Abstract
In response to antineutrophil cytoplasmic antibody (ANCA), activated neutrophils release decondensed chromatin associated with cytoplasmic granular proteins composed of proteolytic enzymes and myeloperoxidase (MPO); these complexes are named neutrophil extracellular traps (NETs). NET formation requires peptidylarginine deiminase 4 (PAD4) to citrullinate chromatin histones and also MPO and neutrophil elastase to aid in decondensation and release of chromatin. Immunostaining of renal biopsy revealed that NET components of citrullinated histone, MPO and PAD4 were concurrently deposited both around fibrinoid necrosis in necrotizing glomerulonephritis in the early focal phase of ANCA-associated polyangiitis and along an interlobular arterial wall.
Keywords: fibrinoid necrotizing vessel wall damage, kidney lesion, myeloperoxidase anti-neutrophil cytoplasmic antibody-associated vasculitis (MAAV), neutrophil extracellular traps (NETs)
Background
Antineutrophil cytoplasmic antibodies (ANCAs) are generated mostly against myeloperoxidase (MPO) and proteinase 3, and are associated with systemic small-vessel vasculitis. ANCA-associated vasculitis (AAV) is an autoimmune disease [1]. In renal tissue, fibrinoid necrosis mainly occurs as a segmental lesion, representative of vasculitis of the glomerular capillaries and interlobular arteries [2]. In vitro and animal studies have given compelling evidence that ANCA interacts with and activates cytokine-primed neutrophils to cause endothelial damage. Whether ANCA acts on the site of the necrotizing lesions remains unanswered. Recently, NETosis, a unique type of cell death of neutrophils, has been discovered. It is characterized by the active release of chromatin fibers, so-called neutrophil extracellular traps (NETs) that trap and kill invading microbes [3]. The unfolded chromatin is complexed with bactericidal proteins (e.g. proteinases and MPO) [3]. Kessenbrock et al. [4] noted ANCA-dependent NET formation and deposition of NETs in the small-vessel vasculitis-inflamed kidney. For NET formation, nuclear localizing peptidylarginine deiminase 4 (PAD4)-mediated histone citrullination is associated with chromatin decondensation and release [5–8]. We investigated the association of NET occurrence with the foci of MPO-AAV. Here, we describe concurrent presentations of the NET components of citrullinated histone, PAD4 and MPO around fibrinoid necrotic foci in a glomerulus and along an interlobular arterial wall with the focal class of MPO-ANCA-associated glomerulonephritis [9].
Case report
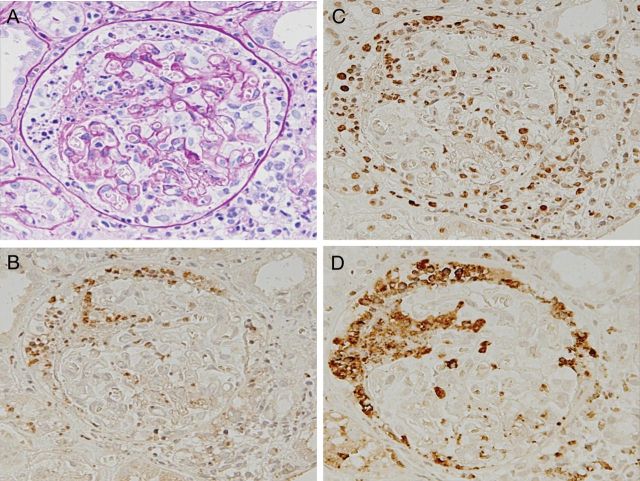
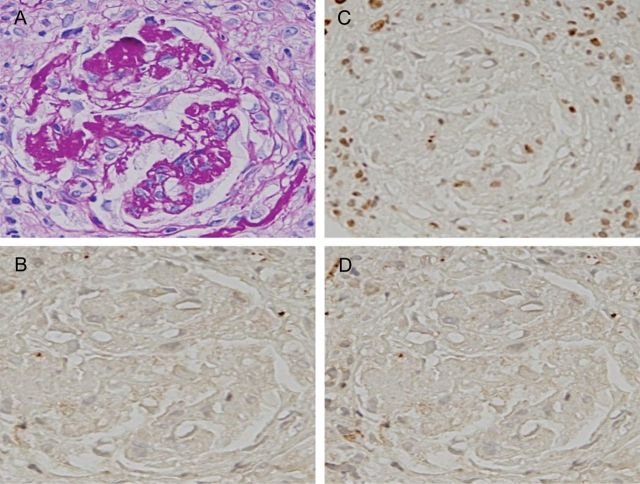
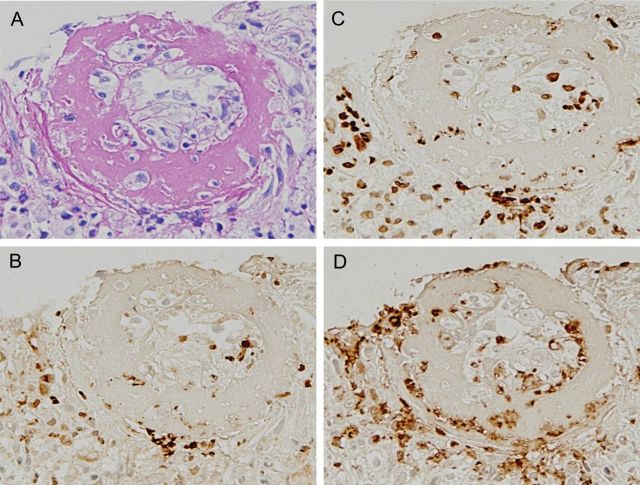
A 70-year-old man who had a low-grade fever was treated with antibiotics and a nonsteroidal anti-inflammatory drug. About 1 week after a fall, general malaise and peripheral edema appeared. He was admitted to our renal department and his condition was diagnosed as rapidly progressive glomerulonephritis. He had no family history of kidney disease. Physical examination findings included the following: height 160 cm; weight 45 kg; body temperature 37.2°C; blood pressure 137/72 mmHg; edema present in both of the lower legs. The rest of the physical examination was unremarkable. A further clinical workup indicated kidney failure with MPO-ANCA-positive vasculitis, supported by the following laboratory data: serum creatinine 2.19 mg/dL (200 µmol/L), eGFR 23.7 (mL/min/1.73 m2); eGFR (mL/min/1.73 m2) = 194 × serum creatinine−1.094 × age−0.287) [10] urea nitrogen 48.0 mg/dL (19 mmol/L), C-reactive protein 7.39 mg/dL, MPO-ANCA 276 EU (reference <20 EU), negative serologic tests for PR-3-ANCA and anti-glomerular basement membrane antibodies, and urinalysis showed protein (+) and blood (3+). Microscopic examination of urine showed numerous erythrocytes and casts. A percutaneous kidney biopsy was performed 1 day after the admission. In light microscopy, 15 glomeruli were included. The renal specimen shows a fibrinoid necrosis with cellular crescent and apoptosis-like appearance of neutrophils (Figure 1A). Then, the serial sections were immunostained for citrullinated histone using anti-citrullinated histone H3 peptide antibodies [8], for MPO using an antiserum against MPO and for PAD4 using anti-PAD4 antibodies in a coupled reaction with peroxidase-conjugated goat anti-rabbit IgG antibodies (Figure 1B–D). Strong positivities for citrullinated histone, MPO and PAD4 were distributed throughout the foci. The three antibodies were recognized to be distributed in cytoplasmic and extracellular sites, as well as the nuclei of the cells of the foci. More exactly, double immunofluorescence staining of the section revealed the codistribution of significant signals of MPO and citrullinated histone in the fibrinoid necrosis of the cellular crescent in the lesion (Supplementary Figure S1). The three other glomeruli with fibrocellular crescents showed no immunostaining for citrullinated histione, MPO and PAD4 (Figure 2). In the 11 glomeruli showing only minor abnormalities, almost no signals were found throughout the sections. The other view field of the sections exhibited a typical fibrinoid necrosis of the interlobular artery (Figure 3A). Some immunostains for citrullinated histone, MPO and PAD4 were distributed in the extracellular and cytoplasmic sites, as well as in the nuclei, of the cells throughout the lesion (Figure 3B–D). More directly, double immunofluorescence staining of the section indicated the codistribution of MPO and PAD4 in the fibrinoid necrosis of the interlobular artery (Supplementary Figure S2). MPO-AAV was recognized as belonging to the focal class, according to the histopathologic classification [9]. No immunoglobulin or complement deposition was found. Immunostaining of renal biopsy sections from a patient with anti-glomerular basement membrane glomerulonephritis revealed no signals of PAD4, citrullinated histone and MPO in the cellular crescent of glomerulus in the lesion (Supplementary Figure S3). The occurrence of NET components is likely to be specific for a type of renal disease.
Fig. 1.
Immunomicrograph of fibrinoid necrosis of the cellular crescent with the early focal phase of MPO-ANCA-associated glomerulonephritis. Consecutive sections of a renal biopsy from a patient with MPO-AAV were stained with periodic acid-Schiff, and immunostained with anti-PAD4 IgG, anti-citrullinated histone IgG and antiserum against MPO. (A) Periodic acid-Schiff stain; (B) PAD4 stain; (C) citrullinated histone stain; (D) MPO stain. Note that the signals appear concurrently with PAD4, citrullinated histones and MPO, distributed in fibrinoid necrosis of the cellular crescent. A–D: ×20.
Fig. 2.
Immunomicrograph of a fibrocellular crescent with the early focal class of MPO-ANCA-associated glomerulonephritis. The view field of fibrocellular crescent for the sections described in Figure 1 is shown. (A) Periodic acid-Schiff stain; (B) PAD4 stain; (C) citrullinated histone stain; (D) MPO stain. No signals for PAD4, citrullinated histones and MPO in fibrocellular crescent were found. A–D: ×20.
Fig. 3.
Immunomicrograph of fibrinoid necrosis of the interlobular artery with the early focal class of MPO-ANCA-associated glomerulonephritis. The view field of necrotizing interlobular artery is shown for the sections described in Figure 1. (A) Periodic acid-Schiff stain; (B) PAD4 stain; (C) citrullinated histone stain; (D) MPO stain. The signals appear concurrently with PAD4, citrullinated histones and MPO, distributed in fibrinoid necrosis of the interlobular artery. A–D: ×20.
First, the patient received steroid pulse therapy consisting of a series of three intravenous infusions of 1 g methylprednisolone plus 40 mg/day oral prednisolone for 30 days, and then (for induction therapy) 50 mg/day oral cyclophosphamide. After 60 days, the patient successfully entered into complete remission, as evidenced by the following laboratory findings: serum creatinine 0.62 mg/dL (60 µmol/L); eGFR 80.5 (mL/min/1.73 m2); C-reactive protein 0.2 mg/dL, MPO-ANCA 20 EU (reference < 20 EU) and urine protein (−) and blood (−). He has remained in remission for 3 years without intermittent relapse.
Immunohistochemistry and double immunofluorescence staining
Sections (2 μm thick) of paraffin-embedded tissue from the renal biopsy were prepared. For antigen retrieval, the sections were microwaved in boiling 10 mM citrate buffer (pH 6.5) for 3 min three times at 1-min intervals and then cooled to room temperature. Endogenous peroxidase was blocked with 3% H2O2 in water for 5 min at room temperature. The sections were immunostained with the following antibodies: 1 μg/mL rabbit anti-human PAD4 IgG [11] in phosphate-buffered saline (PBS) including 0.1% Tween 20 and 1% bovine serum albumin (BSA) and incubated overnight at 4°C, rabbit antiserum against human MPO [12] diluted 1:8000 in PBS including 0.1% Tween 20 and 1% BSA and incubated for 2 h at room temperature, 0.2 μg/mL rabbit anti-citrullinated histone (Abcam, rabbit polyclonal Cit H3 to histone H3: citrulline 2+8+17, -ChlP grade) in PBS including 0.1% Tween 20 and 1% BSA and incubated overnight at 4°C. The bound rabbit antibody was detected using a peroxidase-labeled polymer conjugated to goat anti-rabbit and goat anti-mouse IgG (Envision kit/HRP; DAKO) for 30 min at room temperature and a diaminobenzidine substrate for 10 min at room temperature. For counterstaining, specimens were immersed in a solution of Mayer's hematoxylin for 1 min at room temperature, and then mounted with Malinol medium (Muto Pure Chemicals Co., Ltd.).
For double immunofluorescence staining, the sections were prepared in the same way as described above and were treated with the solution containing 5% donkey serum and 1% BSA in PBS-0.01% Tween 20 for 1 h at room temperature. They were immunostained with the mixture of 25 µL of 1 µg/mL rabbit anti-human PAD4 IgG in PBS including 1% BSA, and 25 µL of 1 µg/mL mouse anti-human MPO monoclonal antibody 3-2H3 [11, 13] in PBS including 1% BSA overnight at 4°C or with the mixture of 25 µL of 0.2 µg/mL rabbit anti-human citrullinated histone in PBS including 1% BSA and 25 µl of 1 µg/mL mouse anti-human MPO monoclonal antibody overnight at 4°C and then the bound antibodies were detected by incubation with the mixture of 25 µL of 10 µg/mL Alex Fluor 594-labeled donkey anti-rabbit IgG (Invitrogen), and 25 µL of 10 µg/mL Alex Fluor 488-labeled donkey anti-mouse IgG (Invitrogen) for 60 min at room temperature. DNA was stained with 0.17 µg/mL Hoechst 33342 for 5 min at room temperature. The sections were mounted by coverslips using a Prolong Gold antifade reagent (Life technologies). Immnofluorescence detected was displayed in pseudo-color.
Discussion
On the basis of the immunostaining results, this work provided convincing evidence for the concurrent appearance of NET components of citrullinated histone, MPO and PAD4 in necrotizing lesions of the early focal phase of ANCA-associated polyangiitis. In NET, neutrophil elastase and MPO cooperatively enhance chromatin decondensation, leading to cell rupture and NET release [13]. MPO component acts cell-autonomously and in part contributes to host defense for innate immunity [14]. Kessenbrock et al. [4] noted netting neutrophils and the deposition of NETs in small-vessel vasculitis-inflamed kidney. These findings together give further evidence for the hypothesis that netting neutrophils [5, 14, 15] are responsible for vessel wall damage in AAV syndromes, characterized by fibrinoid necrotic lesions. As shown in Figures 1 and 3, immunostaining of sections of kidney biopsy highlighted the spontaneous deposition of the NET components of citrullinated histone, PAD4 and MPO in fibrinoid necrotic lesions consisting of cellular crescents with many apoptosis-like neutrophils and fibrinoid necrotic lesions of interlobular arterial walls. However, some signals of citrullinated histones, MPO and PAD4 are not always simultaneously found in both the glomerular capillary tuft and the interlobular artery, suggesting that degradation of NET components in the lesion may occur in a different way and at a different rate. The NET components sustained in the lesions might be involved in developing further immunogenic responses. In particular, MPO could act as an autoantigen in acquired immune response and in innate immunity. To date, no measurable immune complex of MPO has been observed in the kidney tissue in cases of MPO-AAV. This means that NET components should be rapidly cleared. As for substantial NET involvement in systemic lupus erythematosus, delayed clearance of NET is associated with development of lupus nephritis and with the onset of the disease early in childhood [16, 17]. The rate of NET formation in vitro is stimulated by ANCAs. In addition, MPO-ANCAs of individuals have recognition properties with several epitopes of MPO and also different affinities for MPO [18, 19]. Recently, Nakazawa et al. reported that NETs are involved in the pathogenesis of thrombosis, in a patient with microscopic polyangiitis [20]. Determining whether NET occurrence in lesions of AAV is associated with MPO-ANCAs or not will require further research.
In summary, we report that the NET components of citrullinated histone, PAD4 and MPO appear concurrently in fibroid necrosis of both the glomerular capillary tuft and the interlobular artery from a kidney biopsy classified as a focal class from patients with MPO-ANCA-associated glomerulonephritis.
Supplementary data
Supplementary data is available online at http://ckj.oxfordjournals.org.
Funding
This study was supported in part by a Grant-in-Aid for Intractable Vasculitis Disease Research and Progressive Renal Diseases Research, Research on Intractable Diseases, from the Ministry of Labor and Welfare of Japan.
Conflict of interest statement. None declared.
Supplementary Material
Acknowledgements
We are indebted to Dr Clifford A. Kolba (Ed.D., D.O., M.P.H.) and Associate Professor Edward F. Barroga (Ph.D.) of the Department of International Medical Communications of Tokyo Medical University for their editorial review of the English manuscript. We thank the Fellows of Renal Unit of Internal Medicine, Hachioji Medical Center, Tokyo Medical University, Tokyo, Japan: Drs T. Tomiyasu, T. Kojima, G. Hirose, Y. Sudo and N. Yoshikawa.
References
- 1.Jennette JC, Falk RJ. New insight into the pathogenesis of vasculitis associated with antineutrophil cytoplasmic autoantibodies. Curr Opin Rheumatol. 2008;20:55–60. doi: 10.1097/BOR.0b013e3282f16c0a. doi:10.1097/BOR.0b013e3282f16c0a. [DOI] [PubMed] [Google Scholar]
- 2.Bajema IM, Hagen EC, Ferrario F, et al. Immunopathological aspects of systemic vasculitis. Springer Semin Immunopathol. 2001;23:253–265. doi: 10.1007/s002810100074. doi:10.1007/s002810100074. [DOI] [PubMed] [Google Scholar]
- 3.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. doi:10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 4.Kessenbrock K, Krumbholz M, Schönermarck U, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15:623–625. doi: 10.1038/nm.1959. doi:10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Remijsen Q, Kuijpers TW, Wirawan E, et al. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 2011;18:581–588. doi: 10.1038/cdd.2011.1. doi:10.1038/cdd.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li P, Li M, Lindberg MR, et al. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med. 2010;207:1853–1862. doi: 10.1084/jem.20100239. doi:10.1084/jem.20100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Li M, Stadler S, et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. 2009;184:205–213. doi: 10.1083/jcb.200806072. doi:10.1083/jcb.200806072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neeli I, Khan SN, Radic M. Histone deimination as a response to inflammatory stimuli in neutrophils. J Immunol. 2008;180:1895–1902. doi: 10.4049/jimmunol.180.3.1895. [DOI] [PubMed] [Google Scholar]
- 9.Berden AE, Ferrario F, Hagen EC, et al. Histopathologic classification of ANCA associated glomerulonephritis. J Am Soc Nephrol. 2010;21:1628–1636. doi: 10.1681/ASN.2010050477. doi:10.1681/ASN.2010050477. [DOI] [PubMed] [Google Scholar]
- 10.Matuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. doi:10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 11.Nakashima K, Hagiwara T, Yamada M. Nuclear localization of peptidylarginine deiminase V and histone deimination in granulocytes. J Biol Chem. 2002;277:49562–49568. doi: 10.1074/jbc.M208795200. doi:10.1074/jbc.M208795200. [DOI] [PubMed] [Google Scholar]
- 12.Yamada M, Mori M, Sugimura T. Purification and characterization of small molecular weight myeloperoxidase from human promyelocytic leukemia HL-60 cells. Biochemistry. 1981;20:766–771. doi: 10.1021/bi00507a018. doi:10.1021/bi00507a018. [DOI] [PubMed] [Google Scholar]
- 13.Hur SJ, Toda HK, Yamada M. Isolation and characterization of an unprocessed extracellular myeloperoxidase in HL-60 cell cultures. J Biol Chem. 1989;264:8542–8548. [PubMed] [Google Scholar]
- 14.Papayannopoulos V, Metzler KD, Hakkim A, et al. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191:677–691. doi: 10.1083/jcb.201006052. doi:10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Metzler KD, Fuchs TA, Nauseef WM, et al. Myeloperoxidase is required for neutrophil extracellular trap formation: implications for innate immunity. Blood. 2011;117:953–959. doi: 10.1182/blood-2010-06-290171. doi:10.1182/blood-2010-06-290171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hakkim A, Furnrohr B, Amann K, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci USA. 2010;107:9813–9818. doi: 10.1073/pnas.0909927107. doi:10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Mayouf S, Sunker A, Abdwani R, et al. Loss of function variant in DNASE 1 L3 causes a familiar form of systemic lupus erythematosus. Nat Genet. 2011;43:1186–1188. doi: 10.1038/ng.975. doi:10.1038/ng.975. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida M, Sasaki M, Nakabayashi I, et al. Two types of myeloperoxidase-antineutrophil cytoplasmic autoantibodies with a high affinity and low affinity in small vessel vasculitis. Clin Exp Rheumatol. 2009;27:28–32. [PubMed] [Google Scholar]
- 19.Bruner BF, Vista ES, Wynn DM, et al. Epitope specificity of myeloperoxidase antibodies: identification of candidate human immunodominant epitopes. Clin Exp Immunol. 2011;164:330–336. doi: 10.1111/j.1365-2249.2011.04372.x. doi:10.1111/j.1365-2249.2011.04372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakazawa D, Tomaru U, Yamamoto C, et al. Abundant neutrophil extracellular traps in thrombus of patient with microscopic polyangiitis. Front Immunol. 2012;3:33–335. doi: 10.3389/fimmu.2012.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.