Netherlands, Denmark and Sweden, despite evidence of reductions in the past few years. Countries with the lowest prevalence include Italy with 10.3% in 2004 [4], France with 8.9% in 2003 [5], Spain with 8.8% [6] and Germany with 5% [3].
In the French-speaking part of Belgium, the PD technique is clearly underused, reaching a prevalence of just 8.7% in 2009. Between 2000 and 2009, the total number of patients reaching end-stage kidney disease (ESKD) has significantly progressed (+58%) (Figure 1). A large part of the increase was due to the development of in-centre HD (for patients needing close medical supervision and/or nursing care) as well as self-care HD in satellite units. These last ones, also called low-care HD units as patients are actively involved in their treatment (and assisted by a nurse if needed), grew from 8.4 to 19.5%. In the same time interval, PD grew only from 8.3 to 8.7% (data collected by the Registry of ESKD from the French-speaking Belgian Nephrologist Association: http://www.gnfb.be).
Fig. 1.
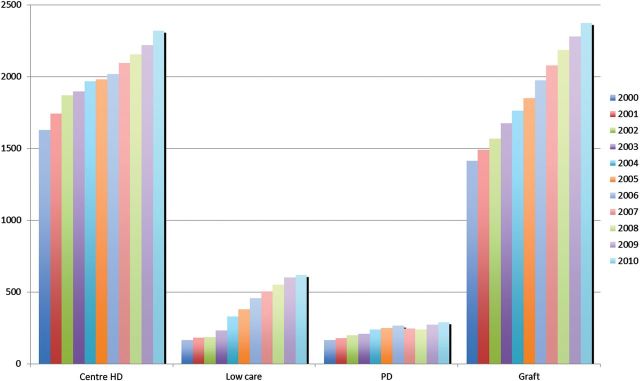
Prevalent ESKD patients in the French-speaking part of Belgium. Patients are distributed according to the different RRT modalities from 2000 to 2010: in-centre HD is the predominant RRT modality when compared with self-care HD in satellite units (also called low-care HD) and PD. The proportion of renal grafts has significantly increased during the last decade.
There are several reasons that could explain why such a low prevalence of patients are treated with PD compared with HD, both medical and non-medical. The medical contraindications exclude around 20–30% of the patients [7]. Regarding the non-medical reasons, several causes are reported in the literature such as reimbursement structures for dialysis, availability of home nurses, the presence of numerous HD centres, ignorance and lack of PD practice by the nephrologists, the proportion of patients referred late to the nephrologist, absence of well-organized pre-dialysis education programmes and epidemiological/sociological aspects. One of the unique aspects of PD, compared with in-centre HD, is the necessity of non-physician extenders to assist in implementing the technique outside the hospital. It is clear that PD requires, even more than HD, the agreement, support and intervention of five to six protagonists: the patient, the nephrologist (and the surgeon), PD nurses, the general practitioner, the family environment and support. The role of each one of them in the decision to choose the PD technique is very important, as Thamer et al. pointed out in an inquiry among American nephrologists [8].
The main objectives of the present report were to evaluate the nephrologists' perception of PD and its use in the French-speaking part of Belgium, to identify the obstacles to PD implementation perceived by the nephrologists themselves and to compare the results with similar inquiries performed in other countries in the last 10 years in order to reflect on new approaches to encourage PD development.
Materials and methods
We designed a 33-item questionnaire (see Supplementary data Appendix S1 or upon request to the corresponding author) for nephrologists in order to record their personal opinions concerning the limited use of PD and to attempt to elicit factors negatively interfering with its implementation.
We searched all electronic addresses of the nephrologists currently professionally active in the French-speaking part of Belgium, gathered information from the three university listings, the professional French-speaking nephrologists association of the country and the Belgian healthcare Ministry.
We implemented this questionnaire into an inquiry management software (Checkmarket®, Turnhout, Belgium) and coordinated the distribution by electronic mail. Using this system, the nephrologists could reply directly on an Internet site and access the site several times if they wished in order to complete their answers. Replies were automatically transferred and stored in an Excel data base. The software also captured responses from the nephrologists who had partially answered the questionnaire. For each question and proposed answers, the respective proportions of nephrologists who positively and negatively responded were extracted by the software. The inquiry was sent on 27 May and completed on 5 July 2009. Reminders were sent to non-responders on June 4 and June 14 2009. A Chi-square test was used to analyse the differences between groups for each question.
Results
Among 140 eligible responders, 113 completed the questionnaire (response rate 80.7%). Due to the low number of paediatric nephrologists and postgraduate students in nephrology, we decided to analyse only the data supplied by nephrologists taking care of adult patients, i.e. 97 responders out of 120 eligible (response rate 80.8%). Five questionnaires were partially completed. Consequently, the response rate for each question was automatically obtained by dividing the number of replies by the total amount of answers. The mean time for completing the questionnaire was 14 min.
Professional profile of the responders
Fifty-one per cent were identified as nephrologists in practice for <10 years, 27% for 10–20 years and 21% for >20 years. The overall majority (83%) was working in large city areas (Brussels, Hainaut and Liège districts). Twenty-two per cent were working in academic hospitals, 26% in regional hospitals linked to an academic institution, 31% in private institutions and 21% in public institutions.
Nephrologists' practice environment and prior education in PD therapy
Half of the medical workload of the responders was devoted to HD care, whereas only 10% was dedicated to PD care. The remaining workload was distributed between transplantation (15%) and other tasks (including nephrology outpatient clinics, 23%).
Only 7% of the responders had personally followed a total of more than 50 PD patients, 24% had personally followed more than 20 PD patients and almost 30% had personally followed less than 5 patients or had no experience on PD, respectively. From a practical point of view, by the time the questionnaire was completed, only 55% were currently taking care of PD patients, 31% occasionally (only as emergency situations) and 11% never experienced this technique (3% did not answer the question).
More than one-third (39%) of nephrologists had not received a specific training in PD; a decreasing tendency in receiving this training was observed in recently graduated nephrologists when compared with their older colleagues. Along the same line, nearly half of the responders (46%) were not aware of the so-called ‘integrated care in dialysis’ concept which proposes that HD, PD and renal transplantation should all be offered to the ESKD patient in an unbiased way, and that all three modalities may be a part of treatment during the patient's lifetime with emphasis that starting with PD preserves residual kidney function and vascular access [3, 9].
Use and efficacy of PD as perceived by the nephrologists
Eighty per cent of the responders thought PD is an RRT as effective as HD and 88% of them considered that PD is underused in Belgium. According to them, the prevalence of PD in Belgium should reach 20–25%.
Main perceived barriers proposed to explain the low prevalence of PD
Among 12 factors frequently reported in the literature, each responder was asked to select 3 as the most important ones susceptible to explain the low prevalence of PD (Table 1). These were as follows: easy access to HD as RRT (29%), patient's refusal to the technique (28%) and lack of motivation of nephrologists (26%).
Table 1.
Representative item of the opinion poll
| Main possible reasons for the low percentage of patients in PD in Belgium when comparing with certain countries | Percentage of positive answers (%) |
| The medical contraindications | 12 |
| The important number of dialysis centres nearby | 22 |
| The ease of using HD as RRT | 29 |
| The lack of motivation of nephrologists | 26 |
| Fear of complications | 6 |
| Late referral | 22 |
| The need to use HD places with priority | 4 |
| The time needed to implement peritoneal dialysis | 10 |
| Lack of PD training (PD technique) | 19 |
| The need to have a nurse team dedicated to the technique | 22 |
| The need to have an experienced surgeon | 4 |
| Patient refusal (by choice) | 28 |
This item is part of a 33-item questionnaire sent to the French-speaking Belgian nephrologists. Three answers were to be chosen by the responders (n = 97). For each proposition, the percentage of positive answers is indicated.
Nephrologists' personal perception of PD
Fifty per cent of responders would select PD as RRT of choice for themselves if they were suffering from ESKD and waiting for a renal transplant (living donor excluded). Furthermore, 77% of nephrologists would prefer a home-based RRT. These proportions were increased with the longer experience in PD care (Figure 2).
Fig. 2.
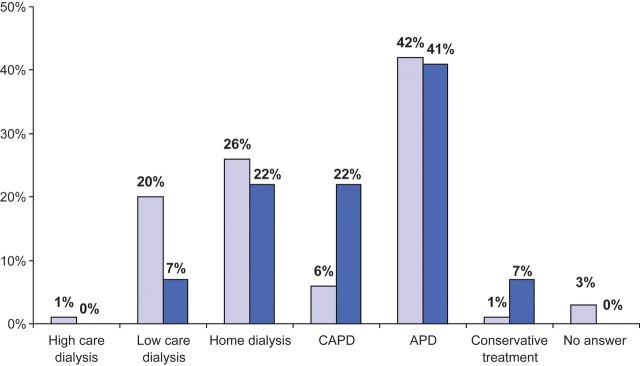
Nephrologists' first choice of RRT if they suffered from ESKD themselves (living donor excluded). The top selected modality is clearly automated PD (APD) whatever the nephrologists' own experience in PD care (< or >20 years, as represented by dark and light blue histograms, respectively). The second most chosen modality is home HD, followed by self-care HD in a satellite unit or continuous ambulatory PD (CAPD), depending on the duration of the responders' own experience in PD care. In-centre HD was the least selected option.
Contraindications to PD
Among responders, 16% thought that contraindications to PD were numerous and 26% considered the association of relative contraindications as an absolute contraindication.
Over 70% of nephrologists considered as indications for PD the difficulty in creating a permanent vascular access, chronic cardiac failure and significant residual renal function. Over 60% of them thought that anuria, bad patient hygiene, chronic respiratory insufficiency, body mass index between 30 and 35, malnutrition with hypoalbuminaemia were at least relative contraindications to PD. Also noticeable is the fact that 44% thought that the general practioners' negative opinion was a relative contraindication to PD modality.
Education delivered to the patients and on-site pre-dialysis team
Half of the responders declared that they would not discuss PD as an optional RRT if they considered the patient a bad candidate for PD (poor understanding and collaboration). In a similar proportion, 46% of responders thought that an early patient referral could facilitate PD presentation as a possible RRT option. In this respect, the majority (94%) felt that availability of an on-site caregiver facilitated the improved quality of delivered information. However, only 50–75% of their patients meet such a team. Seventy-five percent of nephrologists felt they were well supported by a collaborative pre-dialysis team. Almost all of them (98%) thought that a PD project is only feasible by working closely with a team of dedicated nurses.
Complications of PD perceived by the nephrologists as barriers to the technique
The nephrologists' confidence in managing PD complications is quite similar to what it is for HD: on a scale of 1–10, the mean value was 7/10 related to PD versus 8/10 related to HD. Nearly, one-fifth (21%) had fear of insufficient dialysis quality with PD, 16% hesitated proposing PD after a difficult experience, 32% thought that technical failures were frequent and discouraging. Finally, 55% had fear of encapsulating peritoneal sclerosis.
Discussion
The implementation of PD varies considerably across the world. In 2007, Hong Kong and Mexico had the highest rate of PD use with 80% and 66%, respectively. These countries are followed by New Zealand with 36% (US Data Renal Systems, USRDS. Annual Data Report 2009; http://www.usrds.org). PD prevalence among several Western European countries (Great Britain, the Netherlands and Denmark), Korea, Singapore, Australia and Canada is between 20 and 30%. In other industrialized countries such as the USA (7.2%), Germany (5%), France (8.7%), Spain (8.8%) and Japan (3.6%) [10], and also in other developing countries, PD utilization was <10%. While India demonstrated a significant PD growth of 20% per year in 2004 [11], PD prevalence is declining in certain countries of North America and Western Europe (Canada, USA, Germany, Great Britain). Other countries are now reporting increased PD utilization (Singapore and countries of Eastern Europe) or report remaining stable or slight growth, like Belgium.
These differences in PD utilization are immense and medical factors cannot explain the great disparities observed between neighbouring countries such as the Netherlands [12] and Belgium. Non-medical factors such as health policy and PD reimbursement may be contributing factors [9]. Yes, it is indeed difficult to explain the huge disparities between different regions in the same country with the same financial criteria [13]. Van Biesen et al. explained that besides questions of organization, there are mainly two frontline reasons for not initiating PD: either the patients refuse it or the doctors are reluctant to propose PD [3, 14]. Clearly, there is a tight connection between the two, as the patient formulates his opinion based on the information he receives from the doctors and nurses taking care of him. Our report provides further insight into the perception of PD and the barriers to PD as perceived by nephrologists. One strength of the current report is certainly its high response rate (81.4%), which is markedly higher than that observed in other surveys covering the same topic [5, 7, 8, 12, 15–19] and the ability to clearly identify the core aspects of PD development in the French-speaking part of Belgium.
The survey reveals the importance of PD exposure and training: almost 30% of responders had personally followed less than five patients or had no PD experience at all and 39% of responders reported no specific training on PD management, more particularly in younger nephrologists when compared with their senior colleagues. A vicious cycle of less education leading to less knowledge and practice and once again less training is thus launched, probably contributing to reluctant opinions of nephrologists towards PD.
Second, despite this educational gap, the nephrologists' opinion stresses the low use of PD, with 88% of responders claiming PD is underused and should reach a prevalence around 20–25%. This finding is in concordance with other studies reporting similar targets for PD [5, 7, 8, 15, 16]. An interesting point revealed by the present study was the clear preference for home-based RRT if the nephrologists themselves were to suffer from ESKD. Furthermore, it is remarkable that 51% of them would choose PD as their first technique of dialysis.
Finally, the necessity of active nursing teams dedicated to a pre-dialysis promotional and educational programme as well as PD patient management and care was clearly pointed out by a majority of the nephrologists, as already reported [20].
Regarding the complex reasons for low prevalence of PD, it is important to acknowledge other financial aspects in this matter, such as the reimbursement modalities in Belgium. Lameire et al. suggested a significant impact of this latter point on the prevalence of PD [9]. Actually, a significant increase in PD use (70%) was observed between 1994 and 2002, which could be accounted for by improved reimbursement modalities. However, these upgraded reimbursement modalities concerned assisted PD and were effective in 2001. This situation did not result in any significant increase in PD prevalence: 8.3% in 2000, 9.7% in 2006 and 8.7% in 2009 (Figure 1), probably reflecting other problems besides funding.
This survey reveals the three main factors responsible for the low prevalence of PD in the French-speaking part of Belgium—an easy access to HD as RRT (multiple HD centres, short distances, patient refusal and the lack of motivation from the nephrologists themselves. Although several authors insisted on the importance of patient freedom of RRT choice [20, 21], it is quite obvious that the patient's choice is clearly influenced by the nephrologist's perspective, making the item ‘patient refusal’ influenced by both actors. Beyond the fact that information could sometimes be biased, it is also important to know that a full information programme about PD may not even be presented to patients. Indeed, 50% of responders declared that they do not present PD as an alternative modality to HD if they are convinced the patient is not a good candidate (poor capacities of understanding or collaboration). As important is the fact that almost 50% would also propose PD if the patient had been referred earlier, raising again the question of the late referral of the renal patients to the nephrologists. Consistently, this point was also mentioned in 22% of the answers as a cause of low PD prevalence.
Only 16 and 26% of nephrologists, respectively, thought that relative contraindications to PD were numerous and that several relative contraindications made an absolute one. Even though the self-reported personal skill to manage PD complications is similar to HD, there are still some perceived barriers to PD such as the risk of encapsulating peritoneal sclerosis and/or technical drawbacks.
Once again, the Achilles' heel resides in the lack of education and training of the nephrologists. More investment needs to be made in this area in order to motivate the youngest nephrologists in developing PD. Stimulating research aspects in this field should also therefore be proposed to them during their academic training.
The main limitation of the present study is the difficulty in generalizing these conclusions worldwide. Indeed, Belgium is a small country, with peculiar reimbursement modalities and easy access to HD facilities. However, the opinion poll provides interesting data on the way nephrologists think and implement the various RRT modalities.
To conclude, this inquiry reveals that nephrologists from French-speaking Belgium believe PD is clearly underused in the country. Enhanced motivations and implementation of specific trainings for younger nephrologists and of patient's pre-dialysis education programmes have been identified as potential key success factors to increase PD penetration.
Supplementary data
Supplementary data is available online at http://ckj.oxfordjournals.org.
Conflict of interest statement
None declared.
Supplementary Material
Acknowledgements
This work has been presented in part at the 12th Annual Joint Congress of the Société de Néphrologie and Société Francophone de Dialyse (Brussels, Belgium, September 29—1 October 2010) and the 11th Symposium of the RDPLF (Registre de Dialyse Peritonéale de Langue Française) at Montvillargenne, France, April 14–15 2011; a poster presentation of the most significant data was also performed at the 49th ERA-EDTA Congress in Paris, France, on 25 May 2012.
References
- 1.Lo WK. Peritoneal dialysis utilization and outcome: what are we facing? Perit Dial Int. 2007;27:S42–S47. [PubMed] [Google Scholar]
- 2.Van Biesen W, Davies S, Lameire N. An integrated approach to end-stage renal disease. Nephrol Dial Transplant. 2001;16:7–9. doi:10.1093/ndt/16.suppl_6.7. [PubMed] [Google Scholar]
- 3.Van Biesen W, Veys N, Lameire N, et al. Why less success of peritoneal dialysis programmes in Europe? Nephrol Dial Transplant. 2008;23:1478–1481. doi: 10.1093/ndt/gfn123. doi:10.1093/ndt/gfn123. [DOI] [PubMed] [Google Scholar]
- 4.Viglino G, Neri L, Alloati S, et al. Analysis of the factors conditioning the diffusion of peritoneal dialysis in Italy. Nephrol Dial Transplant. 2007;22:3601–3605. doi: 10.1093/ndt/gfm416. doi:10.1093/ndt/gfm416. [DOI] [PubMed] [Google Scholar]
- 5.Bouvier N, Durand PY, Testa A, et al. Regional discrepancies in peritoneal dialysis utilization in France: the role of the nephrologist́s opinion about peritoneal dialysis. Nephrol Dial Transplant. 2009;24:1293–1297. doi: 10.1093/ndt/gfn648. doi:10.1093/ndt/gfn648. [DOI] [PubMed] [Google Scholar]
- 6.Portolés J, Del Peso G, Fernández-Reyes M, et al. Previous comorbidity and lack of patient free choice of technique predict early mortality in peritoneal dialysis. Perit Dial Int. 2009;29:150–157. [PubMed] [Google Scholar]
- 7.Blake PG, Finkelstein FO. Why is the proportion of patients doing peritoneal dialysis declining in North America? Perit Dial Int. 2001;21:107–114. [PubMed] [Google Scholar]
- 8.Thamer M, Hwang W, Fink N, et al. US Nephrologists’ recommendation of dialysis modality: results of a national survey. Am J Kidney Dis. 2000;36:1155–1165. doi: 10.1053/ajkd.2000.19829. doi:10.1053/ajkd.2000.19829. [DOI] [PubMed] [Google Scholar]
- 9.Lameire N, Peeters P, Vanholder R, et al. Peritoneal dialysis in Europe: an analysis of its rise and fall. Blood Purif. 2006;24:107–114. doi: 10.1159/000089446. doi:10.1159/000089446. [DOI] [PubMed] [Google Scholar]
- 10.Kawaguchi Y. Various obstacles to peritoneal dialysis development in Japan: too much money? Too much fear? Perit Dial Int. 2007;27:S56–S58. [PubMed] [Google Scholar]
- 11.Abraham G. An update on PD in the India subcontinent. ISPD Asian Chapter Newslett. 2004;2:1. [Google Scholar]
- 12.Coester A, Krediet R, Dekker F, et al. Nephrologists perception of PD in Netherlands. Perit Dial Int. 2008;28(Suppl 4):S88. Abstract. [Google Scholar]
- 13.Couchoud C, Savoye E, Frimat L, et al. Variability in case mix and peritoneal dialysis selection in fifty-nine French districts. Perit Dial Int. 2008;28:509–517. [PubMed] [Google Scholar]
- 14.Lameire N, Van Biesen W. Epidemiology of peritoneal dialysis: a story of believers and non-believers. Nat Rev Nephrol. 2009;6:75–82. doi: 10.1038/nrneph.2009.210. doi:10.1038/nrneph.2009.210. [DOI] [PubMed] [Google Scholar]
- 15.Jung B, Blake P, Mehta R, et al. Attitudes of Canadian nephrologists toward dialysis modality selection. Perit Dial Int. 1999;19:263–268. [PubMed] [Google Scholar]
- 16.Mendelssohn D, Mullaney S, Jung B, et al. What do American nephrologists think about dialysis modality selection? Am J Kidney Dis. 2001;37:22–29. doi: 10.1053/ajkd.2001.20635. [DOI] [PubMed] [Google Scholar]
- 17.Jassal SV, Krishna G, Mallick N, et al. Attitudes of British Isles nephrologists towards modality selection: a questionnaire study. Nephrol Dial Transplant. 2002;17:474–477. doi: 10.1093/ndt/17.3.474. doi:10.1093/ndt/17.3.474. [DOI] [PubMed] [Google Scholar]
- 18.Troidle L, Kliger A, Finkelstein F. Barriers to utilization of chronic peritoneal dialysis in network # 1, New England. Perit Dial Int. 2006;26:452–457. [PubMed] [Google Scholar]
- 19.Ledebo I. What limits the expansion of self-care dialysis at home? Hemodial Int. 2008;12:S55–S60. doi: 10.1111/j.1542-4758.2008.00298.x. doi:10.1111/j.1542-4758.2008.00298.x. [DOI] [PubMed] [Google Scholar]
- 20.Goovaerts T, Jadoul M, Goffin E. Influence of a Pre-Dialysis Education Programme (PDEP) on the mode of renal replacement therapy. Nephrol Dial Transplant. 2005;20:1842–1847. doi: 10.1093/ndt/gfh905. doi:10.1093/ndt/gfh905. [DOI] [PubMed] [Google Scholar]
- 21.Lo WK. A controversy in PD. Absolute free choice: a modality selection—is it possible? Perit Dial Int. 2009;29:142–143. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


