Abstract
We report a case of a kidney and pancreas transplanted patient, hospitalized for septic hip arthritis. The whole diagnostic work-up including synovial and bone biopsies remained negative. After inefficient empirical anti-bacterial antibiotic treatment, femoral head resection was performed and tissue analysis revealed Aspergillus fumigatus hyphae. Treatment with voriconazole along with hip replacement led to complete recovery. However, drug interaction between immunosuppressive and anti-fungal drugs was complicated by cellular acute graft rejection. Aspergillus fumigatus arthritis is an uncommon and serious infection that should be evoked especially in the case of resistance to anti-microbial antibiotics and/or an atypical clinical picture.
Keywords: Aspergillus fumigatus, arthritis, immunosuppression, transplant infectious diseases
Introduction
Invasive mycoses represent a major cause of morbidity and mortality in immunocompromised patients. Aspergillus fumigatus, the most frequent agent of fungal infection primarily affects the lower respiratory tract, with a 3-month mortality rate of ∼30%. Invasive aspergillosis with osteo-articular involvement is rare and its prognosis may be even worse, due to additional diagnostic challenges and pharmacokinetics concern, as illustrated by the following case report.
Case report
A 43-year-old man was hospitalized in November 2008 for left hip arthritis. He had undergone simultaneous pancreas–kidney transplantation for Type I diabetic nephropathy in January 2008, which was complicated by an early venous thrombosis of the pancreas. His maintenance treatment consisted of tacrolimus (5 mg bid) and mycophenolate mofetil (MMF) (500 mg bid). Corticosteroids were discontinued in August 2008.
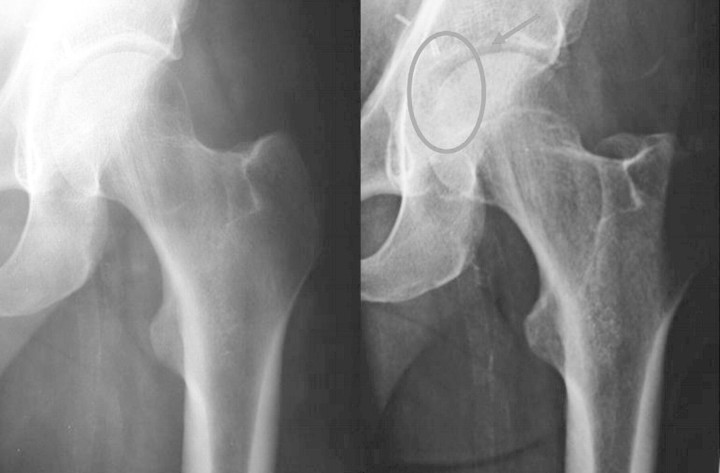
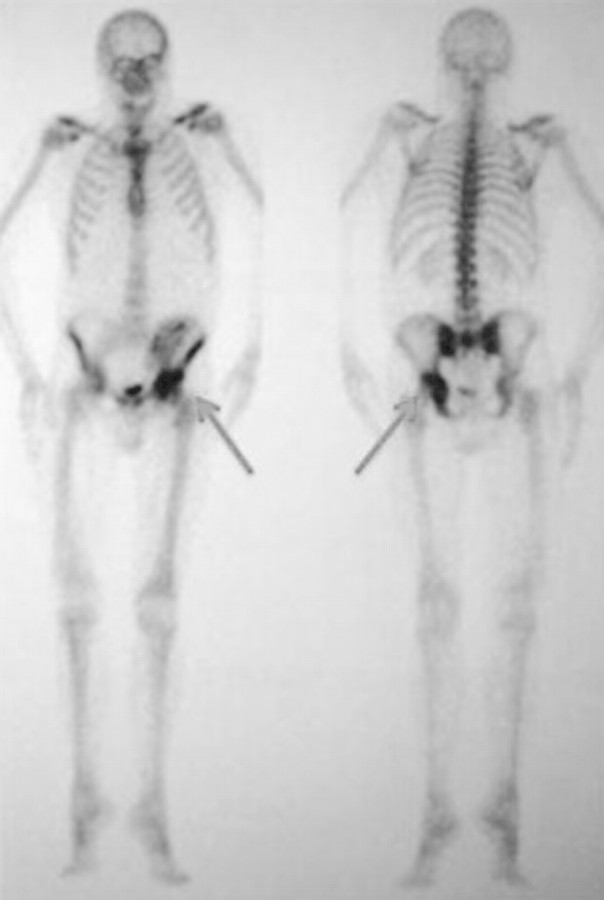
Hip radiographs were normal on admission but revealed a crescentic radiolucent zone on the femoral head when repeated 2 weeks later (Figure 1). Technetium Tc 99-labelled bone scintigraphy revealed an increased uptake at the coxo-femoral joint and ruled out aseptic bone necrosis (Figure 2). Ultrasound-guided synovial biopsy was performed, but no pathogen was found despite specific staining and culture medium for bacteria, mycobacteria and fungi. Histology revealed chronic, non-specific, inflammatory changes with lymphocytic infiltrates and fibrosis. Despite empirical anti-bacterial treatment with a combination of ceftriaxone and fosfomycin, the patient remained febrile and pain increased. Computed tomography (CT) scan-guided synovial and bone biopsies yielded no pathogen. Histology was again non-contributive.
Fig. 1.
Initial (left) and 2 weeks later (right) X-rays: the circle around the radiological image indicates the crescentic radiolucent zone on the femoral head that led to continue with further examination.
Fig. 2.
Bone scintigraphy: increased uptake at the coxo-femoral joint (inflammatory process aspect) that ruled out the hypothesis of an aseptic bone necrosis.
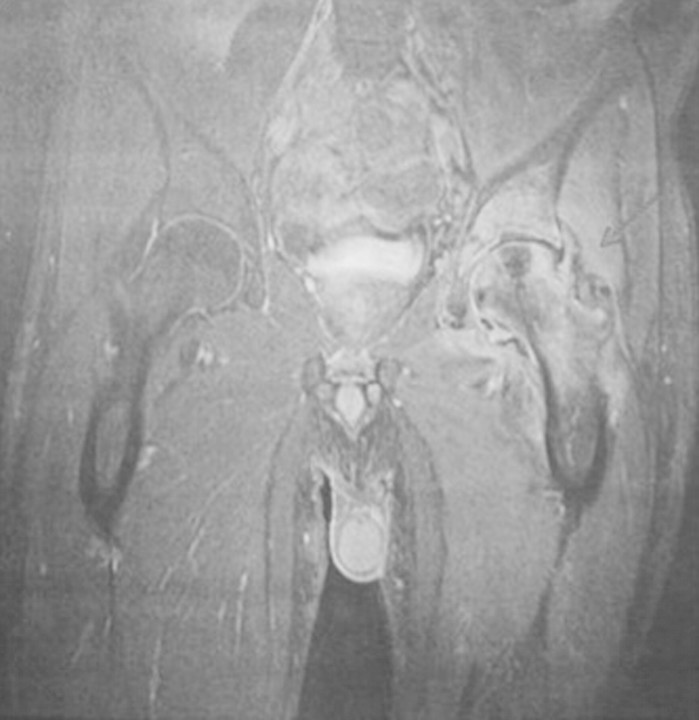
Magnetic resonance imaging revealed severe joint degradation (Figure 3), and a femoral head resection was performed in December 2008, temporarily replaced by a spacer with a high-viscosity bone cement containing gentamycin. Surgical biopsies of bone and synovial tissue revealed branching septated hyphae on haematoxylin–eosin stain and all cultures grew A. fumigatus (Figure 4a and b).
Fig. 3.
Magnetic resonance imaging: severe left femoral head destruction from an acute inflammatory process.
Fig. 4.
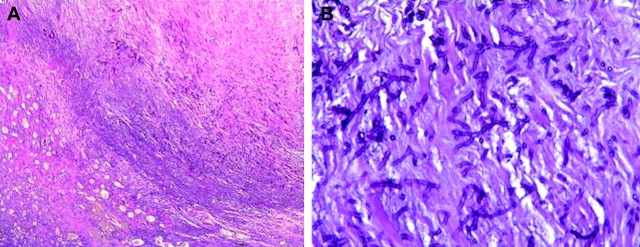
Synovectomy sample (haematoxylin and eosin stain). (a) Magnification ×25. Inflammatory joint rearrangements with presence of abundant fungus. (b) Magnification ×200. Fungus with septate and hyaline hyphae characteristic of Aspergillus species.
A treatment with oral voriconazole, 200 mg bid, was started along with a 50% reduction of tacrolimus doses in view of drug interactions through cytochrome P450.
The source of aspergillosis could not be determined, and no other infectious foci were found. Chest, brain and sinuses CT scans were normal. The patient had never suffered a hip trauma. Galactomannan serum assay and serological testing for Aspergillus antibodies were repeatedly negative.
The patient improved remarkably, and hip replacement by a prosthetic joint was performed 6 months after voriconazole was initiated. Seven samples of bone and synovial tissue found no residual evidence of fungal infection, and voriconazole was discontinued 2 weeks after surgery.
Meanwhile, the patient was re-admitted in March 2009 for pneumonia due to Haemophilus influenzae and Branhamella catarrhalis, and in June 2009 for agranulocytosis, which led to decreasing MMF dosage.
For these reasons, during a few months, tacrolimus plasma concentrations were maintained at low levels (i.e. <5 mg/L). Three days after voriconazole discontinuation, and despite an increase of tacrolimus dosage from 3 to 8 mg bid as we anticipated drug interaction consequences, the patient developed an acute cellular graft rejection. Pulsed high-dose steroids failed to control rejection. Considering the benefit/risk balance, therapy was limited to steroids. Renal function did not improve, so the patient re-started maintenance haemodialysis and was registered again on the kidney transplant waiting list.
Discussion
Aspergillus fumigatus arthritis is an uncommon infection, mostly observed in the setting of disseminated invasive aspergillosis affecting severely immunocompromised patients. Only 16 cases of A. fumigatus arthritis have been reported thus far (not including vertebral osteomyelitis), with the knees and shoulders being the most frequently affected joints [1]. To the best of our knowledge, our case is the first documented observation of hip aspergillosis.
The main conditions associated with osteoarticular aspergillosis are haematological malignancies, stem cell transplantation and renal transplantation (three cases reported). Cases have also been reported following intra-articular administration of corticosteroids [1, 2].
The source of aspergillosis may be a direct inoculation, as in the cases following intra-articular corticosteroid injections. Alternatively, the haematogenous route is the most likely route, when Aspergillus species seed via the bloodstream during disseminated invasive aspergillosis. Surprisingly, there were neither arguments for direct intra-inoculation nor haematogenous seeding in our observation where no source was found and no other infectious foci were identified despite extensive investigations. Of note, among the 16 cases of A. fumigatus arthritis reported, only two had no evidence of disseminated disease, similar to our patient [2–4].
The lessons of this case are useful to transplantation teams. The diagnosis of osteo-articular aspergillosis is difficult because of its rarity, the non-specific clinical and radiological findings and the difficult access to the infected tissue. The delay to diagnosis in this observation was long, due to failure of two attempts to come up with a diagnosis through ultrasound- and CT scan-guided bone and synovial samples. The low yield of microbiologic sampling and the failure of a wide spectrum, empirical anti-bacterial treatment are clues that should prompt clinicians to include invasive mycosis in the differential diagnosis of septic arthritis in immunocompromised patients.
In this population, the treatment ideally combines surgery and prolonged use of anti-fungal agents. Although limited data are available on the optimal medical treatment of osteo-articular invasive aspergillosis, voriconazole, the only azole agent licensed for use in invasive aspergillosis, has the most satisfactory profile for osteo-articular aspergillosis, including efficacy, diffusion and bioavailability allowing oral use for prolonged treatment [5]. However, failure of azole anti-fungal agents has been reported, which lead to using amphotericin B as a salvage therapy [2, 6–8].
Two other points must be considered: (i) if possible, immunosuppressive treatment should be reduced, at least temporarily, as the degree of immunosuppression strongly influences the outcome of invasive aspergillosis; (ii) drug interactions must be kept in mind, taking into account the effect of voriconazole, which interferes with P450 cytochrome oxidase.
Aspergillus fumigatus arthritis in immunocompromised patients requires a combination of surgery and the prolonged use of voriconazole. The price to pay can be rejection following drug interaction with the immunosuppressive regimen.
Acknowledgments
Conflict of interest statement. None declared.
References
- 1.Golmia R, Bello I, Marra A, et al. Aspergillus fumigatus joint infection: a review. Semin Arthritis Rheum. 2011;40:580–584. doi: 10.1016/j.semarthrit.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 2.García-Porrúa C, Blanco FJ, Atanes A, et al. Septic arthritis by Aspergillus fumigatus: a complication of corticosteroid infiltration. Br J Rheumatol. 1997;36:610–611. doi: 10.1093/rheumatology/36.5.610. [DOI] [PubMed] [Google Scholar]
- 3.Baumann PA, Cunningham B, Patel NS, et al. Aspergillus fumigatus infection in a mega prosthetic total knee arthroplasty: salvage by staged reimplantation with 5-year follow-up. J Arthroplasty. 2001;16:498–503. doi: 10.1054/arth.2001.21505. [DOI] [PubMed] [Google Scholar]
- 4.Mekan SF, Saeed O, Khan JA. Invasive aspergillosis with polyarthritis. Mycoses. 2004;47:518–520. doi: 10.1111/j.1439-0507.2004.01031.x. [DOI] [PubMed] [Google Scholar]
- 5.Mouas H, Lutsar I, Dupont B, et al. Voriconazole for invasive bone aspergillosis: a worldwide experience of 20 cases. Clin Infect Dis. 2005;40:1141–1147. doi: 10.1086/428734. [DOI] [PubMed] [Google Scholar]
- 6.Stratov I, Korman TM, Johnson PD. Management of Aspergillus osteomyelitis: report of failure of liposomal amphotericin B and response to voriconazole in an immunocompetent host and literature review. Eur J Clin Microbiol Infect Dis. 2003;22:277–283. doi: 10.1007/s10096-003-0909-3. [DOI] [PubMed] [Google Scholar]
- 7.Franco M, Van Elslande L, Robino C, et al. Aspergillus arthritis of the shoulder in a renal transplant recipient. Failure of itraconazole therapy. Rev Rhum Engl Ed. 1995;62:215–218. [PubMed] [Google Scholar]
- 8.Kirby A, Hassan I, Burnie J. Recommendations for managing Aspergillus osteomyelitis and joint infections based on a review of the literature. J Infect. 2006;52:405–414. doi: 10.1016/j.jinf.2005.08.016. [DOI] [PubMed] [Google Scholar]