Abstract
In oncogenic osteomalacia (OOM), fibroblast growth factor 23 (FGF23) induces renal phosphate wasting and inhibits the appropriate increase of calcitriol. A patient suffering from OOM is described. Serum calcium, phosphate, biointact parathyroid hormone and intact FGF23 as well as the calcitriol and 24,25-vitamin D levels were measured before and after tumour removal. The clinical approach to a patient with hypophosphataemia is discussed and the changes in mineral metabolism after removal of a FGF23-producing tumour are described.
Keywords: FGF23, mineral metabolism, osteomalacia, phosphate
Background
Phosphate homeostasis is maintained by a complex interaction between vitamin D, parathyroid hormone (PTH) and bone-derived fibroblast growth factor 23 (FGF23). Phosphate depletion increases the rate of synthesis of calcitriol, while phosphate loading has the opposite effect [1, 2]. FGF23 decreases tubular phosphate reabsorption by internalization of the sodium–phosphate co-transporters NaPi-IIa and -IIc. It decreases the 1-α hydroxylation of vitamin D by cytochrome P450 (CYP)27B1 as well as increases vitamin D degradation by CYP24 [3, 4]. Oncogenic osteomalacia (OOM) is a rare hypophosphataemic disease caused by unregulated production of FGF23. For a recent review of the condition, see [5]. In this case report, subsequent changes in mineral metabolism before and after removal of a FGF23-producing tumour are described.
Case report
A 73-year-old woman, without any skeletal disease in the family or rickets in her childhood, developed spontaneous fractures, muscle weakness and severe pain. She had a history of oestrogen receptor-positive endometrial adenocarcinoma 13 years earlier, which was cured by hysterectomy, bilateral oophorectomy and treatment with the progestagen megestrol for 11 years. The last 3 years, she developed progressive skeletal pain, muscle weakness and low energy fractures of right hip, tibias, ribs and vertebras. She received treatment by weekly alendronate, calcium carbonate and cholecalciferol 800 IU/day. Biochemical analyses (see Table 1) revealed hypophosphataemia and increased fractional excretion (FE) of phosphate in the urine, calculated as (serum phosphate × urine creatinine)/(urine phosphate × serum creatinine) × 100. The maximal phosphate reabsorption per glomerular filtration rate (TmP/GFR) according to the nomogram of Bivojet was 0.6 mmol/L (1.9 mg/dL) [normal range >0.85 mmol/L (>2.63 mg/dL)]. Alendronate treatment was stopped and treatment with phosphate capsules 1500 mg thrice daily and 1-alfa-calcidol (Etalpha) 1.5 μg daily was initiated 6 months before diagnostic work-up. The calcium level was normal and the PTH level became slightly elevated after start of phosphate substitution. Her calcidiol level was sufficient: 88 nmol/L (35.2 ng/mL). Her creatinine clearance was 91 mL/min/1.73 m2. Urinary excretion of albumin was 24 mg/24 h. There was no glucosuria on dipstick testing. The serum bicarbonate level was 27 mEq/L and the urinary pH was 6. There was no monoclonal immunoglobulin in serum or urine. The FGF23 level was increased to 100 pg/mL. The level of alkaline phosphatase was increased to 3.7 μcat/L (reference range 0.6–1.8). Bone density measured by dual X-ray absorption was 0.603 g/cm2 (T-score −3.3) at the left hip and 0.884 g/cm2 (T-score −2.5) at lumbar spine. For differential diagnoses of hyperphosphaturic hypophosphataemia and their relation to FGF23 level see Table 2.
Table 1.
Laboratory parameters before and after tumour removala
| Time (hour:minute) | Phosphate (mmol/L) | Calcium (mmol/L) | BiPTH (pg/mL) | FGF23 (pg/mL) | 1,25-vitD (pg/mL) | 24,25-vitD (ng/mL) | FE-Pi % | FE-Ca % |
| Normal range: | 0.80–1.50 | 2.15–2.50 | 5.5–30.9 | 10–50 | 20–80 | 5–15 | 1–2 | |
| 0 | 0.87 | 2.32 | 46.4 | 71.3 | 88.5 | 5.6 | 32 | 0.59 |
| 0:35 | ND | ND | 27.3 | 38.9 | 74.8 | 4.3 | ||
| 1:45 | 0.72 | 2.30 | 30 | 34.8 | 80.8 | 5.2 | ||
| 3:05 | 0.79 | 2.29 | ND | 31.7 | 76.2 | 5.2 | ||
| 4:05 | ND | ND | ND | 31 | 74.6 | 5 | ||
| 5:20 | ND | ND | 35.5 | 29 | 90.2 | 6.2 | ||
| 6:15 | 0.75 | 2.21 | ND | 28 | 75.2 | 5.2 | ||
| 10:15 | ND | ND | 40.9 | 28 | 95.2 | 4.5 | ||
| 14:20 | 0.79 | 2.37 | ND | 28 | 122.2 | 5.5 | ||
| 18:20 | ND | ND | ND | 28 | 134 | 5 | ||
| 22:20 | 0.81 | 2.37 | ND | 29 | 162.3 | 3.9 | ||
| 48 | 0.80 | ND | 56.4 | ND | ND | ND | 16 | 0.69 |
| 1 week | 1.36 | 2.37 | 21.8 | 28 | 267 | 3.1 | ||
| 2 weeks | 1.36 | 2.34 | 38.2 | 28 | 211.4 | 2.8 | ||
| 6 months | 1.34 | 2.48 | 10.9 | 27 | 159.1 | 5.5 |
BiPTH, bioactive 1-84 parathyroid hormone; ND, not determined; 1,25 vit D, 1,25-dihydroxyvitamin D; 24,25-vitD, 24,25-dihydroxyvitamin D; FE-Pi and FE-Ca are fractional excretion of phosphate and calcium, respectively, and are calculated as [serum mineral × urine creatinine (mmol/L)/serum creatinine × urine mineral (mmol/L)]. To convert phosphate to mg/dL multiply with 3.1; calcium to mg/dL multiply with 4; BiPTH to pmol/L multiply with 0.9; 1,25 vit to pmol/L multiply with 2.496; 24,25 vit D to nmol/L multiply with 2.496.
Table 2.
Hyperphosphaturic hypophosphataemic disordersa
| Diagnosis or clinical manifestation (OMIM number) | Defective protein (gene) | Tissue | Serum FGF23 |
| XLH (307800) | Phosphate-regulating gene with homology to endopeptidases on the X chromosome (PHEX) | Bone | ↑ |
| ADHR (193100) | Fibroblast growth factor 23 (FGF23) | Osteocytes | ↑ |
| ARHR-1 (241520) | Dentin matrix protein 1 (DMP1) | Bone | ↑ |
| ARHR-2 (613312) | Ectonucleotide pyrophosphatase phosphodiesterase 1 (ENPP1) | Bone | ↑ |
| Hyperphosphaturia, osteoporosis, kidney stones | NaPi co-transporter IIa (SLC34A1) | Proximal tubules | =, ↓ |
| HHRH (241530) | NaPi co-transporter IIc (SLC34A3) | Proximal tubules | =, ↓ |
| Oncogenic osteomalacia | Acquired, unregulated FGF23 production | Mesenchymal tumour | ↑-↑↑ |
| Fanconi syndrome | Genetic or acquired | Proximal tubules | =, ↓ |
OMIM, online mendelian inheritance in man; XLH, X-linked hypophosphataemia; ADHR, autosomal dominant hypophosphataemic rickets; ARHR, autosomal recessive hypophosphataemic rickets; HHRH, hereditary hypophosphataemic rickets with hypercalcuria.
In conclusion, the patient suffered from an acquired form of hypophosphataemic osteomalacia due to excess FGF23, which makes OOM the most likely diagnosis. The challenge in such cases is to localize a small tumour that can be located anywhere in the body.
Radiological and nuclear examinations, including venous sampling for a venous gradient of FGF23 and fluorodeoxy-glucose positron emission tomography, lead to the localization of a small subcutaneous tumour in the sole of the right forefoot. The 1-alfa-calcidol treatment was stopped 3 days before surgery and the phosphate supplementation was gradually tapered from the day after surgery.
Immunoabsorbent assays were used for analyses of bioactive 1-84 PTH (Scantibodies) and intact FGF23 (Kainos; Japan) before extirpation and at 10 time points the first day, as well as after 1 and 2 weeks and 6 months, (see Table 1 and Figure 1). Serum calcitriol and 24,25-vitamin D were measured using liquid chromatography-tandem mass spectrometry recently described [6]. Serum was collected, protected from light and stored in −70°C until analysed.
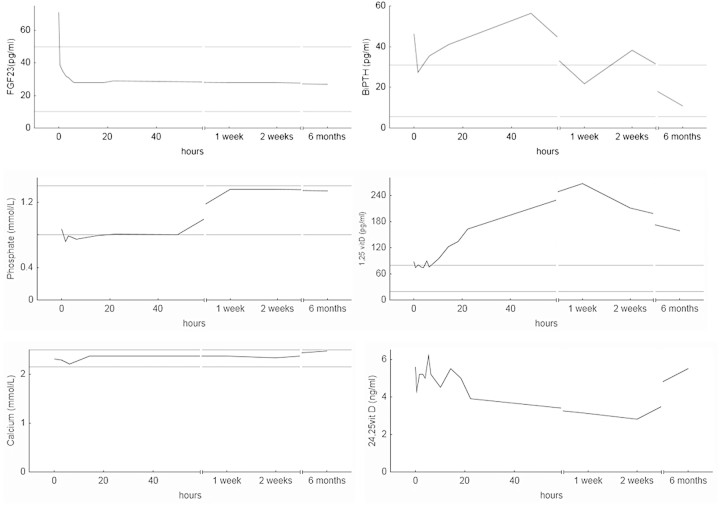
Fig. 1.
Parameters of mineral metabolism before and after removal of an FGF23-producing tumour. Area between horizontal grey lines represent normal range.
Thirty minutes after extirpation of the tumour, serum FGF23 had decreased to normal range. An initial reduction of biointact PTH was followed by a gradual increase to above pre-operative levels. Calcitriol concentration increased 10 h after surgery, reached a maximum after 1 week and remained elevated 6 months after surgery. The 24,25-vitamin D concentration, on the other hand, decreased after ∼20 h and remained lower compared to pre-operative levels at 1 and 2 weeks. The bone mineral density increased 30% in 6 months. Most importantly, the patient's symptoms and general condition improved remarkably.
On histopathological examination, the 11-mm nodular tumour had the features of a mesenchymal tumour with abundant matrix with small spindle-shaped cells and granular calcifications and a cell-rich component of irregular cells with foci of multinucleated osteoclast-like cell in accordance with a phosphaturic mesenchymal tumour of the mixed connective tissue type [7].
Discussion
This case illustrates the clinical picture of a hypophosphataemic syndrome due to excess FGF23 and the time-related changes in the parameters of mineral metabolism as FGF23 is normalized.
Other causes of chronic hypophosphataemia could be excluded by clinical history: there were no congenital or inherited skeletal problems, no malnutrition, malabsorption nor exposure to tubular toxins; and the calcidiol level was normal. For an up-to-date discussion of the approach to the hypophosphataemic patient, see [8].
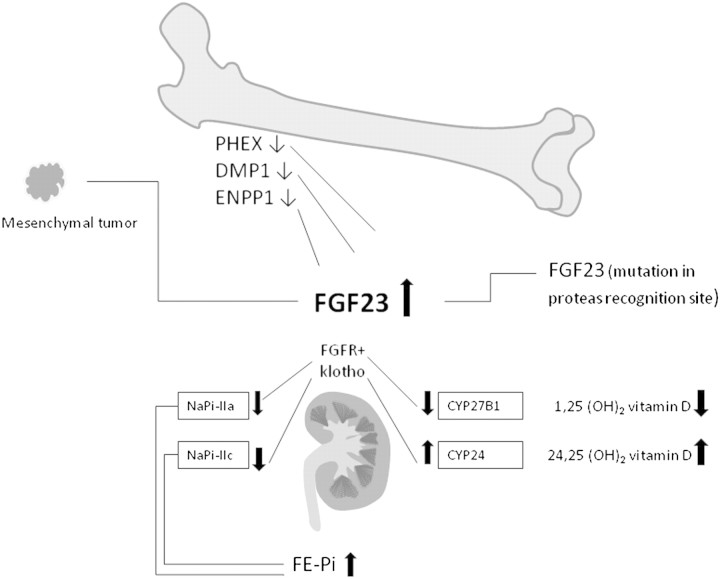
Increased FGF23 may be due to a mutation in the FGF23 gene, making it resistant against enzymatic degradation as in autosomal dominant hypophosphataemic rickets [9]. Mutations in the PHEX, DMP1 or ENPP1 genes, respectively, induce a defect in the normal regulation of FGF23 synthesis in osteocytes by unknown mechanisms. These mutations all give rise to hereditary rickets, see Table 2 and [10]. The recessive disorder hereditary hypophosphataemic rickets with hypercalciuria on the other hand is due to mutations in the SLC34A3 gene, causing decreased activity of the tubular sodium phosphate co-transporter NaPi-IIc [11]. The condition is associated with increased calcitriol and hypercalciuria and a decrease in serum FGF23. Mutations in SLC34A1 resulting in a decrease in NaPi-IIa cause nephrolithiasis, osteoporosis and mild hypophosphataemia [12] (see Figure 2). Acquired chronic hypophosphataemia may be due to general or selective proximal tubular damage or Fanconi syndrome, caused by light chains associated with monoclonal gammopathies or other tubular toxins. FGF23 is normal or decreased in chronic hypophosphataemic disorders not caused by FGF23 excess [13]. The patient in this case had an acquired form of phosphate wasting, the FGF23 level was increased and there were no other features of general tubular dysfunction.
Fig. 2.
Mechanisms of increased FGF23. Hypophosphataemia due to increased FGF23 may be due to paraneoplastic synthesis by mesenchymal tumours, genetic defects affecting the FGF23 synthesis and possibly phosphate sensing in bone cells or a mutation in FGF23 itself rendering it resistant against enzymatic degradation. FGF23 signals via FGF receptors in conjunction with its co-receptor klotho in the kidney downregulating phosphate transporters and decreasing calcitriol synthesis. Mutated or knocked out klotho in animals causes hyperphosphataemia and increased calcitriol, despite high FGF23. A mutation in gene coding for NaPi-IIc causes renal phosphate wasting with a compensatory increase in calcitriol and hypercalciuria due to increased intestinal calcium absorption. Abbreviations: PHEX, phosphate-regulating gene with homology to endopeptidases on the X chromosome; DMP, dentin matrix protein; ENPP, ectonucleotide pyrophosphatase/phosphodiesterase; FE-Pi, fractional excretion of phosphate; ↓ means decreased expression or function; ↑ means increased concetration or function.
After surgery, the phosphate level normalized within 1 week, and the phosphate FE % had decreased already 2 days after surgery (Table 1).
PTH gradually increased to above pre-operative levels the first day after extirpation after a short decrease immediately after surgery. The finding is unexpected as FGF23 inhibits PTH secretion in vitro and in animal experiments [14]. Though, in chronic kidney disease and other situations with increased FGF23, there is development of hyperparathyroidism. In OOM as well as in other states with phosphate wasting due to FGF23 as X-linked hypophosphataemia (XLH), treatment with high doses of phosphate and active vitamin D induces an increase in FGF23 and PTH [15]. Also, in experiments with FGF23-neutralizing antibodies in the hyp mice, an animal homologue of XLH, there was actually an immediate decrease in PTH [16]. The regulation of PTH in hypophosphataemic disorders is incompletely understood. Vitamin D deficiency and calcium malabsorption, aggravated by large phosphate intake, may induce hyperparathyroidism. The fast post-operative decrease in PTH is difficult to explain. It may have been induced by the drop in phosphate level, increasing the free calcium fraction or increasing intra-cellular pre-PTH degradation in the parathyroid gland.
Serum calcitriol level started to increase after ∼10 h mirrored by a decline in 24,25-vitamin D starting after ∼20 h. CYP27B1 is the rate-limiting enzyme in calcitriol synthesis, while CYP24 inactivates calcitriol and its precursor 25-hydroxy vitamin D. CYP24 is stimulated by calcitriol, an effect attenuated by PTH [17]. FGF23 stimulates CYP24 activity in the kidney. Increased CYP24 activity, rather than decreased CYP27b1 activity, seems to be the cause of calcitriol deficiency in uraemic rat [18]. Treatment with FGF23 antibodies in hyp mice increases CYP27B1 expression and decreases the CYP24 activity [16].
FGF23 induced hypophosphataemia due to decreased phosphate reabsorption and prevented an appropriate increase of calcitriol. When FGF23 normalized, phosphate reabsorption increased the activity of CYP27B1 and CYP24 reacted adequately to the low serum phosphate. The calcitriol remained elevated after 6 months, when PTH and FGF23 were in reference range, reflecting an intrinsic regulation of calcitriol production stimulated by a positive phosphate balance due to continuing mineralization of the skeleton and need for increased phosphate reabsorption to maintain homeostasis.
In conclusion, this case verifies the known effects of FGF23, illustrates the utility of FGF23 determination when encountering a patient with hypophosphataemia and points to one of the unsolved questions concerning FGF23, namely its direct versus indirect effects on the parathyroid glands.
Acknowledgments
This clinical project was funded by Uppsala University.
Conflict of interest statement. None declared.
References
- 1.Portale AA, Halloran BP, Murphy MM, et al. Oral intake of phosphorus can determine the serum concentration of 1,25-dihydroxyvitamin D by determining its production rate in humans. J Clin Invest. 1986;77:7–12. doi: 10.1172/JCI112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Portale AA, Booth BE, Halloran BP, et al. Effect of dietary phosphorus on circulating concentrations of 1,25-dihydroxyvitamin D and immunoreactive parathyroid hormone in children with moderate renal insufficiency. J Clin Invest. 1984;73:1580–1589. doi: 10.1172/JCI111365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jonsson KB, Zahradnik R, Larsson T, et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003;348:1656–1663. doi: 10.1056/NEJMoa020881. [DOI] [PubMed] [Google Scholar]
- 4.Yamazaki Y, Okazaki R, Shibata M, et al. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab. 2002;87:4957–4960. doi: 10.1210/jc.2002-021105. [DOI] [PubMed] [Google Scholar]
- 5.Chong WH, Molinolo AA, Chen CC, et al. Tumor-induced osteomalacia. Endocr Relat Cancer. 2011;18:R53–R77. doi: 10.1530/ERC-11-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casetta B, Jans I, Billen J, et al. Development of a method for the quantification of 1alpha, 25(OH)2-vitamin D3 in serum by liquid chromatography tandem mass spectrometry without derivatization. Eur J Mass Spectrom (Chichester, Eng) 2010;16:81–89. doi: 10.1255/ejms.1024. [DOI] [PubMed] [Google Scholar]
- 7.Folpe AL, Fanburg-Smith JC, Billings SD, et al. Most osteomalacia-associated mesenchymal tumors are a single histopathologic entity: an analysis of 32 cases and a comprehensive review of the literature. Am J Surg Pathol. 2004;28:1–30. doi: 10.1097/00000478-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Bacchetta J, Salusky IB. Evaluation of hypophosphatemia: lessons from patients with genetic disorders. Am J Kidney Dis. 2012;59:152–159. doi: 10.1053/j.ajkd.2011.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ADHR consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26:345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 10.Carpenter TO. The expanding family of hypophosphatemic syndromes. J Bone Miner Metab. 2012;30:1–9. doi: 10.1007/s00774-011-0340-2. [DOI] [PubMed] [Google Scholar]
- 11.Bergwitz C, Roslin NM, Tieder M, et al. SLC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am J Hum Genet. 2006;78:179–192. doi: 10.1086/499409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magen D, Berger L, Coady MJ, et al. A loss-of-function mutation in NaPi-IIa and renal Fanconi's syndrome. N Engl J Med. 2010;362:1102–1109. doi: 10.1056/NEJMoa0905647. [DOI] [PubMed] [Google Scholar]
- 13.Endo I, Fukumoto S, Ozono K, et al. Clinical usefulness of measurement of fibroblast growth factor 23 (FGF23) in hypophosphatemic patients: proposal of diagnostic criteria using FGF23 measurement. Bone. 2008;42:1235–1239. doi: 10.1016/j.bone.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Galitzer H, Ben-Dov I, Lavi-Moshayoff V, et al. Fibroblast growth factor 23 acts on the parathyroid to decrease parathyroid hormone secretion. Curr Opin Nephrol Hypertens. 2008;17:363–367. doi: 10.1097/MNH.0b013e328303e172. [DOI] [PubMed] [Google Scholar]
- 15.Imel EA, DiMeglio LA, Hui SL, et al. Treatment of X-linked hypophosphatemia with calcitriol and phosphate increases circulating fibroblast growth factor 23 concentrations. J Clin Endocrinol Metab. 2010;95:1846–1850. doi: 10.1210/jc.2009-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aono Y, Yamazaki Y, Yasutake J, et al. Therapeutic effects of anti-FGF23 antibodies in hypophosphatemic rickets/osteomalacia. J Bone Miner Res. 2009;24:1879–1888. doi: 10.1359/jbmr.090509. [DOI] [PubMed] [Google Scholar]
- 17.Petkovich M, Jones G. CYP24A1 and kidney disease. Curr Opin Nephrol Hypertens. 2011;20:337–344. doi: 10.1097/MNH.0b013e3283477a7b. [DOI] [PubMed] [Google Scholar]
- 18.Helvig CF, Cuerrier D, Hosfield CM, et al. Dysregulation of renal vitamin D metabolism in the uremic rat. Kidney Int. 2010;78:463–472. doi: 10.1038/ki.2010.168. [DOI] [PubMed] [Google Scholar]