Summary
Curcumin appears to sensitize chemotherapy drugs to cancer, but the mechanism is unclear. We demonstrated in 5FU resistant colorectal cell lines that curcumin attenuates drug resistance through inhibition of EMT via upregulation of EMT suppressive miRNAs.
Abstract
Resistance to cytotoxic chemotherapy is a major cause of mortality in colorectal cancer (CRC) patients. Chemoresistance has been linked primarily to a subset of cancer cells undergoing epithelial–mesenchymal transition (EMT). Curcumin, a botanical with antitumorigenic properties, has been shown to enhance sensitivity of cancer cells to chemotherapeutic drugs, but the molecular mechanisms underlying this phenomenon remain unclear. Effects of curcumin and 5-fluorouracil (5FU) individually, and in combination, were examined in parental and 5FU resistant (5FUR) cell lines. We performed a series of growth proliferation and apoptosis assays in 2D and 3D cell cultures. Furthermore, we identified and analyzed the expression pattern of a subset of putative EMT-suppressive microRNAs (miRNAs) and their downstream target genes regulated by curcumin. Chemosensitizing effects of curcumin were validated in a xenograft mouse model. Combined treatment with curcumin and 5FU enhanced cellular apoptosis and inhibited proliferation in both parental and 5FUR cells, whereas 5FU alone was ineffective in 5FUR cells. A group of EMT-suppressive miRNAs were upregulated by curcumin treatment in 5FUR cells. Curcumin suppressed EMT in 5FUR cells by downregulating BMI1, SUZ12 and EZH2 transcripts, key mediators of cancer stemness-related polycomb repressive complex subunits. Using a xenograft and mathematical models, we further demonstrated that curcumin sensitized 5FU to suppress tumor growth. We provide novel mechanistic evidence for curcumin-mediated sensitization to 5FU-related chemoresistance through suppression of EMT in 5FUR cells via upregulation of EMT-suppressive miRNAs. This study highlights the potential therapeutic usefulness of curcumin as an adjunct in patients with chemoresistant advanced CRC.
Introduction
Colorectal cancer (CRC) is one of the most common causes of cancer-related deaths in the USA (1). Although chemotherapy for CRC has improved, resistance to chemotherapy remains a major obstacle to obtaining a cure for this malignancy. 5-fluorouracil (5FU) is an antimetabolite used as the first-line chemotherapeutic agent of various cancers. However, the response rates of 5FU for advanced CRC is only 10–15% (2), whereas combining 5FU with oxaliplatin or irinotecan have only improved the response rates for patients with advanced CRC to 40–50% (3,4). Furthermore, patients who respond to chemotherapy initially will eventually acquire resistance to these treatments, and the mechanisms underlying such acquired chemoresistance remain unclear.
To gain mechanistic insights into chemoresistance in cancer patients, cancer cell lines resistant to cytotoxic chemotherapeutic drugs have been developed for few tumor types. These cell lines feature an epithelial–mesenchymal transition (EMT) phenotype and demonstrate resistance to different chemotherapies suggesting that EMT and chemoresistance are closely inter-related processes (5,6). Loss of E-cadherin is a critical step involved in EMT and is inhibited by zinc finger transcriptional suppressors ZEB, TWIST, SNAIL and SLUG, as these function to facilitate conversion of well-differentiated epithelial cells into mesenchymal cells (7–9). Moreover, EMT is essential during embryogenesis and induces phenotypic changes such as loss of cell adhesion and polarity, and the acquisition of migratory and invasive properties (10). Therefore, EMT is considered as a key process for cancer stem cell generation (11).
MicroRNAs (miRNAs), which regulate expression of multiple genes by antisense complementarity to 3′-UTR region of their target genes, have recently emerged as the major regulators of EMT in embryonic development and cancer (12,13). Well-established EMT-inducing transcription factors such as SNAIL and ZEB1/2 have been shown to be tightly regulated by miRNAs. In particular, the miR-200 family was identified as a potent inducer of epithelial differentiation through suppression of ZEB1/2 (14–16). Similarly to the miR-200 family, miR-34 targets alternative EMT-inducing transcription factor, SNAIL, to mediate mesenchymal-to-epithelial transition (MET) in cancer (17). Although using EMT-suppressive miRNAs to treat patients who develop drug resistance appears to have enormous therapeutic potential, these miRNAs also have the capacity to down-regulate cancer unrelated genes which could lead to potential adverse effects.
Curcumin, a derivative of the spice turmeric curcuma longa, is a well-researched botanical with antitumorigenic properties (18). Recently, we and others have demonstrated that curcumin enhances the sensitivity of cancer cells to chemotherapeutic drugs (19–22), but the mechanisms through which this phenomenon occurs remain unclear. Herein, we for the first time demonstrate that curcumin chemosensitizes CRC cells to 5FU by suppression of EMT through upregulation of EMT-suppressive miRNAs in 5-fluorouracil resistant (5FUR) cell lines. Furthermore, we demonstrate that the curcumin-mediated chemosensitization of 5FU-resistant cells can be further controlled through modulation of miR-200c expression. Finally, quantifying the dynamics of tumor growth in xenograft preclinical animal models, we demonstrate that curcumin is able to re-sensitize CRC resistant cells to 5FU treatment. Collectively, these data indicate that supplementation of curcumin to patients receiving conventional chemotherapy could tremendously benefit from the use of such a safe and cost-effective adjunct treatment strategy.
Materials and methods
Cell lines and materials
HCT116 and SW480 CRC cells were purchased from ATCC. 5FU resistant (5FUR) cell lines were established by a previously described method (19) by treating them with increasing concentrations of 5FU over a duration of 9 months. All cell lines were grown in Iscove’s modified Dulbecco’s medium (Invitrogen) with 10% fetal bovine serum and 1% penicillin and streptomycin, and maintained at 37°C in a humidified incubator (5% CO2). 5FUR cells were maintained in medium containing 5 µM 5FU (Sigma–Aldrich). All cell lines were routinely tested and authenticated using a panel of genetic and epigenetic markers. Both 5FU and curcumin (BCM-95, Dolcas Biotech) were dissolved in DMSO and diluted to appropriate concentrations with tissue culture medium.
Viability, cell cycle, apoptosis and clonogenic assays
Cell cytotoxicity was determined using the 3-(4,5-dimethylthiazole-2-yl_2,5-diphenyl tetrazolium bromide (MTT)) assay as previously described (23). Cells were incubated with various concentrations of curcumin and/or 5FU for 72h. Optical density was determined using the Infinite 200 Pro multi-reader and i-control 1.10 (Tecan Group Ltd,). The combination index (CI) was calculated using Chou-Talalay equation (24) at 50% inhibitory concentration to determine synergism between curcumin and 5FU. Cell cycle analysis was conducted using the Cell Cycle Assay Kit (Millipore) and apoptotic cell fraction was measured using the Annexin V and Dead Cell Assay Kit (Millipore) according to the manufacturer’s instructions. In addition, apoptotic cells were evaluated using the 4′,6-diamidino-2-phenylindole (DAPI) nuclear staining assay as previously described (25). The cells were incubated with curcumin, 5FU or both compounds for 24h and evaluated using fluorescence microscopy (Leica Camera AG). Clonogenic assays were conducted as previously described (23). The number of colonies (>50 cells) was counted using GeneTools (Syngene). All experiments were conducted in replicates and at least three independent times.
miRNA expression and transfection analyses
Expression of miRNAs was analyzed using the TaqMan® real-time PCR assay kit (Applied Biosystems) as previously described (26). Cells were treated with curcumin (10 µM) and/or 5FU (10 µM) for 24h and RNA was extracted using the miRNeasy Mini Kit (Qiagen). All data were analyzed using ΔΔC t method and normalized to RNU6B. In order to transiently induce or inhibit miR-200c expression, hsa-pre-miR-200c or hsa-anti-miR-200c (Ambion), was used to transfect 5FUR cells as previously described (26). Verification of transfection efficiency was conducted using pre-miR-negative-control 1 or anti-miR-negative-control 1 (Ambion), respectively.
Western blotting
Western immunoblotting experiments were performed as previously described (27). Cells were treated with curcumin and/or 5FU for 24h. The list of primary antibodies is provided in Supplementary Table 1, available at Carcinogenesis Online and anti-mouse or anti-rabbit antibodies (Santa Cruz Biotechnology) were used as secondary antibodies. All samples were compared against β-actin (Sigma–Aldrich) as a reference protein. The protein bands on the gels were visualized using GeneTools (Syngene).
Toluidine blue staining of 3D cultures
3D cultures were generated as previously described (22). Cell lines were treated with curcumin (10 µM), 5FU (10 µM) or both agents for 0, 1 or 3 days. The 3D cultures were embedded in Tissue-Tekcassette (Sakura Finetek), cryopreserved at −80°C and cut into 5–7 µm sections using acryomicrotome (Zeiss). Slides were stained with toluidine blue and examined under a light microscope (Leica) and number of apoptotic and degraded cells were quantified manually. Each experiment was performed at least in triplicate or more.
Animal experiments
The 5 week-old male athymic nude mice (Harlan Laboratories) were housed under controlled conditions of light and fed ad libitum. Xenograft tumors were generated by subcutaneous injection of 5×106 HCT116-5FUR cells. Tumor volume was calculated using formula: (π/6) (length × width × height). Once average tumor size reached 50mm3, animals were randomly divided into four groups with 10 animals in each group: (i) control vehicle (DMSO), (ii) 5FU (20mg/kg once every 2 days), (iii) Curcumin (50mg/kg daily) or (iv) 5FU and curcumin. All treatments were injected intraperitoneally daily for 40 days before animals were euthanized; mice in the 5FU group received DMSO every other day. Tumor samples were dissected and stored in RNAlater (Sigma–Aldrich) for later analysis. The animal protocol was approved by Institutional Animal Care and Use Committee, Baylor Research Institute, Dallas, TX.
Mathematical modeling of tumor growth
The parameters underlying xenograft growth were estimated by fitting a mathematical model to the data. The model contains two variables, the resistant cell population, x, and the susceptible cell population y. The model is given by the following pair of ordinary differential equations that describe the development of these populations over time.
where .
Resistant tumor cells grow with a rate r resist, susceptible cells grow in the absence of treatment at a rate r cntrl, and treatment reduces the growth with a rate d(t). This is a time-dependent function (of the Hill type) to account for the observation in the data that a response to treatment can require a certain time-delay. The parameter k denotes the duration of the time delay, and the parameter g denotes the maximal reduction in tumor growth that is achieved after the time-delay. The Hill coefficient α determines how steeply the function d(t) rises from zero to g. The model was fit to the data using least squares fitting procedures, implemented by the software Berkeley-Madonna (Ver. 8.3.18, Berkeley, CA). The fitting procedure was set up as follows. First, the Hill coefficient α was set to 3, and the rest of the parameters obtained by fitting. Next, the Hill coefficient was modified (first increased and then decreased with a fixed increment), and the fitting procedure repeated. We observed that the goodness of fit (measured by the sum of squares), as a function of parameter α, exhibited a very flat valley-like minimum around the value α = 3. Inside this valley, the values of the fitted parameters were exceptionally stable in a wide range of the Hill coefficient values. For values of α less than one and also for very large values of α, the fit was significantly worse, and the fitted parameter values diverged [the latter situation corresponds to a biologically unrealistic situation of a ‘digital’ (not analogue) time-delay]. The parameter values presented here correspond to the value α = 3.
Statistical analysis
All analyses were performed using GraphPad Prism Ver.6.0 (GraphPad Software, Inc.). All data were expressed as mean ± SEM with statistical significance indicated when P < 0.05. Statistical comparisons were determined using unpaired t-test or one-way ANOVA with Tukey’s post hoc tests.
Results
5FU resistant cells acquire mesenchymal characteristics
Two primary CRC cell lines (HCT116 and SW480) were used to generate 5FU resistant cells (5FUR) cells (Figure 1A). After treating these cell lines with 5FU for over 9 months, both cell lines developed altered cellular morphology resembling that of their mesenchymal origin (Figure 1B). We found HCT116 cells developed stronger acquired resistance to 5FU compared with SW480 cell lines. This could be due to higher proliferation rate of HCT116-5FUR cell line compared with SW480-5FUR. We then confirmed 5FU resistance of these cell lines by treating them and their parental cell lines with increasing doses of 5FU and compared their viability to their respective parental cell lines using the MTT assay (Figure 1C). Since miRNAs are heavily involved in the regulation of EMT, we assessed whether an EMT phenotype acquired by these 5FUR cells reflected a change in the expression of miRNAs related to EMT (Figure 1D).The expression of miR-34a, miR-200c, miR-141, miR-429 and miR-101 was decreased in HCT116-5FUR in comparison to its parental cell line, while the SW480-5FUR cell line showed a significant decrease only in levels of miR-34a, miR-200c and miR-429. Finally, we measured the corresponding expression of proteins to ensure that the mesenchymal phenotype acquired by these cells. As expected loss of E-cadherin protein expression, which is a critical event for EMT was observed in 5FUR cell lines, while the ZEB1 protein was significantly upregulated (Figure 1E).
Fig. 1.
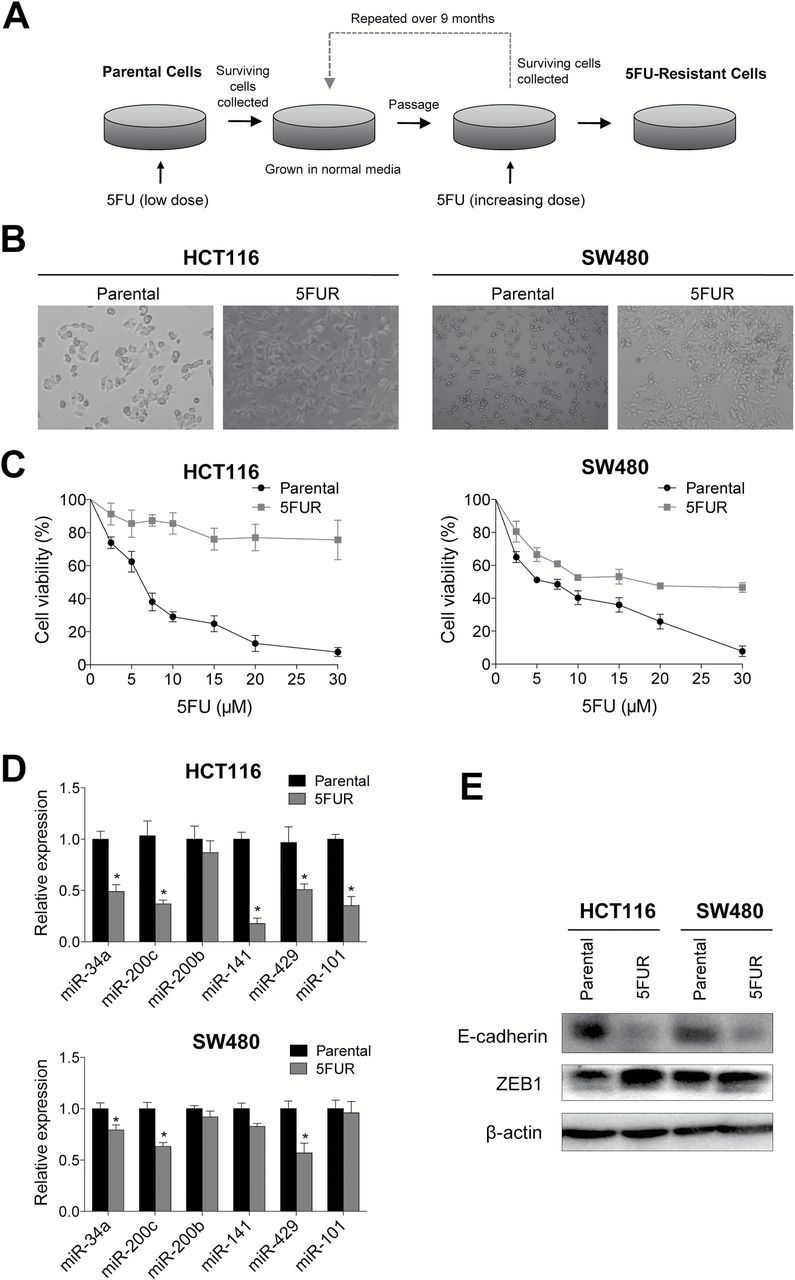
Chemoresistant colorectal cancer cell lines acquire mesenchymal phenotype. (A) Schematic diagram for the establishment of chemoresistant cell lines. (B) Microscopic images illustrating 5FUR cells in comparison to their parental counterparts (left HCT116, right SW480). (C) 5FU sensitivity in 5FUR cell lines in comparison to their respective parental controls. (D) qRT-PCR analysis of miRNA expression changes in 5FUR cell lines compared to parental cell lines. (E) Western blot analysis of E-cadherin and ZEB1 of parental and 5FUR cell lines.*P < 0.05.
Curcumin enhances sensitivity to 5FU in 5FUR cell lines
In order to determine whether curcumin enhances the efficacy of 5FU, we assessed the cytotoxicity of both compounds individually and in combination using both parental and 5FUR HCT116 and SW480 cell lines. 5FU caused greater cytotoxicity than curcumin in both parental cell lines, and the combination of the two compounds moderately increased cytotoxicity (Figure 2A). Chou-Talalay CI showed weak synergism between curcumin and 5FU for HCT116 cell line, while the combined cytotoxicity was additive for SW480 (Figure 2A insets). In contrast, curcumin exerted greater cytotoxicity compared with 5FU in 5FUR cell lines, while the combined curcumin and 5FU treatment further enhanced cytotoxicity. The combined treatment resulted in significant synergistic enhancement in cytotoxicity in both cell lines (CI < 1), indicating that curcumin may sensitize 5FU in the two chemoresistant cell lines (Figure 2A insets).
Fig. 2.
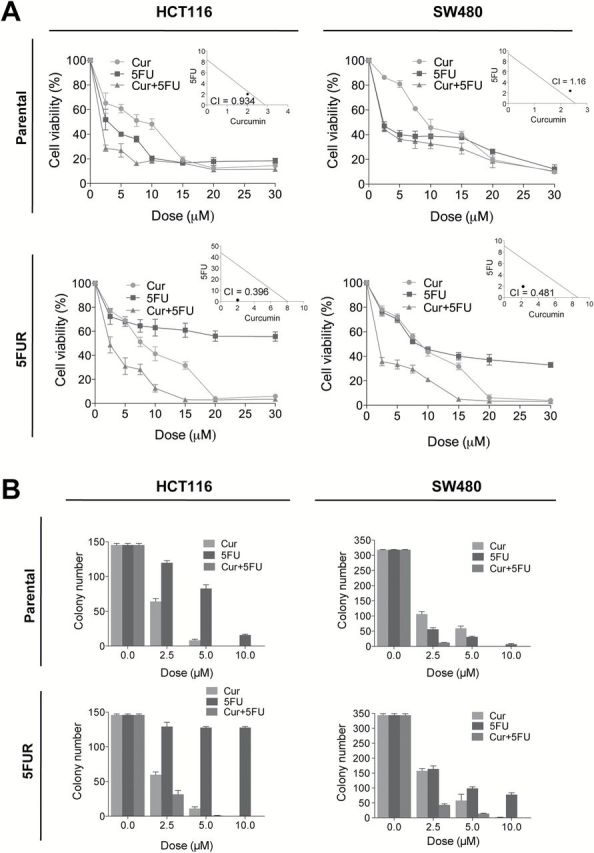
Curcumin enhances 5FU sensitivity in colorectal cancer cells. (A) Cytotoxicity of curcumin and 5FU in HCT116 and SW480 and their respective resistant counterpart cell lines treated with 2.5–30 µM of curcumin and/or 5FU. Inset: Synergy between curcumin and 5FU was calculated by CI. (B) Colony formation assays of HCT116 and SW480 and their respective 5FUR cells treated with curcumin and/or 5FU.
While MTT data suggests that SW480-5FUR cell line appears to respond to 5FU treatment, these data are represented as comparison of cell growth over 3 day incubation between resistant cells treated with 5FU compared with untreated controls. Hence these cells are growing in the presence of 5FU, but at a slower rate. Next, we evaluated the combinatorial effects of curcumin and 5FU on cell growth/survival using a clonogenic assay. In the parental cell lines, both 5FU and curcumin inhibited colony formation, while the combined treatments effectively inhibited colony formation at a low individual dose (Figure 2B). In contrast, 5FU treatment was less effective in inhibiting colony formation in 5FUR cell lines. The addition of curcumin enhanced the ability of 5FU to inhibit colony formation compared with either treatment alone (Figure 2B).
Curcumin sensitized 5FUR cells and resulted in enhanced apoptosis and cell cycle arrest
Next we assessed whether the synergy between curcumin and 5FU resulted in a corresponding increase in cellular apoptosis. Consistent with our previous data, incubation with curcumin or 5FU enhanced apoptosis in parental cell lines (Figure 3A) and the combination of the two agents further elevated the apoptotic cell population (25). In contrast, incubation with 5FU alone did not increase apoptosis in 5FUR cell lines, whereas curcumin enhanced apoptosis. Although incubation of cells with 5FU had a limited effect on apoptosis in 5FUR cell lines, combining curcumin and 5FU significantly elevated apoptosis. We then determined whether the reduced rates of apoptosis observed in 5FUR cells resulted in decreased caspase activity. Both Bcl-2 and Bcl-xL, anti-apoptotic markers, were down-regulated by 5FU in parental cell line, whereas 5FU had limited effect on Bcl-2 and Bcl-xL expression in the resistant cell line (Figure 3B). In contrast, 5FU downregulated PARP1 in both parental and resistant cell lines indicating that 5FU can still inhibit DNA strand break repair in a resistant cell line. We used DAPI staining to confirm that the apoptotic cell population was reduced in 5FUR cells following 5FU treatment, whereas the combined treatment enhanced apoptosis (Figure 3C).
Fig. 3.
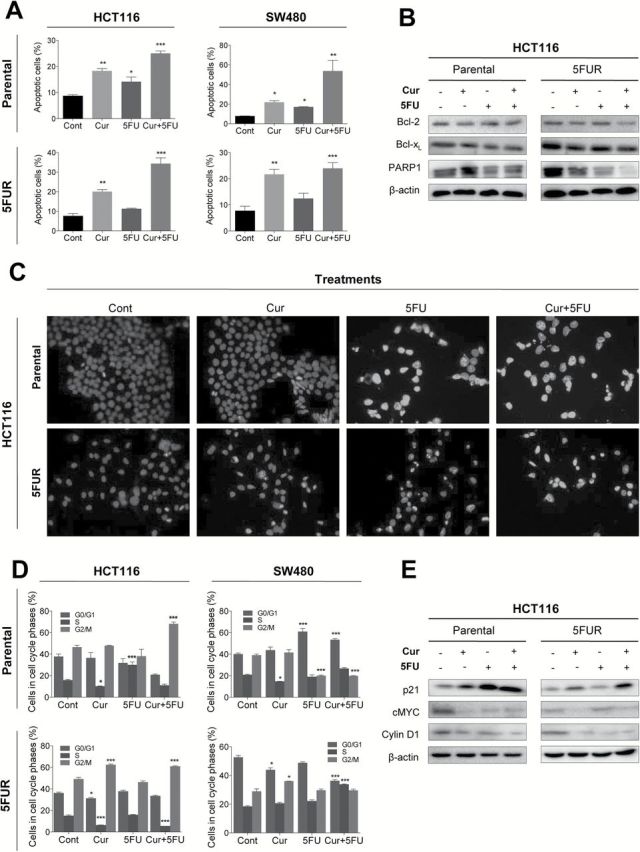
Curcumin, but not 5FU, induces cellular apoptosis and cell cycle arrest in 5FU resistant cell lines. (A) Cells were stained with Annexin V and 7-AAD, and apoptotic cell number was determined by flow cytometry. (B) Western blot analysis of Bcl-2, Bcl-xL and PARP1 in HCT116 and HCT116-5FUR cell lines. (C) Images of apoptotic cells treated with increasing doses of curcumin and/or 5FU determined by DAPI staining. (D) Cell cycle analysis for cells treated with 10 µM curcumin, 10 µM 5FU and the combination, followed by staining with propidium iodide and subjected to flow cytometry analysis for the determination of DNA content. (E) Western blot analysis of p21, cMyc and Cyclin D1 treated with curcumin and/or 5FU in HCT116 or HCT116-5FUR cell lines.*P < 0.05, **P < 0.01, ***P < 0.001 compared to control.
Next, we determined whether curcumin and 5FU exert anti-tumorigenic effects through regulation of the cell cycle. 5FU is a potent inducer of cell cycle arrest by inducing both G0/G1 and G2 arrest in CRC cells (28). In parental cell lines, 5FU treatment induced significant alterations in cell cycle dynamics in the parental HCT116 and SW480 cell lines (Figure 3D), whereas 5FU treatment did not have any significant effect in 5FUR cell lines. In contrast, curcumin moderately affected the cell cycle distribution in both parental and 5FUR cell lines. The combined treatment with curcumin and 5FU significantly altered cell cycle phases in both parental and resistant cell lines. These data indicate that although 5FU treatment alone had little influence on cell cycle regulation in 5FUR cells, combined treatment had a more profound effect on cell cycle dynamics than curcumin treatment alone. To further investigate the combined effects of curcumin and 5FU, we compared the expression of key cell cycle-regulatory proteins in parental and 5FUR cell lines (Figure 3E). In parental cells, 5FU upregulated p21 expression, while inhibiting its downstream targets cMyc and Cyclin D1. In contrast, p21 and cMyc expression remained unaffected by 5FU treatment in HCT116-5FUR cells. The combined treatment resulted in enhanced upregulation of p21 in 5FUR cell lines while downregulating cMyc and Cyclin D1, indicating that curcumin-mediated re-sensitization to 5FU may occur through p21-induced cell cycle arrest.
Curcumin, but not 5FU, inhibits EMT and upregulates EMT-suppressive miRNAs in 5FUR cell lines
Recent reports indicate that curcumin inhibits EMT in breast and renal cancer cells (29,30). Considering 5FUR CRC cell lines displayed a mesenchymal phenotype, we investigated whether curcumin can induce epithelial differentiation. Since curcumin is a potent inducer of cell death, it was hard to delineate whether curcumin alters mesenchymal phenotype of 5FUR cells to epithelial cells microscopically due to curcumin treated cells undergoing various phases of apoptosis. Therefore, we performed western blotting to determine whether curcumin can downregulate a well-established EMT marker, ZEB1. Both curcumin and 5FU treatments resulted in downregulation of ZEB1 in parental cell lines (Figure 4A). However, such treatment in 5FUR cell lines leads to upregulation of ZEB1 (Figure 4A), suggesting that the cells that have adapted to regular 5FU treatment may still be responsive to additional 5FU treatment through upregulation of ZEB1. In contrast, curcumin treatment downregulated ZEB1 expression in 5FUR cell lines, both individually and in combination with 5FU, indicating that curcumin may induce epithelial differentiation in chemoresistant CRC cell lines. Given that miRNAs have emerged as the key regulators of EMT, we next investigated whether curcumin treatment modulates key EMT-suppressive miRNAs in 5FUR CRC cell lines. We examined the miR-200 family cluster as well as other notable miRNAs involved in regulation of EMT and ‘stemness’ in 5FUR cell lines. Curcumin upregulated the expression of miR-200b, miR-200c, miR-141, miR-429 and miR-101, whereas 5FU treatment did not affect expression of these EMT-suppressive miRNAs in 5FUR cells (Figure 4C). MiR-34a expression was the only one upregulated in HCT116-5FUR cells, but not in SW480-5FUR cell line. Since SW480 is p53-mutated, and miR-34a is primarily upregulated by p53 (31), we suspect that curcumin upregulates miR-34a through p53 activation. The expression of these EMT-related miRNAs in the combined curcumin and 5FU treatment group were similar to that of curcumin treatment alone, indicating that curcumin, but not 5FU, regulates EMT-suppressive miRNAs in 5FUR cells. To confirm EMT suppressive activity of curcumin in 5FUR cell lines, we used scratch assays to show that curcumin treatment markedly reduced the ability to migrate (data not shown), whereas 5FU treatment did not affect cell migration.
Fig. 4.
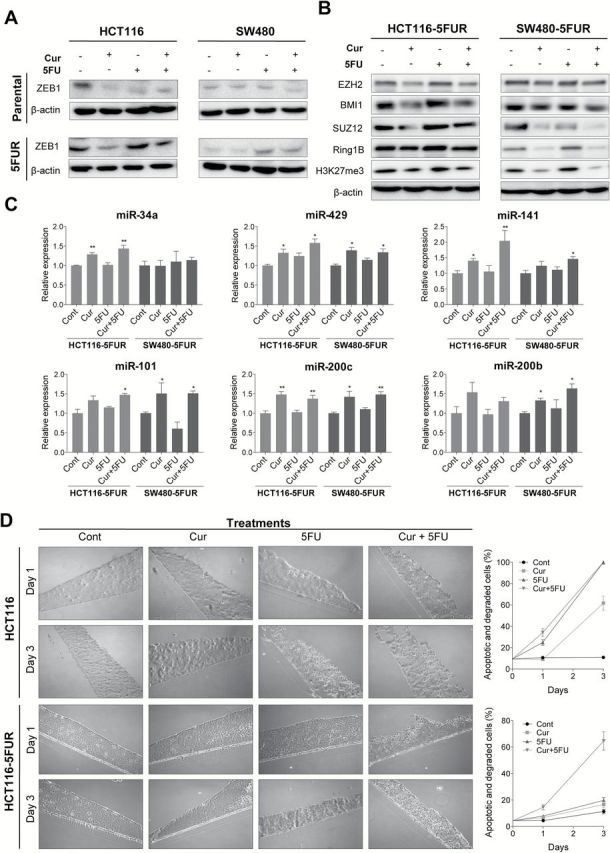
Curcumin suppresses EMT and cancer stemness in 5FUR colorectal cancer cell lines. (A) Western blot analysis of ZEB1 in parental and 5FUR cell lines. (B) Western blot analysis of polycomb repressive complexes subunits and H3K27me3 in 5FUR cell lines. (C) qRT-PCR analysis of miRNA expression in HCT116-5FUR and SW480-5FUR cell lines treated with curcumin and/or 5FU, normalized to RNU6B. (D) Images of 3D cultures stained with toluidine blue 1 and 3 days after treatment with curcumin and/or 5FU in parental and 5FUR HCT116 cells. Apoptotic and degraded cells were quantified (left).
Curcumin inhibits cancer stemness through downregulation of the polycomb repressive complex
Considering EMT and stemness are strongly interrelated and both mechanisms are major contributors to chemoresistance (11), we investigated whether curcumin inhibits ‘cancer stemness’ in CRC 5FUR cell lines. A previous study demonstrated that EMT activity in cancer cells results in upregulation of the polycomb group of epigenetic gene silencers, including BMI-1, SUZ12 and EZH2 (32). Since many of the EMT and polycomb repressive complex (PRC) target genes are regulated by the same cluster of EMT-suppressive miRNAs, we investigated the expression of PRC subunits regulated by miRNAs identified in the present study in 5FUR cell lines. While curcumin and the combined curcumin and 5FU treatments resulted in downregulation of EZH2, BMI1, SUZ12 and Ring1B (Figure 4B), 5FU treatment by itself did not alter the expression of PRC subunits. Furthermore, we showed that inhibition of PRC subunits by curcumin in 5FUR cell lines resulted in downregulation of H3K27me3 (Figure 4B). To ensure that curcumin targets cells with stem-like properties we generated 3D cultures using HCT116 and HCT116-5FUR cell lines. We have demonstrated previously that HCT116-5FUR generated 3D cultures highly expressing stem cell markers ALDH1, CD44 and CD133 (22). While 5FU-induced apoptosis and cell degradation in 3D HCT 116 cultures, its effect was inhibited in HCT116-5FUR cultures (Figure 4D). In contrast, the combined curcumin and 5FU treatment resulted in a significant increase in apoptotic and necrotic cells, confirming that curcumin can enhance 5FU sensitivity against 3D cultures.
EMT-suppressive miRNA modulates 5FU chemosensitivity in CRC cells
Next, we investigated whether modulation of EMT-suppressive miRNAs expression in 5FUR cell lines can regulate the efficacy of 5FU. Since we identified that miR-200c was upregulated by curcumin, we examined whether altering miR-200c expression could modulate 5FU sensitivity in 5FUR cell lines. Transient knockdown of miR-200c in HCT116-5FUR (Supplementary Figure 1A, available at Carcinogenesis Online) resulted in upregulation of ZEB1 and BMI1, both established direct targets of miR-200c (Supplementary Figure 1B, available at Carcinogenesis Online). Next we assessed the functional role of miR-200c in 5FUR cells by MTT and clonogenic assays. MiR-200c inhibition significantly increased 5FU resistance demonstrating enhanced 5FU resistance in miR-200c-knockdown-5FUR cells (Supplementary Figure 1C—left panel, available at Carcinogenesis Online). We then treated these miR-200c-knockdown-HCT116-5FUR cells with curcumin and/or 5FU to confirm that the chemosensitizing property of curcumin is not solely dependent on miR-200c (Supplementary Figure 1C—right panel, available at Carcinogenesis Online). Indeed curcumin and 5FU synergistically enhanced cellular cytotoxicity in the miR-200c-knockdown-5FUR cell line indicating that curcumin enhances chemosensitivity in part through miR-200c (Supplementary Figure 1C—right inset, available at Carcinogenesis Online). Clonogenic assays revealed that miR-200c knockdown resulted in increased colony forming ability. Inhibition of miR-200c expression in 5FUR cells not only enhanced 5FU chemoresistance, but also increased resistance to curcumin as well as the combined treatments (Supplementary Figure 1D, available at Carcinogenesis Online). Similarly, miR-200c knockdown in 5FUR cells contributed to attenuation of apoptosis in all treatment groups (Supplementary Figure 1E, available at Carcinogenesis Online). Since the efficacy of curcumin was attenuated by downregulation of miR-200c, this suggests that the antitumorigenic capacity of curcumin is at least in part mediated through miR-200c.
We next investigated whether over-expression of miR-200c can enhance 5FU sensitivity through inhibition of EMT in a 5FUR cell line. MiR-200c over-expression in HCT116-5FUR (Figure 5A) resulted in upregulation of E-cadherin indicating suppression of EMT, while ZEB1 and BMI1 were downregulated (Figure 5B). The MTT assay confirmed that miR-200c over-expression enhanced 5FU sensitivity (Figure 5C, left panel), while the combination of curcumin and 5FU synergistically enhanced cytotoxicity in these cell lines (Figure 5C right panel and inset). Both clonogenic and apoptosis assays confirmed that over-expression of miR-200c enhanced curcumin and 5FU sensitivity in HCT116-5FUR cells (Figure 5D and E). These data collectively demonstrated that EMT-suppressive miRNAs can dictate sensitivity to 5FU-based chemotherapy.
Fig. 5.
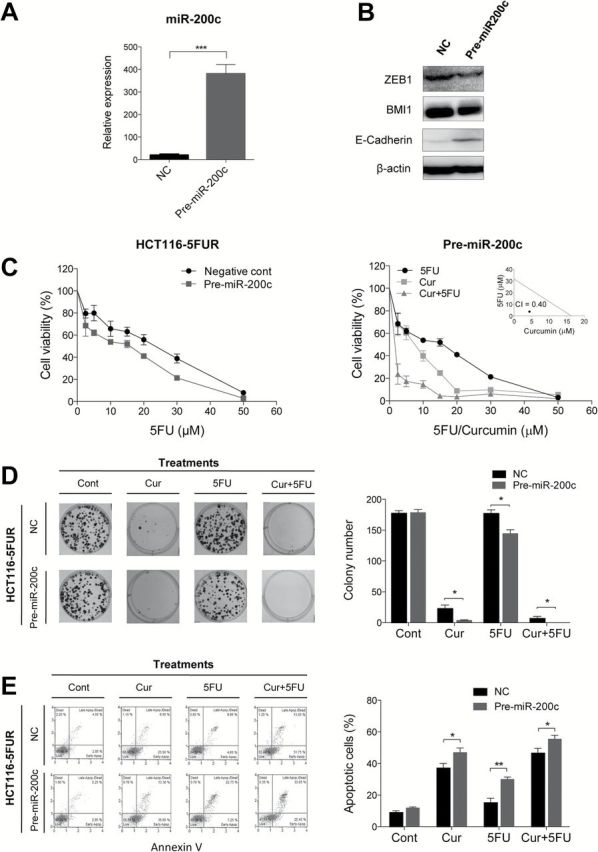
miR-200c over-expression increases 5FU sensitivity in 5FUR colorectal cancer cells. (A) qRT-PCR analysis of miR-200c expression after pre-miR-200c transfection. (B) Western blot analysis of ZEB1, BMI1 and E-cadherin after pre-miR-200c transfection. β-actin served as a loading control. (C) 5FU sensitivity of miR-200c over-expressing HCT116-5FUR cell lines compared to negative control transfected HCT116-5FUR cells (left). Curcumin and 5FU cytotoxicity in miR-200c-overexpressing HCT116-5FUR cells (right). (D) Colony formation assay for miR-200c over-expressing HCT116-5FUR cells or negative controls treated with curcumin and/or 5FU. (E) Cellular apoptosis levels (Annexin V and 7-AAD staining) of miR-200c-over-expressing HCT116-5FUR cells and negative control transfected HCT116-5FUR cell lines treated with curcumin and/or 5FU determined by flow cytometry.
Curcumin mediates 5FU sensitization in a xenograft animal model
Finally, we determined whether curcumin can induce sensitization to 5FU in a xenograft model. We generated chemoresistant xenograft tumors by injecting HCT116-5FUR cells into athymic nude mice followed by curcumin and/or 5FU treatments (Figure 6A). Throughout the experiment, the body weight of the animals was unaffected by any of the treatments (Supplementary Figure 2A, available at Carcinogenesis Online). Although the average tumor volume of 5FU treated animals appeared to be lower than the vehicle treated animals, no statistical differences were observed throughout the study (Figure 6B), indicating that these 5FUR-derived tumors have higher tolerance to 5FU treatment. In contrast, daily curcumin treatment suppressed tumor growth compared with the control group (Figure 6B and C). The combined treatment with 5FU and curcumin further attenuated tumor growth indicating that curcumin mediates 5FU re-sensitization. In addition, miR-200c expression was elevated in the combined curcumin and 5FU treatment group in xenograft tumors confirming that curcumin treatment results in upregulation of miR-200c (Supplementary Figure 2B, available at Carcinogenesis Online).
Fig. 6.
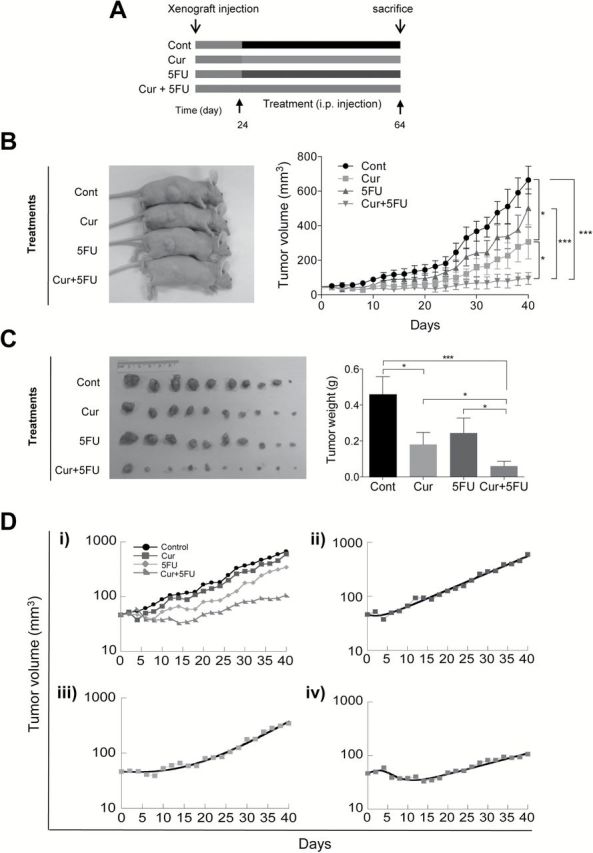
Curcumin sensitizes 5FU against 5FUR derived xenograft tumors. (A) The schematic diagram of the curcumin and 5FU treatment protocol. (B) Representation of tumor sizes (left) and progressive tumor volume increase during treatment period (right). (C) Xenograft tumors collected from experimental animals and average tumor weight with treatments. (D) Mathematical modeling of xenograft tumor growth. All data are represented as normalized averages of the xenograft tumor sizes over time. The tumor volumes in the treated scenarios were scaled such that the tumor volumes upon initiation of treatment were identical to the tumor volumes at day zero in the control setting. (i) all scenarios plotted together. Control = black, 5FU = blue, curcumin = orange, curcumin + 5FU = red (ii–iv). Each treatment setting is plotted individually as indicated. Squares represent observed data points, the thick solid line represents the best model fit. The parameter estimates are given in Supplementary Table 2, available at Carcinogenesis Online. *P < 0.05, **P < 0.01, ***P < 0.001.
Next we used a mathematical model to estimate key parameters that underlie tumor growth dynamics following curcumin, 5FU and the combined treatments. The treated xenografts had the tendency to first show a relatively short phase of decline, followed by exponential growth (Figure 6D). We hypothesize that this decline phase is due to the presence of susceptible cell populations within the xenograft, while the subsequent growth is caused by cell populations that reflect a degree of resistance to the treatment. While resistant cell lines were used to initiate xenograft growth, the initial decline is probably due to the generation of heterogeneity within the growing xenograft before treatment was started, which could have created some susceptible cell variants. Hence, a fraction of the cell population f was assumed to be resistant to treatment, while a fraction 1 − f was assumed to be susceptible. The following key parameters were estimated: In the absence of treatment (control), cells grew exponentially (Figure 6Di) with a rate r cntrl = 0.07 per day, which translates into a tumor doubling time of ~10 days. For the susceptible subpopulation of cells, cells were assumed to grow at a rate r cntrl before treatment, and treatment was assumed to cause a decline of this cell population, which is determined by the rate constant g. Resistant cells, on the other hand, were assumed to grow exponentially during treatment, described by the rate constant r resist. The parameter estimates for the different treatment scenarios are shown in Supplementary Table 2, available at Carcinogenesis Online, and the model fits to the data are shown in Figure 6Dii–iv. For treatment with 5FU only, the fraction of the cell population that is resistant to the drug was estimated to be 67%. This high fraction is expected because a resistant cell line was used to initiate the xenograft. The minority population of susceptible cells was inhibited by the drug with a rate g = 0.4 per day. Not surprisingly, the growth rate of the resistant cell population, r resist, was estimated to be identical to that of the control tumor in the absence of treatment (Supplementary Table 2, available at Carcinogenesis Online). For treatment with curcumin only, 16% of the tumor was estimated to be resistant to treatment, that is the majority of the population was susceptible. However, growth of the susceptible cell population was reduced with a rate g = 0.1, which is lower than observed for 5FU treatment. The growth rate of the resistant cells was found to be higher than that of the control population (Supplementary Table 2, available at Carcinogenesis Online). Finally, while considering treatment with a combination of 5FU and curcumin, the growth of the susceptible cell population was inhibited with a rate g = 0.4, which is identical to the estimate for 5FU treatment alone. About 39% of the tumor cell population was estimated to be resistant. Interestingly, the growth rate of the resistant cells was found to be only r resist = 0.045, which is 30% lower than the growth rate of the control population or the growth rate of the resistant population in the presence of 5FU only (Supplementary Table 2, available at Carcinogenesis Online). Therefore, this analysis indicates that treatment with curcumin modulates the degree of resistance of cells to 5FU. In particular, it reduces the degree of resistance by 30%, thereby confirming that curcumin not only sensitizes resistant cells to chemotherapy in vitro, but also in vivo.
Discussion
In this study, we first demonstrate a novel molecular mechanism for curcumin-mediated chemo-sensitization to 5FU in CRC cell lines through inhibition of EMT. Using a series of systematic assays, we reveal that curcumin induced EMT suppression corresponded with upregulation of multiple EMT-suppressive miRNAs in 5FU-resistant (5FUR) CRC cells. Furthermore, curcumin treatment resulted in the down-regulation of key PRC subunits which are involved in mediating cancer stemness in 5FUR cell lines. Collectively, these results not only highlight curcumin’s potential as an adjunct to conventional chemotherapy in CRC patients, but also suggest that EMT-suppressive miRNAs regulated by curcumin may also be exploited as potential therapeutic targets in patients who develop resistance to 5FU-based chemotherapy. Accordingly, we demonstrated that the alteration of miR-200c expression, a key EMT-suppressive miRNA, dictated 5FU efficacy in 5FUR cells. Finally, by quantifying the dynamics of tumor growth in a xenograft model and by estimating crucial parameters, we provided additional evidence for curcumin-mediated inhibition of tumor growth, thus confirming that curcumin acted as a chemosensitizer in this animal model.
Elucidating the mechanisms of chemoresistance is critical for development of effective therapeutic strategies. In this study, we derived 5FUR cells from common CRC cell lines to investigate the combined effects of curcumin and 5FU. While we primarily focused on the sensitivity of these compounds to 5FUR cells, further research is required to clarify how these cells acquire resistance to chemotherapy drugs. 5FU resistance cell lines were established from single cell clones, and these clones were subsequently expanded over a period of several months. In other words, although originally these cells originated from a single clone, it is likely that over time these cell lines may have evolved into heterogeneous clones, as reflected in our mathematical modeling data which indicates that 5FUR cells are heterogeneous and have varying response to 5FU. Furthermore, for this particular study, we focused primarily on 5FUR cell lines, but we have tested response of these cell lines to other chemotherapeutic drugs such as oxaliplatin in the past in the context of other chemopreventive compounds. Interestingly, we noticed that 5FUR cell lines also demonstrated cross-resistance to other drugs such as oxaliplatin in these models, highlighting the involvement of common acquired resistance pathways in these cell lines. Similarly, we found oxaliplatin and irinotecan resistant cell lines which also demonstrate cross resistance to 5FU. Our findings are in line with a previous study which reported similar cross drug resistance in CRC chemoresistant cell lines (33).
Recently, it has become increasingly clear that EMT not only plays important role in the progression and metastasis of cancer, but is also responsible for inducing the resistant phenotype in cancer cells to conventional chemotherapy (34). In pancreatic cancer, ZEB1 has been identified as the major inducer of EMT by down-regulating E-cadherin and subsequently promoting chemoresistance (35). 5FUR CRC cell lines generated in this study displayed EMT characteristics such as elongated fibroblastoid shape and low expression of E-cadherin confirming that EMT plays a critical role in CRC chemoresistance. Although the mechanisms by which prolonged 5FU treatment leads to the acquisition of EMT phenotype remain unknown, residual remnants of cancer following conventional chemotherapy display a mesenchymal phenotype (36). The present study demonstrates for the first time that curcumin attenuated EMT in 5FUR CRC cell lines, and also re-sensitized 5FUR cells to 5FU. Moreover, we provide further insights into the potential EMT inhibitory mechanisms of curcumin by identifying a group of EMT-suppressive miRNAs upregulated by curcumin.
We have recently demonstrated that curcumin targets cancer stem-like cells in chemoresistant CRC cell lines (22). Alternation from EMT to MET generates cellular plasticity in cancer cells, which presumably contributes to higher cancer stem cell population in chemoresistant cells (37). The polycomb group is a class of chromatin modifying enzymes which regulate gene expression by methylating both DNA and core histones, and this process has been shown to directly regulate developmental factors that maintain embryonic stem cell self-renewal and pluripotency (38). PRC subunits such as BMI1 and EZH2 are often over-expressed in various cancers (39) and are required for the formation and maintenance of cancer stem cells (40). The present study demonstrated that curcumin inhibited both PRC1 and PRC2 by downregulating multiple PRC subunits in 5FUR cell lines including BMI1, SUZ12 and EZH2. Since EMT and PRC are both, in part, regulated by the same miRNAs (41), it is not surprising that curcumin is capable of suppressing both pathways. We therefore propose a potential mechanism in which curcumin sensitizes to chemotherapeutic drugs such as 5FU by simultaneously targeting EMT and cancer stemness (Supplementary Figure 2C, available at Carcinogenesis Online). The ability of curcumin to reverse EMT while targeting cancer stem cells could have significant therapeutic implications in the management of CRC patients.
Modulation of EMT and elimination of cancer stem cells while killing the bulk of the tumor cells using a combination of drugs has been proposed as a key strategy for cancer treatment (34). Accordingly, the present study supplemented conventional chemotherapy with curcumin to induce differentiation of mesenchymal-like cells and suppress cancer stemness. The combined curcumin and 5FU treatment resulted in a synergistic enhancement of cytotoxicity in 5FUR cells confirming that curcumin chemosensitizes to 5FU-based drug regimens. Furthermore, we alternatively explored this combinatorial adjunctive therapeutic concept by examining the efficacy of 5FU in relation to the expression level of an EMT-suppressive miRNA in 5FUR cells. As expected, miR-200c expression dictated 5FU sensitivity in chemoresistant cells confirming that EMT-suppressive miRNAs could be used therapeutically as chemosensitizers. The miR-200 family is a well-established EMT inhibitor, but there are conflicting data on the role of miR-200 as a suppressor or promoter of cancer (42,43), thereby raising concern over the therapeutic use of miRNAs, as they can potentially downregulate key tumor suppressors. Interestingly, botanicals such as curcumin regulate the expression of individual miRNAs by a relatively small increment. However, these botanicals have the ability to simultaneously regulate multiple putative tumor suppressive and oncogenic miRNAs. Although it is still unclear whether the combined modulation of miRNAs or targeting one key miRNA has a superior therapeutic effect, a recent study showed that coordinated regulation of multiple miRNAs resulted in synergistic tumor growth suppression in pancreatic cancer (44). In theory, co-regulation of multiple miRNAs could minimize unwanted upregulation of oncogenes, while maintaining upregulation of tumor suppressor genes. Therefore, targeting multiple miRNAs could be more effective and perhaps a safer strategy for CRC treatment than targeting individual miRNAs.
The present study generated xenografts using 5FUR cells to confirm the chemosensitizing property of curcumin in vivo.
Although it is difficult to convincingly correlate intraperitoneal concentrations of curcumin to the equivalent in vitro concentration, considering the challenges with curcumin absorption, short half-life and the nature and spectrum of its metabolites with antitumorigenic effects. In this study, the primary considerations we had were to use lowest possible concentration of each agent, and the dose that was consistent with previous studies (45–48). In addition using a dose translation from animal to human, 50mg/kg curcumin dose in mice equates to human equivalent dose of 283mg (for a 70kg human body weight), which is a physiologically achievable dose (49). In order to quantify the chemosensitizing effect exerted by curcumin, we fitted a mathematical model to the experimental data to estimate key parameters. Our data revealed that 5FU is significantly more potent at inhibiting tumor growth compared with curcumin, but had a low susceptible cell population. In contrast curcumin treatment displayed a significantly higher susceptible cell population, but low tumor growth inhibitory rates compared with 5FU. The combined treatment showed a reduction in the degree of 5FU resistance by approximately 30%, confirming that curcumin functions as a chemosensitizer. Although curcumin has been shown to enhance the antitumorigenic activity of paclitaxel and gemcitabine in cervical and pancreatic cancer xenograft models (50,51), this is the first in vivo study to confirm that curcumin acts as a chemosensitizer.
In conclusion, we demonstrate that curcumin-mediated sensitization to 5FU in CRC cells occurs through the suppression of EMT and PRC, which are regulated by miRNAs. Together with the demonstration that miR-200c expression dictates 5FU sensitivity in CRC 5FUR cells, we highlighted the therapeutic potential of curcumin through modulation of EMT-suppressive miRNAs. Targeting both EMT and cancer stemness is proposed to be a promising therapeutic strategy in overcoming chemoresistance, and our study provides strong evidence for use of curcumin as an adjunct to 5FU-based chemotherapy in CRC—a strategy that may eventually be applicable to other human tumors in future as well.
Supplementary material
Supplementary Tables 1 and 2 and Figures 1 and 2 can be found at http://carcin.oxfordjournals.org/
Funding
National Cancer Institute, National Institute of Health (R01 CA72851 and R01 CA181572) and a pilot grant from Baylor Research Institute.
Conflict of interest statement: None declared.
Supplementary Material
Glossary
Abbreviations
- 5FU
5-fluorouracil
- 5FUR
5-fluorouracil resistant
- CRC
colorectal cancer
- EMT
epithelial–mesenchymal transition
- miRNA
microRNA
- PRC
polycomb repressive complex
References
- 1. Jemal A., et al. (2011). Global cancer statistics. CA Cancer J. Clin., 61, 69–90. [DOI] [PubMed] [Google Scholar]
- 2. Johnston P.G., et al. (2001). Capecitabine: a novel agent for the treatment of solid tumors. Anticancer Drugs, 12, 639–646. [DOI] [PubMed] [Google Scholar]
- 3. Giacchetti S., et al. (2000). Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J. Clin. Oncol., 18, 136–147. [DOI] [PubMed] [Google Scholar]
- 4. Douillard J.Y., et al. (2000). Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet, 355, 1041–1047. [DOI] [PubMed] [Google Scholar]
- 5. Wang Z., et al. (2010). Targeting miRNAs involved in cancer stem cell and EMT regulation: an emerging concept in overcoming drug resistance. Drug Resist. Updat., 13, 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thiery J.P., et al. (2009). Epithelial–mesenchymal transitions in development and disease. Cell, 139, 871–890. [DOI] [PubMed] [Google Scholar]
- 7. Yang J., et al. (2004). Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell, 117, 927–939. [DOI] [PubMed] [Google Scholar]
- 8. Comijn J., et al. (2001). The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol. Cell, 7, 1267–1278. [DOI] [PubMed] [Google Scholar]
- 9. Cano A., et al. (2000). The transcription factor snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol., 2, 76–83. [DOI] [PubMed] [Google Scholar]
- 10. Espinoza I., et al. (2013). Deadly crosstalk: Notch signaling at the intersection of EMT and cancer stem cells. Cancer Lett, 341, 41–45. [DOI] [PubMed] [Google Scholar]
- 11. Mani S.A., et al. (2008). The epithelial–mesenchymal transition generates cells with properties of stem cells. Cell, 133, 704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brabletz T. (2012). EMT and MET in metastasis: where are the cancer stem cells? Cancer Cell, 22, 699–701. [DOI] [PubMed] [Google Scholar]
- 13. Hermeking H. (2012). MicroRNAs in the p53 network: micromanagement of tumour suppression. Nat. Rev. Cancer, 12, 613–626. [DOI] [PubMed] [Google Scholar]
- 14. Burk U., et al. (2008). A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep., 9, 582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park S.M., et al. (2008). The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev., 22, 894–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bracken C.P., et al. (2008). A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial–mesenchymal transition. Cancer Res., 68, 7846–7854. [DOI] [PubMed] [Google Scholar]
- 17. Siemens H., et al. (2011). miR-34 and SNAIL form a double-negative feedback loop to regulate epithelial–mesenchymal transitions. Cell Cycle, 10, 4256–4271. [DOI] [PubMed] [Google Scholar]
- 18. Hatcher H., et al. (2008). Curcumin: from ancient medicine to current clinical trials. Cell. Mol. Life Sci., 65, 1631–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu Y., et al. (2009). Elimination of colon cancer stem-like cells by the combination of curcumin and FOLFOX. Transl. Oncol., 2, 321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kunnumakkara A.B., et al. (2009). Curcumin sensitizes human colorectal cancer to capecitabine by modulation of cyclin D1, COX-2, MMP-9, VEGF and CXCR4 expression in an orthotopic mouse model. Int. J. Cancer, 125, 2187–2197. [DOI] [PubMed] [Google Scholar]
- 21. Patel B.B., et al. (2008). Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in mediating growth inhibition of colon cancer cells by modulating EGFR and IGF-1R. Int. J. Cancer, 122, 267–273. [DOI] [PubMed] [Google Scholar]
- 22. Shakibaei M., et al. (2014). Curcumin chemosensitizes 5-fluorouracil resistant MMR-deficient human colon cancer cells in high density cultures. PLoS One, 9, e85397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Takahashi M., et al. (2012). Boswellic acid exerts antitumor effects in colorectal cancer cells by modulating expression of the let-7 and miR-200 family. Carcinogenesis, 33, 2441–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chou T.C. (2010). Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res., 70, 440–446. [DOI] [PubMed] [Google Scholar]
- 25. Shakibaei M., et al. (2013). Curcumin enhances the effect of chemotherapy against colorectal cancer cells by inhibition of NF-κB and Src protein kinase signaling pathways. PLoS One, 8, e57218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hur K., et al. (2013). MicroRNA-200c modulates epithelial-to-mesenchymal transition (EMT) in human colorectal cancer metastasis. Gut, 62, 1315–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jascur T., et al. (2011). N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) triggers MSH2 and Cdt2 protein-dependent degradation of the cell cycle and mismatch repair (MMR) inhibitor protein p21Waf1/Cip1. J. Biol. Chem., 286, 29531–29539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yoshikawa R., et al. (2001). Dual antitumor effects of 5-fluorouracil on the cell cycle in colorectal carcinoma cells: a novel target mechanism concept for pharmacokinetic modulating chemotherapy. Cancer Res., 61, 1029–1037. [PubMed] [Google Scholar]
- 29. Li R., et al. (2013). Curcumin inhibits transforming growth factor-β1-induced EMT via PPARγ pathway, not Smad pathway in renal tubular epithelial cells. PLoS One, 8, e58848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang T., et al. (2013). Curcumin inhibits LPS-induced EMT through downregulation of NF-κB-Snail signaling in breast cancer cells. Oncol. Rep., 29, 117–124. [DOI] [PubMed] [Google Scholar]
- 31. Chen F., et al. (2012). Effect of microRNA-34a in cell cycle, differentiation, and apoptosis: a review. J. Biochem. Mol. Toxicol., 26, 79–86. [DOI] [PubMed] [Google Scholar]
- 32. Tellez C.S., et al. (2011). EMT and stem cell-like properties associated with miR-205 and miR-200 epigenetic silencing are early manifestations during carcinogen-induced transformation of human lung epithelial cells. Cancer Res., 71, 3087–3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dallas N.A., et al. (2009). Chemoresistant colorectal cancer cells, the cancer stem cell phenotype, and increased sensitivity to insulin-like growth factor-I receptor inhibition. Cancer Res., 69, 1951–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Singh A., et al. (2010). EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene, 29, 4741–4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arumugam T., et al. (2009). Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res., 69, 5820–5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Creighton C.J., et al. (2009). Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc. Natl. Acad. Sci. U. S. A., 106, 13820–13825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Polyak K., et al. (2009). Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat. Rev. Cancer, 9, 265–273. [DOI] [PubMed] [Google Scholar]
- 38. Boyer L.A., et al. (2006). Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature, 441, 349–353. [DOI] [PubMed] [Google Scholar]
- 39. Mills A.A. (2010). Throwing the cancer switch: reciprocal roles of polycomb and trithorax proteins. Nat. Rev. Cancer, 10, 669–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Richly H., et al. (2011). Roles of the Polycomb group proteins in stem cells and cancer. Cell Death Dis., 2, e204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brabletz T. (2012). To differentiate or not—routes towards metastasis. Nat. Rev. Cancer, 12, 425–436. [DOI] [PubMed] [Google Scholar]
- 42. Korpal M., et al. (2011). Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nat. Med., 17, 1101–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pecot C.V., et al. (2013). Tumour angiogenesis regulation by the miR-200 family. Nat. Commun., 4, 2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Frampton A.E., et al. (2014). MicroRNAs cooperatively inhibit a network of tumor suppressor genes to promote pancreatic tumor growth and progression. Gastroenterology, 146, 268.e18–277.e18. [DOI] [PubMed] [Google Scholar]
- 45. Moon C.M., et al. (2014). Nonsteroidal anti-inflammatory drugs suppress cancer stem cells via inhibiting PTGS2 (cyclooxygenase 2) and NOTCH/HES1 and activating PPARG in colorectal cancer. Int. J. Cancer, 134, 519–529. [DOI] [PubMed] [Google Scholar]
- 46. Lei X., et al. (2012). Thymoquinone inhibits growth and augments 5-fluorouracil-induced apoptosis in gastric cancer cells both in vitro and in vivo . Biochem. Biophys. Res. Commun., 417, 864–868. [DOI] [PubMed] [Google Scholar]
- 47. Orr W.S., et al. (2013). Curcumin potentiates rhabdomyosarcoma radiosensitivity by suppressing NF-κB activity. PLoS One, 8, e51309. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48. Lin L., et al. (2011). Targeting colon cancer stem cells using a new curcumin analogue, GO-Y030. Br. J. Cancer, 105, 212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Reagan-Shaw S., et al. (2008). Dose translation from animal to human studies revisited. FASEB J., 22, 659–661. [DOI] [PubMed] [Google Scholar]
- 50. Kunnumakkara A.B., et al. (2007). Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res., 67, 3853–3861. [DOI] [PubMed] [Google Scholar]
- 51. Sreekanth C.N., et al. (2011). Molecular evidences for the chemosensitizing efficacy of liposomal curcumin in paclitaxel chemotherapy in mouse models of cervical cancer. Oncogene, 30, 3139–3152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


