Abstract
Idiopathic olfactory loss (IOL) is a common olfactory disorder. Little is known about the pathophysiology of this disease. Previous studies demonstrated decreased olfactory bulb (OB) volume in IOL patients when compared with controls. The aim of our study was to investigate structural brain alterations in areas beyond the OB. We acquired T1-weighted magnetic resonance images from 16 patients with IOL and from 16 age- and sex-matched controls on a 3T scanner. Voxel-based morphometry (VBM) was performed using VBM8 toolbox and SPM8 in a Matlab environment. Psychophysical testing confirmed that patients had higher scores for Toyota and Takagi olfactometer and lower scores for Sniffin’ Sticks olfactory test than controls (t = 46.9, P < 0.001 and t = 21.4, P < 0.001, respectively), consistent with olfactory dysfunction. There was a significant negative correlation between the 2 olfactory tests (r = −0.6, P = 0.01). In a volume of interest analysis including primary and secondary olfactory areas, we found patients with IOL to exhibit gray matter volume loss in the orbitofrontal cortex, anterior cingulate cortex, insular cortex, parahippocampal cortex, and the piriform cortex. The present study indicates that changes in the central brain structures proximal to the OB occur in IOL. Further investigations of this phenomenon may be helpful to elucidate the etiology of IOL.
Key words: gray matter volume, human, idiopathic, magnetic resonance imaging, olfactory loss, voxel-based morphometry
Introduction
Olfactory dysfunction has myriad etiologies, such as sinonasal disease, infections of the upper respiratory tract, head trauma, toxic exposures, neurodegenerative disease, and congenital abnormalities. Some patients are diagnosed with idiopathic olfactory loss (IOL) when the cause remains unknown despite an extensive evaluation, including detailed questionnaires, psychophysical testing of olfactory performance and olfactory pathway, and morphology assessment by magnetic resonance imaging (MRI) or olfactory event-related potentials. Previous reports have indicated that IOL may be related to cognitive impairment. Some patients with IOL may develop Parkinson’s disease (PD) (Haehner et al. 2007). In addition, IOL may be an early sign of Alzheimer’s disease (AD) and PD (Koss et al. 1988; Haehner et al. 2007). For the AD patients, the olfactory impairment is central rather than peripheral (Koss et al. 1988). In our smell and taste center, 13.6% of all patients presenting with olfactory problems were diagnosed with IOL (Chen et al. 2013).
Voxel-based morphometry (VBM) is an elegant method to investigate brain volume, including gray matter, white matter, and cerebrospinal fluid volume (Ashburner and Friston 2000). Using VBM, several studies have investigated the relationship between structure of brain and olfactory function in healthy subjects. Olfactory performance correlates with both the cortical thickness of distinct structures like the orbitofrontal cortex (OFC), the insular cortex (IC), and the volume of the right OFC in healthy people (Frasnelli et al. 2010; Seubert et al. 2013). In addition, a study showed sex differences in the gray matter volume of the normal olfactory system.(Garcia-Falgueras et al. 2006).
Olfactory bulb (OB) volume, and morphological alterations in the primary and secondary olfactory brain areas have been demonstrated to be related to various types of olfactory dysfunction, for example, in patients with postviral anosmia (Mueller et al. 2005; Rombaux et al. 2006), posttraumatic anosmia (Mueller et al. 2005), congenital anosmia (Frasnelli et al. 2013; Yao et al. 2013), and parosmia (Bitter et al. 2011). Very little is known about brain structure changes in patients with IOL. A study suggests that IOL patients have decreased OB volume as compared with controls (Rombaux et al. 2010). Cortical brain areas beyond the OB have received less attention in the case of IOL. Bitter et al. (2010a) reported the reduction of gray matter volume in 17 anosmic patients (8 idiopathic, 4 postinfectious, 5 posttraumatic patients) compared with 17 controls. They did not separate IOL patients from other anosmic subjects or evaluate specific areas in the olfactory pathway related to IOL.
The present investigation was undertaken because the reason for olfactory loss in patients with IOL remains unclear. Study of imaging abnormalities in IOL, may offer insight into disease pathophysiology. The aim of this study was to evaluate structural changes in brain areas of patients with IOL compared with control subjects. We hypothesized that there are specific changes in both primary and secondary olfactory cortices in IOL patients.
Materials and methods
Subjects
Sixteen adult IOL patients (9 male, 7 female) and sex- and age-matched controls subjects were included in this study. Standard otorhinolaryngological evaluations, including medical history and examination including nasal endoscopy, were performed. At the onset of olfactory loss, all patients had no history of brain trauma, acute infection of the upper respiratory airway, sinonasal or brain disease, drug or toxic exposure, nor evidence of malingering. All patients had no other neurological or psychiatric deficits except for the loss of sense of smell. The Mini-Mental-State-Examination (MMSE) showed normal cognitive function in both control subjects and patients. All participants were right-handed according to the Edinburgh Handedness Inventory (Oldfield 1971). This study was approved by the local ethics committee. Written informed consent was obtained from all participants.
Psychophysical testing of olfactory performance
Psychophysical testing of olfactory function was performed with the Toyota and Takagi (T&T) olfactometer and Sniffin’ Sticks (SS) tests, 2 validated measures for each subject (Daiichi Pharmaceutical Co. Ltd; Burghart Gmbh) (Kobal et al. 1996; Hummel et al. 1997; Yang et al. 2010). Standard administration was performed according to the manufacturer’s instructions for the T&T olfactometer. Average odor threshold was obtained by dividing the sum of identification threshold for 5 odorants by 5. The SS test battery was performed by the use of standard methods (Kobal et al. 1996; Hummel et al. 1997). The test was composed of 3 parts: threshold to n-butanol (T), odor discrimination (D), and odor identification (I). The final score was expressed as the sum of the T, D, and I values.
Acquisition and analysis of structural MRI data
All MRI data were acquired on a 3.0 Tesla scanner (Magnetom Trio Tim system; Siemens) using a standard 12-channel head coil. T1-weighted images were acquired using an MP-RAGE sequence (repetition time = 1900ms; echo time = 2.52ms; flip angle = 9°; field of view = 250×250mm; 176 sagittal slices; voxel size = 1×1 × 1mm, and total acquisition time = 5:40min).
Preprocessing of T1-weighted images was performed using SPM8 (Wellcome Trust Centre for Neuroimaging, UCL; http://www.fil.ion.ucl.ac.uk/spm) implemented in the VBM8 Toolbox (Christian Gaser, Department of Psychiatry, University of Jena; http://dbm.neuro.uni-jena.de/vbm) under Matlab 7.12 space (The MathWorks Inc.). Standard routines and default parameters of the VBM8 toolbox were applied. Images were bias corrected, preregistered to standardized Montreal Neurological Institute (MNI) space and segmented. Segmentation in SPM8 was based on a modified gaussian mixture model to avoid misclassification. The warping to MNI space was performed using nonlinear transformation. Gray matter segments were modulated by the Jacobian determinate of the deformations to account for local expansion and compression introduced by nonlinear transformation. Finally, the gray matter images were smoothed with an 8-mm full-width at half-maximum isotropic gaussian kernel. This was done to reduce errors related to intersubject variability in local anatomy and to render the imaging data more normally distributed.
Statistical analysis
Voxel-wise gray matter volume of the patient group were compared with those of the control group. We assumed a t-distribution of the 2 group data and used independent 2-sample t-tests. In order to avoid possible edge effects between different tissue types, we excluded all voxels with gray matter values of less than 0.2 (absolute threshold masking). In order to investigate subtle changes in the primary and secondary olfactory areas (volume of interest [VOI]), we applied a threshold of P < 0.01 (uncorrected) with an extent of 100 voxels. Functional masks (http://flavor.monell.org/~jlundstrom/index_ALE.html) (Seubert et al. 2013) were used to select olfactory regions including piriform cortex (PC) and OFC. The insula, cingulum, hippocampus, parahippocampal, and thalamus was selected by mean of the structural masks. These areas were selected due to their role in olfaction (Figure 1) (Bitter et al. 2011).
Figure 1.
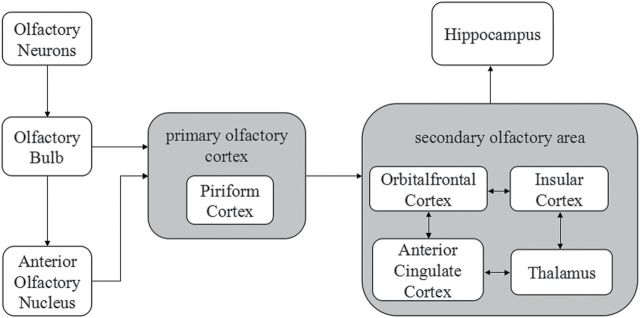
Schematic diagram of the major central olfactory pathway (based on references Gottfried 2006; Bitter et al. 2011). Regions in gray represent the primary and secondary olfactory cortex. The PC is the major part of the primary olfactory cortex. The secondary olfactory cortex mainly includes OFC, IC, ACC, and thalamus. Projections between the secondary olfactory cortex subregions are reciprocal.
All statistical analyses were performed using SPSS version 17.0 software (SPSS Inc.). The significance level was set at 0.05. The independent sample t-test was used to confirm that both groups were equivalent in terms of age. Psychophysical results of olfactory function and brain volume were expressed as mean ± standard deviation and submitted to independent sample t-test with group as factors. Pearson statistics were used for correlation analyses between different olfactory tests.
Results
The age of the IOL group ranged from 29 to 61 years (mean = 48.6±9.9 years). The age of controls ranged from 32 to 60 years (mean = 52.5±8.7 years). There were no significant differences in age between IOL and controls group (t = 1.19, P = 0.25). The mean duration of symptoms from sensory problem recognition to clinical evaluation was 3.5 years (range 0.5–10 years) in the IOL group. The MMSE showed an average value of 29.1±0.5 in controls and 29.3±0.7 in patients groups, with no significant difference between the 2 groups (t = 0.59, P = 0.56).
T&T olfactometer and TDI (odor Threshold, Discrimination, Identification) scores were respectively 5.8±0.2, 9.4±3.6 for IOL patients versus 0.6±0.4, 29.9±1.3 for controls. T&T scores were significantly negative correlated with TDI scores (r = −0.6, P = 0.01), indicating as expected, that greater T&T scores generally were associated with lower TDI scores. The higher T&T scores represents the worse olfactory function, and TDI scores conversely. Both olfactory tests were significantly different between the patients and controls groups (t = 46.9, P < 0.001 and t = 21.4, P < 0.001, respectively), indicating that olfactory function in IOL patients was worse than controls (Table 1).
Table 1.
Subjects: IOL patients and controls
| IOL patients | Controls | |
|---|---|---|
| n | 16 | 16 |
| Gender | M (9)/F (7) | M (9)/F (7) |
| Age (years) | 48.6±9.9 | 52.5±8.7 |
| MMSE | 29.3±0.7 | 29.1±0.5 |
| TDI scores | 9.4±3.6* | 29.9±1.3* |
| T&T scores | 5.8±0.2* | 0.6±0.4* |
Results are shown as mean ± standard deviation. There is significant negative correlation between TDI scores and T&T scores (r = −0.6, P = 0.01).
*P < 0.05.
The average total brain volume of the IOL patients was 1139.6±76.7cm3 with a mean gray matter volume of 560.5±49.6cm3. The control group showed 1141.9±95.8cm3 total brain volume and a mean of 599.8±48.4cm3. Both whole brain and gray matter volume alterations were not significantly different between 2 groups (t = 0.07, P = 0.94 and t = 1.96, P = 0.06, respectively). The volume of the region of interest (ROIs) showed significant differences between 2 groups in several regions with an exception of thalamus (Table 2).
Table 2.
The gray matter volume of the ROIs in IOL patients and controls
| Region | Side | IOL patients (cm3) | Controls (cm3) | t value | P value |
|---|---|---|---|---|---|
| PC | L | 0.59±0.13 | 0.71±0.09 | 3.00 | 0.005 |
| R | 0.65±0.12 | 0.74±0.08 | 2.27 | 0.031 | |
| OFC | L | 15.48±2.34 | 17.79±1.89 | 3.08 | 0.004 |
| R | 15.12±1.98 | 19.39±2.36 | 5.54 | 0.000 | |
| IC | L | 5.97±0.88 | 7.11±0.86 | 3.67 | 0.001 |
| R | 6.02±0.83 | 7.03±0.75 | 3.63 | 0.001 | |
| Cingulate cortex | L | 8.93±1.53 | 10.39±1.24 | 2.97 | 0.006 |
| R | 10.13±1.05 | 11.51±1.67 | 2.92 | 0.007 | |
| Hippocampus | L | 2.33±0.23 | 2.54±0.23 | 2.63 | 0.013 |
| R | 2.68±0.30 | 2.89±0.23 | 2.26 | 0.031 | |
| PPA | L | 4.95±0.58 | 5.71±0.89 | 2.84 | 0.008 |
| R | 6.42±0.97 | 7.43±0.75 | 3.31 | 0.002 | |
| Thalamus | L | 4.81±0.81 | 5.19±0.84 | 1.33 | 0.194 |
| R | 5.33±0.70 | 5.51±0.94 | 0.61 | 0.545 |
Results are shown as mean ± standard deviation.
In the VOI analysis of primary and secondary olfactory areas, significant gray matter volume decreases were demonstrated for the OFC bilaterally, the anterior cingulate cortex (ACC) bilaterally, the IC bilaterally, parahippocampal cortex (PPA) bilaterally, and the left PC (Figure 2, Table 3). In the VOI analysis, no significant volume increases of gray matter could be observed in primary and secondary olfactory areas.
Figure 2.
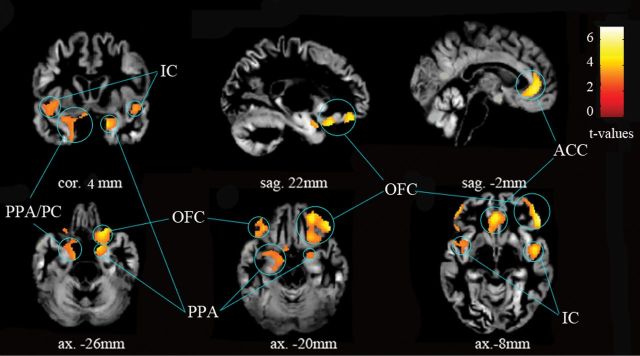
Gray matter reductions in IOL patients group versus controls group—volume of interest analysis for primary olfactory cortex and secondary olfactory area with a threshold set at P < 0.01 (uncorrected) and an extent of 100 voxels. All coordinates are given in MNI space.
Table 3.
Reductions in gray matter for patients with IOL compared with healthy controls
| Region | Side | MNI coordinates (mm) | t value peak | Cluster size (voxels) | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| OFC | R | 25 | 22 | −21 | 4.79 | 1590 |
| L | −35 | 32 | −21 | 3.87 | 566 | |
| ACC | R | 3 | 39 | −3 | 4.54 | 704 |
| L | −2 | 40 | −3 | 4.71 | 1204 | |
| IC | R | 46 | −8 | −3 | 4.01 | 607 |
| L | −41 | −18 | 7 | 3.97 | 839 | |
| PPA | R | 24 | 7 | −23 | 3.63 | 348 |
| L | −16 | −5 | −23 | 3.39 | 318 | |
| PC | L | −22 | 0 | −12 | 2.75 | 156 |
The result is thresholded at P < 0.01 (uncorrected), and only clusters exceeding a size of 100 voxels are reported. All coordinates are given in MNI space.
Discussion
Here we show that IOL is associated with altered structure of brain areas involved in olfactory processing. Our results demonstrate that the patients with IOL have decreased gray matter volume in the primary olfactory cortex (PC) and the secondary olfactory areas (IC, OFC, ACC, PPA).
Several studies investigated brain structure changes in subjects with olfactory dysfunction. Anosmic or hyposmic subjects exhibited gray matter volume loss in the IC, the ACC, the OFC, the cerebellum, the fusiform gyrus, the precuneus, the middle temporal gyrus, and the PC (Bitter et al. 2010a, 2010b). The bilateral gyrus rectus and OFC volume are increased in perfumers, who have superior olfactory abilities (Delon-Martin et al. 2013). All of these studies showed a positive association between gray matter volume and olfactory function. Similarly, gray matter volume loss in the left anterior insula was found in parosmic patients (Bitter et al. 2011). However, one study found subjects with congenital anosmia to exhibit increased gray matter in the left PC and bilateral OFC (Frasnelli et al. 2013).
To our knowledge, there is no systematic report on brain structure change in IOL patients with an exception of a study showing decreased OB volume for IOL patients when compared with controls (Rombaux et al. 2010). We report here for the first time on central structure changes in patients with IOL.
The PC forms the major part of the primary olfactory cortex. We could see atrophy of the left PC only, but none in the right PC. The same finding was observed in congenital anosmia patients, where gray matter volume is also altered in left PC (Frasnelli et al. 2013). This may indicate an important role for the left PC in olfactory processing. However, this result is inconsistent with previous reports in patients with PD where a positive correlation between olfactory performance and gray matter volume was observed exclusively in the right but not in the left PC (Wattendorf et al. 2009). The reason for this discrepancy is unclear and may be due to other effects of neurodegenerative disease. Frasnelli et al. (2013) speculated that structural features of left side olfactory areas seem to be determined by the presence or absence of olfactory input postnatally, as occurs during development.
The OFC receives major projections from the PC and constitutes the most important olfactory target area with the heteromodal association cortex (Gottfried and Zald 2005). Our ROI analyses showed the most atrophic brain areas were located in the right OFC. This result is consistent with reports on heathy adults that showed a significant positive correlation of gray matter volume and olfactory function scores in the right OFC (Seubert et al. 2013). In other settings, various authors have explored the contributions of the OFC to the formation of the olfactory percept. Although earlier lesion studies suggested an essential role for all aspects of olfactory processing (Potter and Butters 1980), more recent studies have pointed out that some complex olfactory tasks, such as distinctions between the smells of predator and kin, cannot be accomplished without an intact OFC, especially the right OFC (Gottfried and Zelano 2011). Furthermore, the OFC is not only responsible for olfactory processing, but also for several other brain functions. A meta-analysis linked thickness of the OFC to depression by showing that subjects suffering from major depressive disorder exhibit thinning of the OFC (Koolschijn et al. 2009). Interestingly, subjects with anosmia exhibit significantly higher depression scores compared with normosmic controls (Croy et al. 2012). It is therefore tempting to speculate that the missing olfactory input leads to reduced gray matter in OFC, and then a disposition toward depression.
The IC region is a secondary olfactory area. In our study, the gray matter volume loss was demonstrated in both left and right ICs. This result is consistent with the involvement of the IC during olfactory tasks in functional imaging studies (Gottfried 2006). In particular, the IC contributes to odor quality coding. Hence, reduced gray matter volume in IC could be related to an impaired performance in correctly assessing quality of sensory information including gustatory and olfactory sensations (Bitter et al. 2011).
We found significant gray matter reductions in the ACC. The ACC is a well-known secondary olfactory area. Besides the IC and OFC, ACC is a key node in the “flavor network” (Small and Prescott 2005). Furthermore, human imaging studies showed that reward-related stimuli, such as the taste of sucrose and the texture of oral fat, activated the ACC (Zald and Pardo 2000). Therefore, it is not surprising IOL patients have reduced gray matter of IC and ACC. The PPA, as part of the limbic system, is involved in the odor memory and odor quality discrimination learning (Martin et al. 2007). In mild cognitive impairment patients, olfactory deficits are associated with the reduction in hippocampal volume (Mariqliano et al. 2013). Our results showing PPA volume loss in IOL patients are consistent with their findings.
Human olfactory performance is determined by complex interactions between early sensory processing in the periphery, and higher order cortical integration of perceptual and cognitive input (Mainland et al. 2002). It has been speculated that different causes of olfactory loss would vary in the extent to which they recruit peripheral and central brain structures. We find gray matter volume loss in the primary olfactory cortex and secondary olfactory areas in IOL patients. These results support the hypothesis that the central components of the olfactory system play an important role in IOL. Further studies are needed to determine if these findings are primary or secondary effects.
Serveral limitations of this study should be acknowledged. First, the threshold of the analysis (P < 0.01 uncorrected for multiple t-tests and extent threshold of 100 voxels) is liberal. Given that there is little information on this topic, we cast a wide net in this exploratory study. Thus, our data must be interpreted with caution. Significant gray matter loss was only observed in the right OFC using independent 2-sample t-test at P < 0.001 (uncorrected) across the whole brain. In further studies, large series with VBM comparisons between IOL and normal controls are warranted to confirm our initial findings. As the Seubert et al. emphasized, an additional limitation is that the functionally derived ROI approach might present an insufficient spatial resolution for isolation of smaller functional subregions, for example, the absence of structural–functional associations in the PC (Seubert et al. 2013).
In summary, studying changes in the central brain structures may help to elucidate the etiology of olfactory loss in patients with IOL. Further investigations directed toward this topic are needed.
Funding
This work was supported by the China Nature Scientific Foundation [81271062]; the Beijing Municipal Science & Technology Commission [2012218048]; and the High-end Foreign Experts Recruitment Program [GDW20121100042].
Conflict of Interest statement
The authors report no financial interests or potential conflicts of interest.
References
- Ashburner J, Friston KJ. 2000. Voxel-based morphometry–the methods. Neuroimage. 11(6 Pt 1):805–821. [DOI] [PubMed] [Google Scholar]
- Bitter T, Gudziol H, Burmeister HP, Mentzel HJ, Guntinas-Lichius O, Gaser C. 2010a. Anosmia leads to a loss of gray matter in cortical brain areas. Chem Senses. 35(5):407–415. [DOI] [PubMed] [Google Scholar]
- Bitter T, Brüderle J, Gudziol H, Burmeister HP, Gaser C, Guntinas-Lichius O. 2010b. Gray and white matter reduction in hyposmic subjects–A voxel-based morphometry study. Brain Res. 1347:42–47. [DOI] [PubMed] [Google Scholar]
- Bitter T, Siegert F, Gudziol H, Burmeister HP, Mentzel HJ, Hummel T, Gaser C, Guntinas-Lichius O. 2011. Gray matter alterations in parosmia. Neuroscience. 177:177–182. [DOI] [PubMed] [Google Scholar]
- Croy I, Negoias S, Novakova L, Landis BN, Hummel T. 2012. Learning about the functions of the olfactory system from people without a sense of smell. PLoS One. 7(3):e33365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Wei Y, Miao X, Li K, Ren Y, Liu J. 2013. Clinical features of olfactory disorders in patients seeking medical consultation. Med Sci Monit. 19:444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delon-Martin C, Plailly J, Fonlupt P, Veyrac A, Royet JP. 2013. Perfumers’ expertise induces structural reorganization in olfactory brain regions. Neuroimage. 68:55–62. [DOI] [PubMed] [Google Scholar]
- Frasnelli J, Lundström JN, Boyle JA, Djordjevic J, Zatorre RJ, Jones-Gotman M. 2010. Neuroanatomical correlates of olfactory performance. Exp Brain Res. 201(1):1–11. [DOI] [PubMed] [Google Scholar]
- Frasnelli J, Fark T, Lehmann J, Gerber J, Hummel T. 2013. Brain structure is changed in congenital anosmia. Neuroimage. 83:1074–1080. [DOI] [PubMed] [Google Scholar]
- Garcia-Falgueras A, Junque C, Giménez M, Caldú X, Segovia S, Guillamon A. 2006. Sex differences in the human olfactory system. Brain Res. 1116(1):103–111. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, Zald DH. 2005. On the scent of human olfactory orbitofrontal cortex: meta-analysis and comparison to non-human primates. Brain Res Brain Res Rev. 50(2):287–304. [DOI] [PubMed] [Google Scholar]
- Gottfried JA. 2006. Smell: central nervous processing. Adv Otorhinolaryngol. 63:44–69. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, Zelano C. 2011. The value of identity: olfactory notes on orbitofrontal cortex function. Ann N Y Acad Sci. 1239:138–148. [DOI] [PubMed] [Google Scholar]
- Haehner A, Hummel T, Hummel C, Sommer U, Junghanns S, Reichmann H. 2007. Olfactory loss may be a first sign of idiopathic Parkinson’s disease. Mov Disord. 22(6):839–842. [DOI] [PubMed] [Google Scholar]
- Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. 1997. ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. 22(1):39–52. [DOI] [PubMed] [Google Scholar]
- Kobal G, Hummel T, Sekinger B, Barz S, Roscher S, Wolf S. 1996. “Sniffin’ sticks”: screening of olfactory performance. Rhinology. 34(4):222–226. [PubMed] [Google Scholar]
- Koss E, Weiffenbach JM, Haxby JV, Friedland RP. 1988. Olfactory detection and identification performance are dissociated in early Alzheimer’s disease. Neurology. 38(8):1228–1232. [DOI] [PubMed] [Google Scholar]
- Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, Hulshoff Pol HE, Kahn RS. 2009. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp. 30(11):3719–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainland JD, Bremner EA, Young N, Johnson BN, Khan RM, Bensafi M, Sobel N. 2002. Olfactory plasticity: one nostril knows what the other learns. Nature. 419(6909):802. [DOI] [PubMed] [Google Scholar]
- Mueller A, Rodewald A, Reden J, Gerber J, von Kummer R, Hummel T. 2005. Reduced olfactory bulb volume in post-traumatic and post-infectious olfactory dysfunction. Neuroreport. 16(5):475–478. [DOI] [PubMed] [Google Scholar]
- Martin C, Beshel J, Kay LM. 2007. An olfacto-hippocampal network is dynamically involved in odor-discrimination learning. J Neurophysiol. 98(4):2196–2205. [DOI] [PubMed] [Google Scholar]
- Mariqliano V, Gualdi G, Servello A, Marigliano B, Volpe LD, Fioretti A, Pagliarella M, Valenti M, Di Biasi C, Ettorre E, et al. 2013. Olfactory deficit and hippocampal volume loss for early diagnosis of Alzheimer disease: a pilot study. Alzheimer Dis Assoc Disord. 28(2):194–197. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. 1971. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 9(1):97–113. [DOI] [PubMed] [Google Scholar]
- Potter H, Butters N. 1980. An assessment of olfactory deficits in patients with damage to prefrontal cortex. Neuropsychologia. 18(6):621–628. [DOI] [PubMed] [Google Scholar]
- Rombaux P, Mouraux A, Bertrand B, Nicolas G, Duprez T, Hummel T. 2006. Olfactory function and olfactory bulb volume in patients with postinfectious olfactory loss. Laryngoscope. 116(3):436–439. [DOI] [PubMed] [Google Scholar]
- Rombaux P, Potier H, Markessis E, Duprez T, Hummel T. 2010. Olfactory bulb volume and depth of olfactory sulcus in patients with idiopathic olfactory loss. Eur Arch Otorhinolaryngol. 267(10):1551–1556. [DOI] [PubMed] [Google Scholar]
- Small DM, Prescott J. 2005. Odor/taste integration and the perception of flavor. Exp Brain Res. 166(3-4):345–357. [DOI] [PubMed] [Google Scholar]
- Seubert J, Freiherr J, Frasnelli J, Hummel T, Lundström JN. 2013. Orbitofrontal cortex and olfactory bulb volume predict distinct aspects of olfactory performance in healthy subjects. Cereb Cortex. 23(10):2448–2456. [DOI] [PubMed] [Google Scholar]
- Wattendorf E, Welge-Lüssen A, Fiedler K, Bilecen D, Wolfensberger M, Fuhr P, Hummel T, Westermann B. 2009. Olfactory impairment predicts brain atrophy in Parkinson’s disease. J Neurosci. 29(49):15410–15413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Wei Y, Yu D, Zhang J, Liu Y. 2010. Olfactory and gustatory function in healthy adult Chinese subjects. Otolaryngol Head Neck Surg. 143(4):554–560. [DOI] [PubMed] [Google Scholar]
- Yao L, Yi X, Wei Y. 2013. Gray matter alteration in isolated congenital anosmia patient: a voxel-based morphometry study. Eur Arch Otorhinolaryngol. 270(9):2569–2573. [DOI] [PubMed] [Google Scholar]
- Zald DH, Pardo JV. 2000. Functional neuroimaging of the olfactory system in humans. Int J Psychophysiol. 36(2):165–181. [DOI] [PubMed] [Google Scholar]


