Abstract
According to conflict-monitoring models, conflict serves as an internal signal for reinforcing top-down attention to task-relevant information. While evidence based on measures of ongoing task performance supports this idea, implications for long-term consequences, that is, memory, have not been tested yet. Here, we evaluated the prediction that conflict-triggered attentional enhancement of target-stimulus processing should be associated with superior subsequent memory for those stimuli. By combining functional magnetic resonance imaging (fMRI) with a novel variant of a face-word Stroop task that employed trial-unique face stimuli as targets, we were able to assess subsequent (incidental) memory for target faces as a function of whether a given face had previously been accompanied by congruent, neutral, or incongruent (conflicting) distracters. In line with our predictions, incongruent distracters not only induced behavioral conflict, but also gave rise to enhanced memory for target faces. Moreover, conflict-triggered neural activity in prefrontal and parietal regions was predictive of subsequent retrieval success, and displayed conflict-enhanced functional coupling with medial-temporal lobe regions. These data provide support for the proposal that conflict evokes enhanced top-down attention to task-relevant stimuli, thereby promoting their encoding into long-term memory. Our findings thus delineate the neural mechanisms of a novel link between cognitive control and memory.
Keywords: cognitive control, conflict, fMRI, memory, prefrontal cortex
Introduction
The influential conflict-monitoring model of cognitive control proposes that conflict in information processing serves as a signal for reinforcing top-down control processes associated with the current task set (Botvinick et al. 2001). Specifically, it is argued that when task-irrelevant (distracter) stimuli elicit representations that clash with those stemming from task-relevant (target) stimuli, a conflict signal is generated in the dorsal anterior cingulate cortex (dACC) that triggers an up-regulation in top-down attentional gain on the processing of target information, mediated by the dorsolateral prefrontal cortex (dlPFC), thus resolving the conflict (Botvinick et al. 2001). To date, predictions of this model have been derived and tested exclusively in terms of immediate attentional processing of conflicting (or incongruent) versus nonconflicting (or congruent) stimuli. For instance, the neural substrates and immediate behavioral consequences of preparing for and experiencing conflict (MacDonald et al. 2000), and those of having experienced conflict on the previous trial (Botvinick et al. 1999; Kerns et al. 2004; Egner and Hirsch 2005), have all been investigated extensively. In contrast, to our knowledge, there have been no attempts to test the model's implications for offline, long-term (memory) consequences of conflict, even though subsequent memory can provide a rich window onto cognitive-control processes (e.g., Richter and Yeung 2012).
Intriguingly, a key tenet of the conflict-monitoring model, namely that conflict triggers enhanced attention to task-relevant stimulus information, leads to a straightforward yet somewhat counterintuitive prediction for subsequent memory of that information: Specifically, target information stemming from an incongruent trial should, due to conflict-driven attentional enhancement (cf., Chun and Turk-Browne 2007), be recalled with greater accuracy than those stemming from a congruent trial. This prediction contrasts with the perhaps more intuitive assumption that the additional control operations required to overcome interference from incongruent distracters might impair the processing of, and subsequent memory for, target stimuli due to limited processing resources (Uncapher and Wagner 2009). Moreover, if the behavioral hypothesis of conflict-enhanced memory for target stimuli was to be confirmed, one would expect this effect to be mediated by prefrontal and parietal brain regions involved in attentional enhancement during conflict resolution, presumably in conjunction with medial-temporal lobe (MTL) structures responsible for encoding long-term memories (e.g., Eichenbaum et al. 2007). In the present study, we tested these predictions.
To this end, we developed a novel experimental protocol that consisted of acquiring functional magnetic resonance imaging (fMRI) data during a face-word Stroop task (e.g., Egner and Hirsch 2005; Egner et al. 2008) that employed trial-unique target stimuli (faces), combined with an incidental memory test for those faces (cf., Richter and Yeung 2012). This design allowed us, first, to test the basic prediction of conflict-enhanced, target-stimulus memory we derived from the conflict-monitoring model, and secondly, to delineate its neural substrates by employing a difference-based-on-memory (DM) analysis approach (Paller et al. 1987; Wagner et al. 1998; Paller and Wagner 2002). This approach can isolate brain regions that are more active during encoding of items that are subsequently remembered compared with those that are subsequently not-remembered (forgotten).
Materials and Methods
Participants
Twenty healthy individuals performed a face-word Stroop task in the fMRI scanner (mean age = 22.5 years; range = 18–35 years; 8 males; 6 left-handed). All participants were recruited from the student population of Ghent University and gave written informed consent. The experimental protocol was approved by the ethical committee of the Ghent University Hospital.
Experimental Procedure
The face-word stimuli consisted of male and female faces (Glasgow Face Database, Bruce et al. 1999), overlaid by a word that could be congruent, incongruent, or neutral with respect to the gender of the face. Like in the classic color-naming Stroop task, incongruent displays in this task are responded to more slowly than congruent ones (MacLeod and MacDonald 2000; Egner and Hirsch 2005; Egner et al. 2008). In the present task, we also included neutral stimuli in order to be able to dissociate memory effects attributable to a semantic mismatch between target stimuli and distracters (present in both neutral and incongruent trials) from those related to response conflict—which is exclusively triggered by incongruent trials. Prior to the fMRI session, participants performed a familiarization task on a standard laptop computer, in which all faces that were later used in the Stroop task were presented in a random sequence. We chose this procedure based on recent observations that completely novel stimuli can reduce typical behavioral costs in this kind of task (Krebs et al. 2013), likely due to an automatic capture of attention by novelty that reduces the influence of the irrelevant information. Also, such a procedure helps to avoid potential floor effects in the incidental retrieval of unfamiliar stimuli (Krebs et al. 2009). It should be noted that this preexposure is identical for all experimental conditions, thus ruling out any differential effects on later memory performance. During familiarization, all faces were displayed 3 times in a random order for 1500 ms, with a stimulus-onset asynchrony (SOA) of 2300 ms. To ensure that participants paid attention to all pictures, they were asked to indicate for each face presentation whether they had seen the current face before or not via a button-press response with the index and middle finger of the right hand, respectively (button assignments were counterbalanced across participants). All faces were presented in the center of a white screen (visual angle 5 × 6°). Throughout the entire task, a black fixation dot (0.3°) was visible in the center of the screen, and participants were instructed to keep accurate fixation.
Face-Word Stroop Task
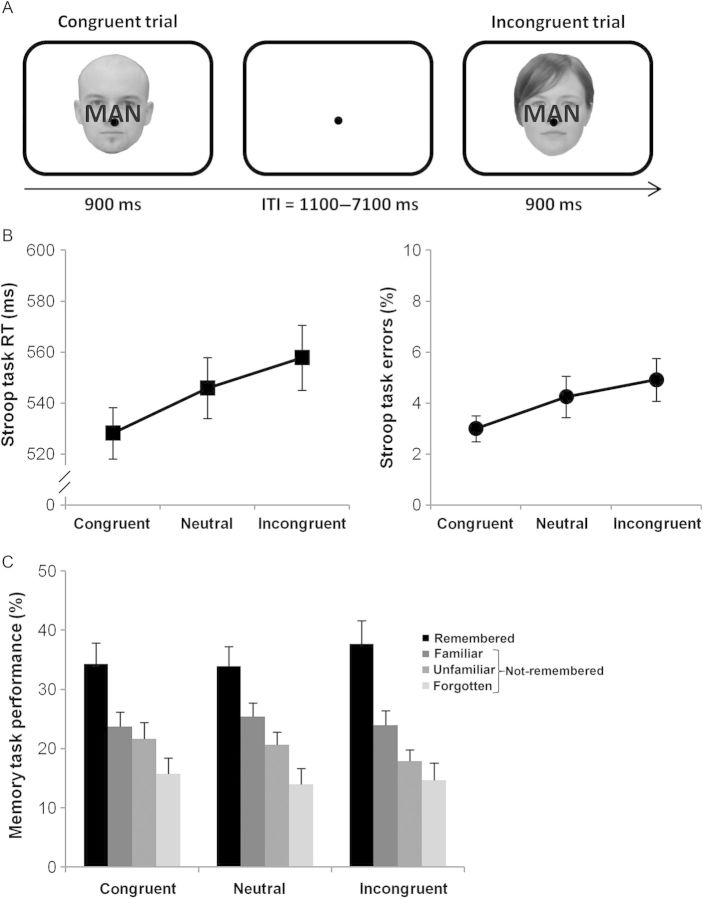
In the actual Stroop task inside the MR scanner (Fig. 1A), all 180 familiarized faces were presented once in a random order for 900 ms each in the center of a white screen (5 × 6°). Each face was overlaid with a congruent, an incongruent, or a neutral word in red ink (Dutch words for man, woman, and house: “man,” “vrouw,” and “huis”; capitalized Arial font, 6 × 2°). These trial types occurred with a probability of 33% each. Participants were asked to attend to the face while ignoring the word and to decide as quickly as possible whether the face was male or female by pressing with the left or right index finger, respectively (button assignments were counterbalanced across participants). Throughout the entire experiment, a black fixation dot (0.3°) was visible in the center of the screen, and participants were instructed to keep accurate fixation. To allow for effective event-related, blood-oxygen-dependent (BOLD) response estimation (Hinrichs et al. 2000), trial onsets were randomly varied with an SOA between 2000 and 8000 ms in steps of 500 ms (mean SOA = 3000 ms). Specifically, SOAs were pseudoexponentially distributed with 75% between 2000 and 3000 ms, 17% between 3500 and 5000 ms, and 8% between 5500 and 8000 ms. This SOA distribution resulted in a variable intertrial interval of 1100–7100 ms (Fig. 1A). Response times (RT) and error rates in the Stroop task were analyzed via 1-way repeated-measures analyses of variance (rANOVAs) and planned follow-up paired t-tests testing for differences between congruent, neutral, and incongruent trials.
Figure 1.
Face-word Stroop task trial structure and behavioral results. (A) Each trial consisted of a face stimulus (task-relevant), overlaid by a word (task-irrelevant) that could be congruent, neutral, or incongruent with respect to the gender of the face (ITI, intertrial interval). (B) Both RTs and error rates in the Stroop task were modulated by the congruency of the overlaid word, with linearly increasing behavioral costs from congruent to neutral to incongruent words. (C) Confident subsequent memory, as indexed by “remembered” (“definitely old”) responses, was greater for faces that were overlaid by incongruent compared with congruent and neutral words. Error bars represent the standard error of the mean (SEM).
Note that the signature of conflict–control we employed in the present study was given by conventional conflict or interference scores, that is, the difference in performance (and neural activity) between incongruent and congruent and/or neutral trials. This metric captures both the generation/detection as well as the within-trial resolution of conflict—given that we only analyzed trials where a correct answer was given (cf., Scherbaum et al. 2011). While an approximate segregation of conflict-detection versus conflict-resolution processes could, in principle, be achieved by analyzing first-order congruency sequence (conflict adaptation) effects (Gratton et al. 1992; Botvinick et al. 2001; Egner 2007), this type of analysis would result in a substantial reduction in trial counts in the present design, especially since our study also involved neutral trials, as well as a subsequent DM analysis. Since this approach would, therefore, have greatly reduced the power of our key analyses, we opted instead to focus exclusively on the standard trial interference effects. An added benefit of this approach is that it coincides with the way that the large majority of previous fMRI studies of conflict processing have analyzed their data, thus rendering the present results more comparable to much of the prior literature.
Incidental Memory Task
After an additional fMRI task and anatomical scans that were unrelated to the present study, which also served as encoding-retrieval delay, participants performed an incidental memory test on a standard laptop computer (∼1 h after the Stroop task). Half of all previously seen faces and 60 completely novel faces were presented once in a random order for 2000 ms each in the center of a white screen (5 × 6°) with an SOA of 3000 ms. Novel faces were included to promote a deliberate decision on each picture thus preventing automated responses. The gender of both familiar and novel faces was completely balanced (50%), as was the former trial category of the familiar faces, that is, congruent, neutral, and incongruent (33%). Participants were asked to indicate whether they remembered the current face by pressing 1 of the 4 number buttons with the 4 fingers of the right hand according to a 4-point scale (4, “definitely old”; 3, “probably old”; 2, “probably new”; 1, “definitely new”). These 4 options and the corresponding buttons were displayed at the bottom of the screen. On the next day, an analogous retrieval task was employed that included the other half of all previously seen faces and 60 novel faces (∼24 h after the face-word Stroop task). Including retrieval day as an additional factor in the behavioral analysis did not yield any significant main effect or interaction with the effect of interest (P > 0.5), which led us to collapse retrieval data across the 2 days, which had the added benefit of yielding higher trial counts per experimental condition in the fMRI analysis. For the analysis, the response categories were labeled as follows: 4, remembered; 3, familiar; 2, unfamiliar; and 1, forgotten. Given that all faces had the same level of familiarity at the start of the face-word Stroop task, we focused on the potential neural mechanisms that give rise to remembering specific faces with high confidence. To this end, we focused on congruency-induced differences in the “remembered” (or “definitely old”) response rates, and considered the remaining responses as “not-remembered.” Memory rates were analyzed via a 1-way rANOVA and planned follow-up paired t-tests testing for differences between congruent, neutral, and incongruent trials.
fMRI Acquisition and Preprocessing
Data were acquired using a 3-T Siemens Magnetom Trio MRI system (Siemens Medical Systems, Erlangen, Germany) with a 32-channel head coil. For all participants, an anatomical T1-weighted 3D magnetization-prepared rapid acquisition with gradient echo sequence (time repetition [TR] = 1550 ms, time echo [TE] = 2.39 ms, TI = 900 ms, acquisition matrix = 256 × 256, FOV [field of view] = 220 mm, flip angle = 9°, voxel size = 0.86 × 0.86 × 0.9 mm) was acquired to enable coregistration, normalization, and localization of areas of interest. During the face-word Stroop task, T2*-weighted echo-planar images (EPIs) were acquired in 33 slices with an interleaved scanning order (TR = 2000 ms, TE = 30 ms, acquisition matrix = 64 × 64, FOV = 192 mm, flip angle = 80°, voxel size = 3 × 3 × 3 mm, no interslice gap). The first 4 EPI volumes were discarded to allow a steady magnetization to be reached. Images were preprocessed and further analyzed using Statistical Parametric Mapping (SPM8; University College, London, UK). Anatomical images were spatially normalized to the SPM T1-template image and resliced to a voxel size of 1 × 1 × 1 mm. Functional EPIs were slice-time corrected and realigned to the first acquired EPI. Next, EPIs were normalized based on the T1-derived normalization parameters, resliced to a final voxel size of 2 × 2 × 2 mm, and smoothed with an isotropic full-width half-maximum Gaussian kernel of 6 mm.
fMRI Analysis
A standard 2-stage procedure was used for statistical analysis. In the first stage, a general linear model (Friston et al. 1995) was employed for each individual participant. BOLD responses were modeled by delta functions at stimulus onset, which were then convolved with a standard hemodynamic response function (HRF). The model included regressors for a total of 6 experimental conditions, as well as 6 movement regressors derived from the realignment procedure. Stimulus onsets for the experimental conditions were derived by dividing the Stroop-task conditions (congruent, neutral, and incongruent) based on later remembered versus not-remembered face stimuli (mean trial numbers [range] for remembered versus not-remembered: congruent 34 [13–63] versus 61 [35–83]; neutral 34 [12–62] versus 60 [35–80]; incongruent 38 [12–72] versus 57 [23–77]). The comparison between neural activity during the encoding of subsequently remembered and “forgotten” (or not-remembered) pictures is often referred to as the “DM” effect (Paller and Wagner 2002) and allows one to identify brain regions that contribute to successful long-term memory. Time series were corrected for slow drifts by applying a high-pass filter of 128 s.
On the second level, considering the highly selective memory benefit for incongruent trials (see Results), we sought to pin down neural activity that is unique to incongruent trials during encoding in the face-word Stroop task. Specifically, BOLD activity in response to incongruent trials was compared with congruent and neutral ones via a 1-sample t-test [incongruent − ([congruent + neutral]/2)]. Activations were deemed significant if they survived a family-wise error (FWE) correction at the cluster level (P < 0.05), based on an auxiliary uncorrected voxel-wise height threshold of P < 0.001. Coordinates of significant local maxima are reported in a standard stereotaxic reference space of the Montreal Neurological Institute (MNI) system. Next, we tested whether frontal and parietal conflict–control regions that were significantly more active in the presence of conflict in the voxel-wise analysis, that is, in incongruent compared with both congruent and neutral trials, were contributing to the improved memory for faces stemming from incongruent trials. To this end, we conducted a region of interest (ROI) analysis using the Marsbar toolbox (Brett et al. 2002). ROIs were defined as spheres (diameter = 5 mm) around selected local activity maxima yielded by the voxel-wise analysis [incongruent − ([congruent + neutral]/2)]. Specifically, in keeping with the existing literature on conflict processing (e.g., Kerns et al. 2004; Egner and Hirsch 2005; Nee et al. 2007; Egner et al. 2008), the ROI analysis was focused on the medial and lateral prefrontal cortex as well as parietal cortex (see Results section for details). Parameter estimates (beta values) of incongruent remembered and incongruent not-remembered target stimuli were extracted from these ROIs and compared via paired t-tests in a DM analysis. Note that the latter test is independent of the contrast by which the ROIs were defined.
Functional Connectivity (PPI) Analysis
Finally, to explore functional connectivity profiles of ROIs that are contributing to the successful retrieval of faces from incongruent trials, we performed psycho-physiological interaction (PPI) analysis (Friston et al. 1997) using the automated generalized PPI toolbox (McLaren et al. 2012). Individual PPI terms were created by multiplying the BOLD time series from a given “seed” ROI with the psychological vector reflecting the experimental conditions [e.g., incongruent − ([congruent + neutral]/2)]. After convolution with the HRF, mean correction, and orthogonalization, the interaction regressor (PPI term) went into a second-level analysis to determine condition-dependent changes of functional connectivity over and above any main effect of task or any main effect of activity in the corresponding brain areas. To this end, the individuals' PPI contrast images were submitted to 1-sample t-tests, revealing brain regions whose activity covaried with activity in a given seed region. Statistical thresholding was analogous to the regular fMRI analysis (FWE correction at the cluster level with a threshold of P < 0.05), except for a small-volume correction (SVC) approach for determining potential activations in a priori ROIs, namely the hippocampus (HIP) and bordering parahippocampal gyrus (PHG), as these regions are strongly implicated in long-term memory formation (e.g., Eichenbaum et al. 2007). To this end, an anatomical mask encompassing bilateral HIP and PHG was created using the MRIcron software package (Rorden and Brett 2000).
Results
Behavior—Face-Word Stroop Task
As anticipated, in the face-word Stroop task, both RT and error rates were modulated by the congruency of the irrelevant distracter word stimulus (Fig. 1B). Specifically, participants were significantly slower to respond correctly in incongruent and neutral trials when compared with congruent ones (F2,38 = 10.52, P < 0.001; planned t-tests: incongruent > congruent t(19) = 4.0, P < 0.005; neutral > congruent t(19) = 3.3, P < 0.005; incongruent > neutral t(19) = 1.8, P > 0.05), and committed significantly more errors in incongruent compared with congruent trials (F2,38 = 5.51, P < 0.01; planned t-tests: incongruent > congruent t(19) = 3.0, P < 0.01; neutral > congruent t(19) = 1.9, P > 0.05; incongruent > neutral t(19) = 1.6, P > 0.1). As can be seen clearly in Figure 1B, there was moreover a significant linear trend for increasing RTs (F1,19 = 16.01, P < 0.005) as well as increasing error rates (F1,19 = 9.21, P < 0.01) from congruent, over neutral, to incongruent trials. While the interference effect may not be as large as in the traditional color-naming Stroop task, it is comparable to effects observed with similar face-word Stroop paradigms (e.g., Egner et al. 2010). The reason for this might be related to the categorical decisions that are more complex than simply naming a color, hence reducing the impact of the irrelevant dimension on RT and accuracy.
Thus, our novel face-word Stroop task with trial-unique target stimuli produced typical conflict effects, which in turn allowed us to investigate whether incidental recall of these target stimuli varied as a function of distracter word congruency.
Behavior—Incidental Memory Task
An overview of the retrieval data can be found in Figure 1C. Note that all error trials in the face-word Stroop task were excluded from this analysis, as were missed trials in the retrieval task itself. In total, no more than 6% of all trials were excluded (averaged across conditions). The rANOVA of memory rates revealed a significant modulation by word congruency during face encoding in the face-word Stroop task (F2,38 = 3.72, P < 0.05). Planned t-tests revealed that faces from incongruent trials yielded significantly better memory rates (38%) compared with those from congruent (34%; t(19) = 2.29, P < 0.05) and neutral trials (34%; t(19) = 2.23, P < 0.05). We note that while a numerical difference of 4% is modest in size, the effects were statistically robust due to being very consistent across participants. Only 4 of 20 participants showed an effect in the opposite direction in 1 of the 2 comparisons (incongruent < congruent or incongruent < neutral) or in both. Taken together, the behavioral results show that incongruent task-irrelevant words caused performance detriments (longer RTs and lower accuracy) in the face-word Stroop task itself, but promoted subsequent memory of the task-relevant face stimuli from correct trials. This novel finding of conflict-enhanced target memory is in line with predictions derived from conflict-monitoring models, which holds that conflict triggers controlled re-focusing on task-relevant stimulus information. While within-trial effects of such re-focusing have recently been described (Scherbaum et al. 2011), the present study is the first that describes changes in the long-term representation of the target stimuli. These data set the stage for our fMRI analyses, which were geared at characterizing the neural substrates of this conflict-triggered boost to target memory.
Note that face stimuli in congruent, neutral, and incongruent trials were displayed for the same duration, thus ruling out the possibility that the memory benefit for incongruent trials could be driven by differential exposure times. However, it could potentially be argued that face stimuli on these trials were subject to longer “processing time,” as reflected in prolonged RTs. To rule out that the observed memory benefit for incongruent trials during the face-word Stroop task was mainly a result of differences in “time-on-task,” we conducted 2 additional analyses. First, to test for effects of the individual RT level, memory rates were correlated with RTs in the face-word Stroop task across participants. None of the correlations (averaged across word congruency, congruent only, neutral only, and incongruent only) reached significance (all P > 0.5). Secondly, incongruent face-word Stroop trials were sorted based on later memory success (remembered vs. not-remembered). There was no significant RT difference between later remembered and not-remembered incongruent target stimuli (paired t-test P > 0.5), indicating that the memory benefit in incongruent trials was not promoted by longer RTs per se during the encoding of the target stimuli, but rather by the (conflicting) relationship between distracter and target stimuli and the resolution of this conflict.
fMRI Results
Based on the observation of a unique memory benefit for target stimuli in incongruent trials, our fMRI analysis strategy was to first identify brain regions involved in conflict–control processes, that is, regions that were more active during incongruent than congruent and neutral Stroop trials. Areas identified based on this comparison thus represent candidate regions that might have mediated the conflict-enhanced encoding of target faces we observed in the behavioral data. Incongruent compared with congruent and neutral trials elicited several robust activation foci in the medial and lateral frontal cortex (Fig. 2A and Table 1), including bilateral presupplementary motor area (pre-SMA) and dACC, bilateral dlPFC, and right inferior frontal gyrus (IFG, at the trend level). Such activations in the medial and lateral PFC are commonly observed in Stroop and other interference paradigms and are thought to mediate the detection and resolution of conflict (Miller and Cohen 2001; Botvinick et al. 2004; Ridderinkhof et al. 2004). Additional activation clusters were observed in posterior regions, namely bilateral inferior parietal lobe (IPL), bilateral precuneus, bilateral fusiform gyrus (FG), and right middle temporal gyrus (MTG). Again, these findings align closely with prior literature, where parietal cortex areas have frequently been implicated in conflict–control, particularly in relation to Stroop-like stimulus conflict (e.g., Egner and Hirsch 2005; Liston et al. 2006; Egner et al. 2007). Moreover, the activation observed in the FG likely reflects the conflict-driven modulation of (target) face processing (Egner and Hirsch 2005).
Figure 2.
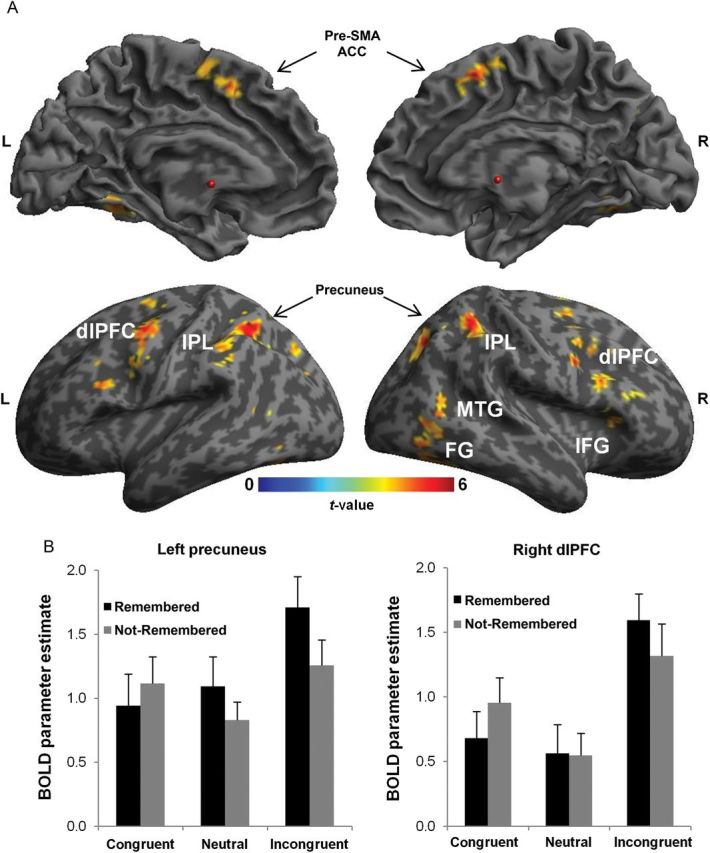
fMRI results. (A) BOLD activity is shown for the contrast of incongruent − ([congruent + neutral]/2) Stroop trials (for display purposes, thresholded at P < 0.001, uncorrected). Note that only activations that survived an FWE correction at the cluster level (Table 1) were considered for the subsequent ROI-based DM analysis. L indicates left hemisphere, R indicates right hemisphere, and the red dot indicates MNI coordinates x, y, z = 0, 0, 0. (B) Two of these conflict-sensitive regions, that is, left precuneus and right dlPFC, exhibited a significant difference between subsequently remembered and not-remembered faces that was exclusive to incongruent trials. BOLD parameter estimates extracted from spherical ROIs around the local cluster maxima (left precuneus MNI x, y, z = −26, 72, 36; right dlPFC MNI x, y, z = 46, 8, 36). Error bars represent the SEM.
Table 1.
Overview of activation clusters for incongruent − ([congruent + neutral]/2)
| FWE-corrected cluster P-value | Cluster size k | Peak voxel t-value | Peak voxel MNI |
Hemisphere | Region | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| 0.000 | 814 | 5.01 | −32 | −52 | 54 | L | IPL |
| 4.22 | −46 | −42 | 46 | L | IPL | ||
| 4.18 | −56 | −40 | 36 | L | IPL | ||
| 4.08 | −42 | −48 | 58 | L | IPL | ||
| 0.000 | 1270 | 4.80 | 46 | 8 | 36 | R | dlPFC |
| 4.32 | 8 | 10 | 54 | R | Pre-SMA/dACC | ||
| 4.16 | 32 | 0 | 62 | R | Medial frontal gyrus | ||
| 4.09 | −6 | 10 | 50 | L | Pre-SMA/dACC | ||
| 3.89 | 4 | 18 | 48 | R | Pre-SMA/dACC | ||
| 3.69 | −6 | 0 | 56 | L | Pre-SMA/dACC | ||
| 3.48 | 40 | 16 | 24 | R | IFJ | ||
| 3.48 | 56 | 24 | 30 | R | dlPFC | ||
| 3.47 | 8 | 2 | 62 | R | Pre-SMA/dACC | ||
| 0.000 | 508 | 4.68 | 42 | −46 | 56 | R | IPL |
| 3.98 | 38 | −40 | 40 | R | IPL | ||
| 3.41 | 54 | −48 | 48 | R | IPL | ||
| 0.000 | 847 | 4.56 | −38 | −6 | 44 | L | dlPFC |
| 4.53 | −56 | 10 | 32 | L | dlPFC | ||
| 4.35 | −30 | −6 | 60 | L | Medial frontal gyrus | ||
| 4.30 | −48 | −2 | 38 | L | dlPFC | ||
| 4.09 | −36 | 16 | 22 | L | IFJ | ||
| 3.92 | −48 | 28 | 36 | L | alPFC | ||
| 3.59 | −24 | −2 | 52 | L | Medial frontal gyrus | ||
| 3.55 | −30 | −12 | 46 | L | Medial frontal gyrus | ||
| 0.000 | 300 | 4.41 | −40 | −62 | −12 | L | FG |
| 4.18 | −38 | −44 | −18 | L | FG | ||
| 3.44 | −30 | −62 | −20 | L | FG | ||
| 0.001 | 240 | 4.36 | 22 | −62 | 40 | R | Precuneus |
| 3.82 | 32 | −62 | 34 | R | Precuneus | ||
| 0.000 | 485 | 4.34 | 50 | −64 | 0 | R | MTG |
| 4.27 | 40 | −60 | −16 | R | FG | ||
| 4.05 | 42 | −70 | −12 | R | FG | ||
| 3.82 | 44 | −62 | 10 | R | MTG | ||
| 3.71 | 32 | −52 | −12 | R | FG | ||
| 3.44 | 32 | −72 | −8 | R | FG | ||
| 0.021 | 161 | 4.28 | −26 | −72 | 36 | L | Precuneus |
| 4.12 | −26 | −78 | 24 | L | Precuneus | ||
| 0.074 | 116 | 4.19 | 42 | 24 | 6 | R | IFG |
| 3.43 | 54 | 14 | 8 | R | IFG | ||
MNI: coordinates according to the Montreal Neurological Institute system; L: left hemisphere; R: right hemisphere; IPL: inferior parietal lobe; dlPFC: dorsolateral prefrontal cortex; Pre-SMA: presupplementary motor area; dACC: dorsal anterior cingulate cortex; IFJ: inferior frontal junction; alPFC: anterolateral prefrontal cortex; FG: fusiform gyrus; MTG: middle temporal gyrus; IFG: inferior frontal gyrus.
To determine whether such conflict-related activity was predictive of subsequent memory success in incongruent trials, we performed an ROI-based DM analysis. Specifically, we tested which of the frontal and parietal regions that are most closely associated with conflict–control may have contributed to subsequent memory for faces seen in incongruent trials, by contrasting ROI-based BOLD activation for incongruent trials that were subsequently remembered with those that were not-remembered (i.e., a DM analysis). Note that we focus on incongruent trials in this comparison, because it renders the DM analysis independent of the conflict contrast that was employed to define these ROIs in the first place. However, it is important to consider whether any differences in trial numbers in the original conflict contrast (i.e., more remembered faces from incongruent trials compared with congruent or neutral ones) may have biased the results of the ROI-based DM analysis. First, the numerical difference in trial numbers is very small and thus unlikely to significantly bias the data. Secondly, any potential bias due to such a difference would work in favor for the conditions with fewer trials, here congruent remembered and neutral remembered ones, due to a higher level of noise (Kriegeskorte et al. 2009). Hence, it is highly unlikely that any DM effects in incongruent trials revealed by the ROI analysis are affected by the contrast used for defining the ROIs. To account for multiple comparisons, the DM analysis carried out in candidate conflict–control ROIs derived from the voxel-wise comparison [incongruent − ([congruent + neutral]/2] was subjected to Bonferroni-correction (P < 0.007 for 7 comparisons). The respective center coordinates of the selected ROIs can be found in Table 1 (pre-SMA/dACC, left and right dlPFC, left and right IPL, and left and right precuneus). This ROI analysis revealed that activity in 2 of 7 regions was predictive of the memory benefit for faces presented in incongruent trials (incongruent remembered > incongruent not-remembered), specifically right dlPFC (t(19) = 2.26, P < 0.005; ROI centered on MNI x, y, z = 46, 8, 36) and left precuneus (t(19) = 3.23, P < 0.006; ROI centered on MNI x, y, z = −26, 72, 36). To test whether these effects were exclusive for incongruent trials, neural activity in right dlPFC and left precuneus was analyzed via rANOVAs with factors word congruency [incongruent vs. ([congruent + neutral]/2)] and memory success (remembered vs. not-remembered). This analysis revealed a significant interaction between conflict and memory in the right dlPFC (P < 0.05) and a trend for an interaction in the left precuneus (P = 0.07), in both cases reflecting activity differences between remembered and not-remembered incongruent trials, but no such difference for congruent and neutral trials. Moreover, to rule out that the DM effect in incongruent trials was the result of an ROI-selection bias, due to the slightly higher number of remembered faces from incongruent trials compared with congruent or neutral ones in the original contrast, we analyzed 4 participants with an opposite behavioral pattern separately (more faces remembered from congruent or neutral trials compared with incongruent ones). This analysis still revealed greater activity in dlPFC and precuneus for incongruent remembered compared with incongruent not-remembered trials (1-tailed t-tests: right dlPFC t(3) = 3.26; P < 0.05; left precuneus t(3) = 3.23; P < 0.05) and no difference for congruent or neutral trials (all P > 0.1), indicating that the DM effect in these regions was not tied to greater trial numbers for incongruent remembered trials.
None of the remaining ROIs (centered around local maxima in pre-SMA/dACC, left dlPFC, left and right IPL, and right precuneus) revealed any trend for a DM effect in incongruent trials (all P > 0.1). These data suggest that while conflict processing in incongruent trials elicited enhanced activity in a large network of brain regions thought to be involved in cognitive-control processes, the successful long-term encoding of the respective target faces appeared to be subserved specifically by the right dlPFC and left parietal cortex. We further confirmed this anatomical specificity by conducting a whole-brain search for additional regions displaying DM effects, but no such areas were detected at the FWE-corrected threshold.
Analogous to the behavioral analysis in which we tested whether memory rates were dependent on RTs (or time-on-task) in the face-word Stroop task, we tested whether the observed activity difference between remembered and not-remembered incongruent target stimuli in the right dlPFC and left precuneus ROIs was correlated with RTs in the face-word Stroop task. No significant correlations between BOLD signal and RTs were observed for incongruent remembered minus incongruent not-remembered items (all P > 0.1), indicating that the increased activity for later remembered items in these regions was not merely attributable to prolonged processing time during incidental encoding of the target stimuli.
In summary, we found that incongruent trials were associated with enhanced memory for target information, as well as with activation in fronto-parietal regions, 2 of which (right dlPFC and left precuneus) displayed a DM effect, whereby greater activity during conflict was predictive of greater subsequent memory for the target stimuli, suggesting a direct involvement of these regions in bringing about the conflict-driven memory improvement. In a final analysis, we sought to corroborate this interpretation.
Functional Connectivity (PPI) Results
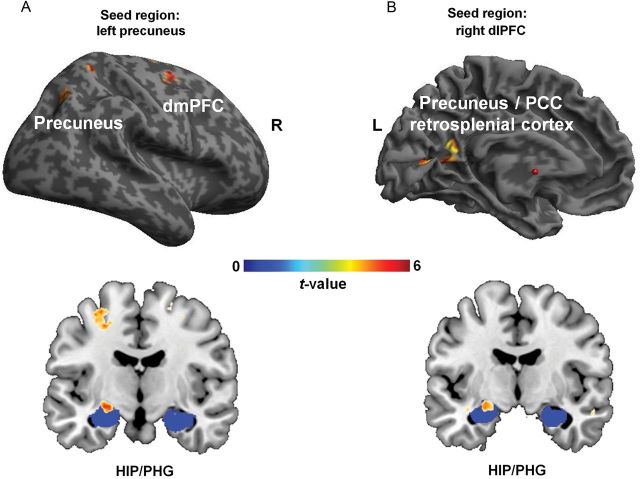
If conflict-sensitive brain regions, in particular the right dlPFC and left precuneus foci identified in the DM analysis above, were indeed involved in mediating the conflict-enhanced encoding of target stimuli, one would assume that they were engaged in some form of top-down modulation of encoding processes in the MTL. We tested this prediction by conducting condition-specific functional connectivity (PPI) analysis (Friston et al. 1997; McLaren et al. 2012). Specifically, employing the right dlPFC and left precuneus sites as seed ROIs, we tested whether their functional coupling with other brain regions was significantly modulated by conflict [incongruent − ([congruent + neutral]/2)]. Given our specific predictions regarding memory encoding, we also used a statistical SVC for investigating functional connectivity specifically with the MTL. To this end, we used the clusters in the right dlPFC and left precuneus (see Fig. 2A) as seed ROIs in separate PPI analysis and searched for brain regions that exhibited increased connectivity in incongruent compared with congruent and neutral trials (Fig. 3 and Table 2). We found that the right dlPFC displayed significantly greater coupling during incongruent trials with a cluster including the retrosplenial cortex, the precuneus, and the posterior cingulate cortex (PCC), as well as with a cluster in the left HIP/PHG (SVC within an anatomical mask). The analogous analyses using the left precuneus as seed region also revealed a conflict-enhanced functional coupling between that region and the left HIP/PHG (SVC within the anatomical mask), as well as with the right dorsomedial PFC (dmPFC) and the right precuneus. Thus, both of the regions that displayed a DM effect for conflict-enhanced target memory also showed increased functional coupling under conflict with MTL regions known to be crucial to episodic memory encoding.
Figure 3.
Functional connectivity (PPI) results. (A) The functional coupling between left precuneus (seed region) and right dmPFC and right precuneus, as well as with the left HIP/PHG (SVC within the anatomical mask in blue, MNI y = −10), was increased for the contrast of incongruent − ([congruent + neutral]/2) Stroop trials. (B) The right dlPFC (seed region) was coupled with a cluster that included parts of the retrosplenial cortex, precuneus, and PCC, as well as with the left HIP/PHG (SVC within anatomical mask in blue, MNI y = −8) for the contrast of incongruent − ([congruent + neutral]/2) Stroop trials. L indicates left hemisphere, R indicates right hemisphere, red dot indicates MNI x, y, z = 0, 0, 0 (for display purposes, thresholded at P < 0.001, uncorrected).
Table 2.
Overview of the functional connectivity results for right dlPFC and left precuneus
| FWE-corrected cluster P-value | Cluster size k | Peak voxel t-value | Peak voxel MNI |
Hemisphere | Region | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Seed region: right dlPFC, incongruent − ([congruent + neutral]/2) | |||||||
| 0.002 | 183 | 5.50 | −14 | −56 | 4 | L | Retrosplenial cortex |
| 5.43 | −22 | −56 | 20 | L | Precuneus/PCC | ||
| 4.41 | −10 | −48 | 16 | L | Precuneus/PCC | ||
| 0.045a | 12 | 4.27 | −24 | −8 | −10 | L | HIP/PHG |
| 4.13 | −24 | −6 | −14 | L | HIP/PHG | ||
| Seed region: left precuneus, incongruent − ([congruent + neutral]/2) | |||||||
| 0.026 | 104 | 5.21 | 26 | 0 | 62 | R | dmPFC |
| 4.24 | 30 | −6 | 50 | R | dmPFC | ||
| 0.042 | 93 | 4.65 | 30 | −64 | 46 | R | Precuneus |
| 0.025a | 16 | 4.86 | −24 | −10 | −10 | L | HIP/PHG |
| Seed region: right dlPFC, incongruent remembered–not-remembered | |||||||
| 0.060a | 8 | 4.08 | 32 | −8 | −24 | L | HIP/PHG |
| Seed region: left precuneus, incongruent remembered–not-remembered | |||||||
| 0.006 | 162 | 6.62 | −26 | 24 | 24 | L | vmPFC |
| 4.64 | −30 | 36 | 18 | L | vmPFC | ||
| 4.04 | −34 | 44 | 22 | L | vmPFC | ||
MNI: coordinates according to the Montreal Neurological Institute system; L: left hemisphere; R: right hemisphere; PCC: posterior cingluate cortex; HIP: hippocampus; PHG: parahippocampal gyrus; dmPFC: dorsomedial prefrontal cortex; vmPFC: ventromedial prefrontal cortex.
Additional SVC based on the anatomical mask.
Finally, in addition to assessing conflict-driven coupling per se, we also explored whether the right dlPFC and left precuneus ROIs displayed connectivity modulations as a function of subsequent memory, by comparing the connectivity patterns for subsequently remembered versus not-remembered incongruent trial target faces. For this comparison, we found increased functional connectivity between left precuneus and a cluster in the left ventromedial PFC (vmPFC). The analogous analysis using the right dlPFC as a seed region did not yield any significant clusters at our conservative, whole-brain FWE-corrected threshold. However, we observed increased coupling between right dlPFC and a cluster in the right MTL at a trend level (P = 0.06; SVC with the anatomical mask) for remembered versus not-remembered incongruent items.
Discussion
The present study investigated the influence of conflict on the long-term encoding of target stimuli in a face-word Stroop task that employed trial-unique target stimuli. We found that irrelevant incongruent information (words), which led to typical behavioral costs in the Stroop task itself, gave rise to improved subsequent memory for the relevant target stimuli (faces). The comparison of fMRI activity in subsequently remembered and not-remembered incongruent trials revealed that the conflict-induced memory benefit was selectively associated with activity modulations in the dlPFC and the precuneus, both of which are part of the conflict–control network in the present as well as in previous studies (e.g., Nee et al. 2007). Finally, under conditions of conflict, both of these regions displayed enhanced functional coupling with each other, as well as with the left MTL.
The behavioral observation of conflict-induced benefits in target-stimulus memory is, to the best of our knowledge, a completely novel finding. On the one hand, this observation seems counterintuitive, given that processing of target information is clearly hindered by incongruent distracters (as evidenced by the ubiquitous congruency effect). On the other hand, it is actually highly consistent with cognitive-control models postulating that interfering information triggers a top-down reinforcement of the ongoing task set and increases attention to the target stimulus (Egner and Hirsch 2005), which, in turn, appears to lead to a deeper encoding thereof. Such a mechanism would be consistent with observed mnemonic benefits due to augmented attentional selection (Chun and Turk-Browne 2007). While this appears to be a feasible explanation for the observed results, it is important to consider whether the memory benefit could simply be attributed to longer processing time (as reflected in longer mean RTs) in incongruent trials during the face-word Stroop task. Our data do not support this idea: We neither observed any relationship between RT and memory benefits in any of the conditions, nor a relationship between RT and brain activity in the regions predicting later retrieval success. Moreover, such a time-on-task explanation would predict that memory rates should be highest for incongruent, intermediate for neutral, and lowest for congruent trials. However, in the present study, memory was selectively better only for incongruent trials, supporting the notion that the memory benefit resulted from within-trial conflict–control processes that are unique to incongruent trials. While the absence of a relationship between RTs in the conflict task and memory benefit in incongruent trials speaks against a simple time-on-task confound, such a relationship would, in fact, be predicted by conflict-resolution models as conflict resolution may take time. We can only speculate why there is no such relationship in our data set. First of all, an absent correlation is not an ultimate proof of an absent relationship (this could be due to a lack of statistical power). Secondly, it seems plausible to assume that an increased attentional focus, which is in our opinion the basis for the DM effect, might as well speed up conflict resolution and ultimately RTs in a subset of incongruent trials. This would, of course, dilute a systematic correlation between the 2 measures, and there is no way to dissociate these processes.
Recall that participants had been preexposed to all faces before the actual conflict task, which raises the possibility that the memory benefits in incongruent trials might be caused by active retrieval of the face during conflict resolution. While we cannot conclusively rule out this possibility, given that we have no measure of active retrieval during conflict resolution, it appears rather unlikely. Since participants are not aware of the subsequent memory test, they are likely focusing on the gender judgment during the conflict task rather than trying to actively retrieve the faces. It should furthermore be noted that the present memory effects also cannot be attributed to a Von Restorff effect (Wallace 1965), which refers to encoding benefits for novel or distinctive stimuli, since all face stimuli in our task were equally familiar to the participants, and the proportions of congruent, neutral, and incongruent trial types were balanced. An intriguing counterpart to the conflict-enhanced memory for target stimuli we observed in the present study has recently been reported in a behavioral task-switching study by Richter and Yeung (2012), in the shape of relatively impaired incidental memory for target stimuli (relative to distracters) from task-switching when compared with task-repetition trials. These opposing effects of conflict-enhanced and switch-impaired target-stimulus memory are perfectly in line with the assumptions that conflict serves as a task-set reinforcement or shielding signal (thus enhancing target processing, Botvinick et al. 2001; Goschke and Dreisbach 2008), and that task-switching necessarily involves a loosening of task-set shielding (thus impairing target processing) in order for its contents to be updated (Goschke 2000; Dreisbach and Wenke 2011). In other words, the converse effects on subsequent memory for target stimuli due to encountering conflict versus performing a task-switch are direct reflections of the stability-promoting nature of conflict-triggered control and the flexibility-inducing nature of task-switch operations. Both effects highlight a previously untapped potential for exploring the architecture of cognitive-control processes via subsequent memory measures that will hopefully be further exploited in future studies. Another closely related aspect that could be addressed in follow-up studies is the influence of trial history. Specifically, it has been demonstrated that encountering conflict on one trial can improve performance on the subsequent incongruent trial (Egner 2007). While it would be very interesting to test whether such conflict-adaptation effects would interact with the conflict-driven memory benefit, the present paradigm is not really suited for this purpose. Specifically, using first-order trial history as an additional factor will yield insufficient trial numbers for the DM analysis.
In further contextualizing our findings, it should be noted that the present results of conflict-enhanced target-stimulus memory pertain to a very different situation than, and are perfectly compatible with, a previously reported semantic congruency benefit for associative memory (van Kesteren et al. 2010). Specifically, the latter describes a finding whereby semantic congruency benefits the intentional integration of several task-relevant stimuli into a multisensory association, whereas our finding deals with implicit encoding benefits for target stimuli in the presence of to-be-ignored incongruent distracters.
The notion that enhanced memory for target stimuli stemming from incongruent trials in the present study was a consequence of conflict-triggered control mechanism receives strong support from our fMRI results. Specifically, activity in the right dlPFC, a region that has been repeatedly implicated in conflict–control processes in previous studies (e.g., Kerns et al. 2004; Egner and Hirsch 2005; Egner et al. 2008), was selectively increased for incongruent compared with congruent and neutral items, as well as for subsequently remembered compared with not-remembered items (DM effect) from incongruent trials. This region, thus, satisfies traditional neural signatures of both conflict–control and memory-formation processes. An analogous effect was observed in the precuneus, a region in the medial posterior parietal cortex that is known to be implicated in attentional selection and orienting, and which displays strong anatomical connectivity with the dlPFC (Cavanna and Trimble 2006). Taken together, the DM effects in dlPFC and precuneus, as well as the finding of conflict-enhanced functional coupling between these regions, strongly suggest that both regions are involved in a processing cascade whereby conflict triggers top-down control to overcome interference by increasing attention to the target stimulus, which promotes elaborate encoding thereof and, ultimately, leads to a more robust long-term representation. Although the present data cannot establish whether the precuneus activity was temporally subsequent to (and evoked by) the conflict-induced dlPFC signal, it certainly represents a plausible possibility, as it is well established that the dlPFC can impose top-down biases on parietal regions in the service of selecting task-relevant stimulus information when directing attention to different spatial locations (Grent-'t-Jong and Woldorff 2007), as well as to different stimulus features (e.g., Banich et al. 2000). Notably, the behavioral and neural modulations observed in the present study arise from immediate, that is, within-trial, attentional enhancements (cf., Scherbaum et al. 2011).
Furthermore, the present results are also compatible with an elaboration of the conflict-monitoring model, proposing that behavioral adaptation to conflict is mediated by arousal (Verguts and Notebaert 2009)—assuming that moderate arousal has beneficial effects on the encoding of target information into long-term memory, an assumption that has received substantial support in the emotional memory literature (Mather and Sutherland 2011). However, the absence of a DM effect in the dACC, the ROI that is most closely associated with arousal (Critchley 2005), suggests that mere arousal is not the key driving force for the DM effect in the present study. The absence of a DM effect in the dACC, moreover, indicates that the memory benefit in incongruent trials is not critically dependent on initial conflict detection (see, e.g., Botvinick et al. 1999; MacDonald et al. 2000; Kerns et al. 2004). Rather, the memory benefit seems to arise from top-down attentional enhancement during conflict-resolution processes, which are subserved by dorsolateral prefrontal and parietal regions (see, e.g., MacDonald et al. 2000; Kerns et al. 2004; Egner and Hirsch 2005; Liston et al. 2006; Egner et al. 2007). In other words, most incongruent trials will elicit a conflict signal in the dACC, but only those that also trigger a high degree of within-trial control in response to that conflict (thus recruiting dlPFC and parietal regions, resulting in attentional enhancement) will be remembered more vividly.
A key additional piece of supportive evidence for the suggested roles of the dlPFC and precuneus in mediating conflict-driven memory benefits for target stimuli is that incongruent trials enhanced the functional coupling of either region with the MTL—a region that has long been explicitly associated with long-term memory formation (Eichenbaum et al. 2007). In addition to this general boost in coupling between conflict–control and memory-related regions in incongruent trials, which were overall remembered more accurately than congruent and neutral trials, we observed a marginally significant relationship between this coupling and retrieval success across trials. Specifically, the functional coupling between dlPFC and MTL was differentially enhanced for incongruent remembered versus not-remembered target stimuli, indicating that the conflict-triggered prefrontal activation promoted MTL-supported memory formation. However, this effect was not overly robust, which could be due to a lack of statistical power for the DM PPI analysis (in spite of significant findings in the standard DM analysis). Specifically, all face stimuli had been familiarized before the Stroop task, and our DM analyses are thus examining quite subtle differential effects of the truly remembered items compared with the sum of all familiar, unfamiliar, and truly forgotten items, which represent the majority of trials. A second possibility relates to an overall low variability in the conflict-driven coupling across trials, which would mitigate the observation of DM PPI effects. Thirdly, we may be observing a true null effect here, in that the encoding success may not primarily depend on conflict–control interactions with the medial MTL, but with the vmPFC, which displayed enhanced functional coupling with the precuneus for incongruent remembered versus not-remembered target stimuli. This possibility is consistent with the previously observed overall shift from HIP-dependent to HIP-independent processes with progressing consolidation (e.g., McClelland et al. 1995). Specifically, by the time interference occurs in the present paradigm, all stimuli had already been encountered several times, which might attenuate the initial hippocampal encoding signal at least to some extent. In contrast, encoding-related activity in the PFC is known to “increase” with progressing consolidation (Nieuwenhuis and Takashima 2011).
Above and beyond the potential interactions between dlPFC and precuneus, on the one hand, and memory-related regions, on the other hand, it is moreover important to consider that dlPFC and precuneus have been directly linked to memory formation in their own right. First, successful long-term encoding has been associated with dlPFC activity in general (see Wagner 2002; Blumenfeld and Ranganath 2007; Kim 2011), and with right dlPFC activity for objects (as compared with, e.g., words) in particular (Wagner 2002). Moreover, successful long-term encoding appears to critically depend on the interaction between prefrontal cortex and MTL, as demonstrated in a lesion study using the above-mentioned von Restorff paradigm (Parker et al. 1998). The increased functional coupling between dlPFC and HIP in the present study is well in line with this observation and provides an interesting link between these different memory paradigms. Secondly, the precuneus, together with the PHG and the retrosplenial cortex, is considered to hold an important role in episodic memory formation, especially with regard to integrating different contextual inputs (Ranganath and Ritchey 2012). The functional coupling between these 3 regions in the present study might hence be related to dissociating relevant and irrelevant pieces of information with regard to long-term encoding.
In conclusion, then, we present in the current paper the first behavioral and neural evidence for conflict-enhanced memory of target stimuli. Our results suggest that conflict induced by incongruent distracter stimuli triggers a reinforcement of top-down attention (via fronto-parietal cortices) toward relevant target stimuli (cf., Botvinick et al. 2001; Egner and Hirsch 2005), which at the same time serves to enhance encoding of those target stimuli into long-term memory in conjunction with MTL and vmPFC regions. These data characterize a novel link between ongoing cognitive-control operations and associative processing in the form of incidental memory encoding.
Funding
This research was supported by the NIH (R01MH087610, T.E.), a post-doctoral fellowship from the Flemish Research Foundation (FWO11/PDO/016, R.M.K.), and the Multidisciplinary Research Platform “The integrative neuroscience of behavioral control” (Ghent University).
Notes
Conflict of Interest: None declared.
References
- Banich MT, Milham MP, Atchley R, Cohen NJ, Webb A, Wszalek T, Kramer AF, Liang ZP, Wright A, Shenker J, et al. fMRI studies of Stroop tasks reveal unique roles of anterior and posterior brain systems in attentional selection. J Cogn Neurosci. 2000;12:988–1000. doi: 10.1162/08989290051137521. [DOI] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C. Prefrontal cortex and long-term memory encoding: an integrative review of findings from neuropsychology and neuroimaging. Neuroscientist. 2007;13:280–291. doi: 10.1177/1073858407299290. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-P. Region of interest analysis using an SPM toolbox (abstract). Available on CD-Rom. NeuroImage. 2002;16:497. [Google Scholar]
- Bruce V, Henderson Z, Greenwood K, Hancock PJB, Burton AM, Miller P. Verification of face identities from images captured on video. J Exp Psychol Appl. 1999;5:339–360. [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chun MM, Turk-Browne NB. Interactions between attention and memory. Curr Opin Neurobiol. 2007;17:177–184. doi: 10.1016/j.conb.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Dreisbach G, Wenke D. The shielding function of task sets and its relaxation during task switching. J Exp Psychol Learn Mem Cogn. 2011;37:1540–1546. doi: 10.1037/a0024077. [DOI] [PubMed] [Google Scholar]
- Egner T. Congruency sequence effects and cognitive control. Cogn Affect Behav Neurosci. 2007;7:380–390. doi: 10.3758/cabn.7.4.380. [DOI] [PubMed] [Google Scholar]
- Egner T, Delano M, Hirsch J. Separate conflict-specific cognitive control mechanisms in the human brain. NeuroImage. 2007;35:940–948. doi: 10.1016/j.neuroimage.2006.11.061. [DOI] [PubMed] [Google Scholar]
- Egner T, Ely S, Grinband J. Going, going, gone: characterizing the time-course of congruency sequence effects. Front Psychol. 2010;1:154. doi: 10.3389/fpsyg.2010.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner T, Etkin A, Gale S, Hirsch J. Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cereb Cortex. 2008;18:1475–1484. doi: 10.1093/cercor/bhm179. [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nat Neurosci. 2005;8:1784–1790. doi: 10.1038/nn1594. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Ann Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Goschke T. Involuntary persistence and intentional reconfiguration in task-set switching. In: Monsell S, Driver J, editors. Control of cognitive processes: attention and performance XVIII. Cambridge, MA: MIT Press; 2000. pp. 331–355. [Google Scholar]
- Goschke T, Dreisbach G. Conflict-triggered goal shielding: response conflicts attenuate background monitoring for prospective memory cues. Psychol Sci. 2008;19:25–32. doi: 10.1111/j.1467-9280.2008.02042.x. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. Optimizing the use of information: strategic control of activation of responses. J Exp Psychol Gen. 1992;121:480–506. doi: 10.1037//0096-3445.121.4.480. [DOI] [PubMed] [Google Scholar]
- Grent-'t-Jong T, Woldorff MG. Timing and sequence of brain activity in top-down control of visual-spatial attention. PLoS Biol. 2007;5:e12. doi: 10.1371/journal.pbio.0050012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichs H, Scholz M, Tempelmann C, Woldorff MG, Dale AM, Heinze H-J. Deconvolution of event-related fMRI responses in fast-rate experimental designs: tracking amplitude variations. J Cogn Neurosci. 2000;12:76–89. doi: 10.1162/089892900564082. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, III, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kim H. Neural activity that predicts subsequent memory and forgetting: a meta-analysis of 74 fMRI studies. NeuroImage. 2011;54:2446–2461. doi: 10.1016/j.neuroimage.2010.09.045. [DOI] [PubMed] [Google Scholar]
- Krebs RM, Fias W, Achten E, Boehler CN. Picture novelty attenuates semantic interference and modulates concomitant neural activity in the anterior cingulate cortex and the locus coeruleus. NeuroImage. 2013;74:179–187. doi: 10.1016/j.neuroimage.2013.02.027. [DOI] [PubMed] [Google Scholar]
- Krebs RM, Schott BH, Schutze H, Duzel E. The novelty exploration bonus and its attentional modulation. Neuropsychologia. 2009;47:2272–2281. doi: 10.1016/j.neuropsychologia.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Matalon S, Hare TA, Davidson MC, Casey BJ. Anterior cingulate and posterior parietal cortices are sensitive to dissociable forms of conflict in a task-switching paradigm. Neuron. 2006;50:643–653. doi: 10.1016/j.neuron.2006.04.015. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, III, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- MacLeod CM, MacDonald PA. Interdimensional interference in the Stroop effect: uncovering the cognitive and neural anatomy of attention. Trends Cogn Sci. 2000;4:383–391. doi: 10.1016/s1364-6613(00)01530-8. [DOI] [PubMed] [Google Scholar]
- Mather M, Sutherland MR. Arousal-biased competition in perception and memory. Perspect Psychol Sci. 2011;6:114–133. doi: 10.1177/1745691611400234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O'Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. NeuroImage. 2012;61:1277–1286. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Ann Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J. Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cogn Affect Behav Neurosci. 2007;7:1–17. doi: 10.3758/cabn.7.1.1. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis IL, Takashima A. The role of the ventromedial prefrontal cortex in memory consolidation. Behav Brain Res. 2011;218:325–334. doi: 10.1016/j.bbr.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Paller KA, Kutas M, Mayes AR. Neural correlates of encoding in an incidental learning paradigm. Electroencephalogr Clin Neurophysiol. 1987;67:360–371. doi: 10.1016/0013-4694(87)90124-6. [DOI] [PubMed] [Google Scholar]
- Paller KA, Wagner AD. Observing the transformation of experience into memory. Trends Cogn Sci. 2002;6:93–102. doi: 10.1016/s1364-6613(00)01845-3. [DOI] [PubMed] [Google Scholar]
- Parker A, Wilding E, Akerman C. The Von Restorff effect in visual object recognition memory in humans and monkeys. The role of frontal/perirhinal interaction. J Cogn Neurosci. 1998;10:691–703. doi: 10.1162/089892998563103. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Ritchey M. Two cortical systems for memory-guided behaviour. Nat Rev Neurosci. 2012;13:713–726. doi: 10.1038/nrn3338. [DOI] [PubMed] [Google Scholar]
- Richter FR, Yeung N. Memory and cognitive control in task switching. Psychol Sci. 2012;23:1256–1263. doi: 10.1177/0956797612444613. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Scherbaum S, Fischer R, Dshemuchadse M, Goschke T. The dynamics of cognitive control: evidence for within-trial conflict adaptation from frequency-tagged EEG. Psychophysiology. 2011;48:591–600. doi: 10.1111/j.1469-8986.2010.01137.x. [DOI] [PubMed] [Google Scholar]
- Uncapher MR, Wagner AD. Posterior parietal cortex and episodic encoding: insights from fMRI subsequent memory effects and dual-attention theory. Neurobiol Learn Mem. 2009;91:139–154. doi: 10.1016/j.nlm.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kesteren MT, Rijpkema M, Ruiter DJ, Fernandez G. Retrieval of associative information congruent with prior knowledge is related to increased medial prefrontal activity and connectivity. J Neurosci. 2010;30:15888–15894. doi: 10.1523/JNEUROSCI.2674-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verguts T, Notebaert W. Adaptation by binding: a learning account of cognitive control. Trends Cogn Sci. 2009;13:252–257. doi: 10.1016/j.tics.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Wagner AD. Cognitive control and episodic memory: contributions from prefrontal cortex. In: Squire LR, Schacter DL, editors. Neuropsychology of memory. 3rd ed. New York: Guilford Press; 2002. [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Wallace WP. Review of the historical, empirical, and theoretical status of the Von Restorff phenomenon. Psychol Bull. 1965;63:410–424. doi: 10.1037/h0022001. [DOI] [PubMed] [Google Scholar]