Abstract
Objectives
To measure the receptive risks of malaria in Somalia and compare decisions on intervention scale-up based on this map and the more widely used contemporary risk maps.
Design
Cross-sectional community Plasmodium falciparum parasite rate (PfPR) data for the period 2007–2010 corrected to a standard age range of 2 to <10 years (PfPR2–10) and used within a Bayesian space–time geostatistical framework to predict the contemporary (2010) mean PfPR2–10 and the maximum annual mean PfPR2–10 (receptive) from the highest predicted PfPR2–10 value over the study period as an estimate of receptivity.
Setting
Randomly sampled communities in Somalia.
Participants
Randomly sampled individuals of all ages.
Main outcome measure
Cartographic descriptions of malaria receptivity and contemporary risks in Somalia at the district level.
Results
The contemporary annual PfPR2–10 map estimated that all districts (n=74) and population (n=8.4 million) in Somalia were under hypoendemic transmission (≤10% PfPR2–10). Of these, 23% of the districts, home to 13% of the population, were under transmission of <1% PfPR2–10. About 58% of the districts and 55% of the population were in the risk class of 1% to <5% PfPR2–10. In contrast, the receptivity map estimated 65% of the districts and 69% of the population were under mesoendemic transmission (>10%–50% PfPR2–10) and the rest as hypoendemic.
Conclusion
Compared with maps of receptive risks, contemporary maps of transmission mask disparities of malaria risk necessary to prioritise and sustain future control. As malaria risk declines across Africa, efforts must be invested in measuring receptivity for efficient control planning.
Article summary
Article focus
Cross-sectional PfPR prevalence survey data for the period 2007–2010 in Somalia.
Bayesian geostatistical models estimating the receptive and contemporary malaria transmission in Somalia.
Implications of the two malaria risk maps for malaria control planning in Somalia.
Key messages
It is feasible to use PfPR community prevalence data in space and time to estimate the receptive and contemporary risks of malaria within the same model framework.
Malaria receptivity maps are critical to inform the scale-up and sustaining of interventions where disease has declined or is highly seasonal.
Efforts must be invested in helping malaria endemic countries in Africa measure their receptive risks.
Strengths and limitations of this study
The annual PfPR surveys provide unique opportunities to measure receptivity in Somalia.
Improving the spatial and temporal distributions of PfPR data and exploring probabilistic approaches of selecting potential maximum risks will improve the measurement of receptivity.
Introduction
Malaria receptivity is a measure of the intrinsic vector transmission potential of an area.1 Interest in measuring malaria receptivity has emerged following the resurgence of the malaria elimination agenda2 3 and the need to quantify the risks posed by human population movement leading to the reintroduction of transmission.4 5 However, understanding receptivity is equally important to decision-making for countries that are implementing control. In low stable endemic countries, national programmes need to understand the risks posed by withdrawal of interventions from areas that are historically high transmission.3 6 In unstable transmission areas where parasite exposure is highly seasonal and prone to climatic anomalies, targeting interventions to prevent the risk of epidemics are a priority.1 The empirical malaria risk maps that are commonly available to countries to support malaria control planning are those that represent the contemporary distribution of risk under control7 8 and are therefore of limited value in defining the epidemic potential or the receptive rebound risks of withdrawing or failing to sustain interventions.
Measuring receptivity for malaria control countries ideally requires empirical data from a period of no control and under the optimum transmission conditions. There is hardly any country in Africa that has remained universally control-naive over the past 100 years.9 Alternatively, nationally representative empirical data on malaria transmission during the pre-Roll Back Malaria (RBM) era, or before intervention scale-up reached critical thresholds for a given country, may represent the best approximation of receptivity. Furthermore, such information must be resolved to administrative decision-making units to which malaria resources are allocated to make them relevant for policy. In this study, we use community Plasmodium falciparum parasite prevalence data from 2007 to 2010 within a model-based geostatistical (MBG) framework to develop contemporary and receptive risk maps and resolve endemicity to districts in Somalia.
Methods
Country context
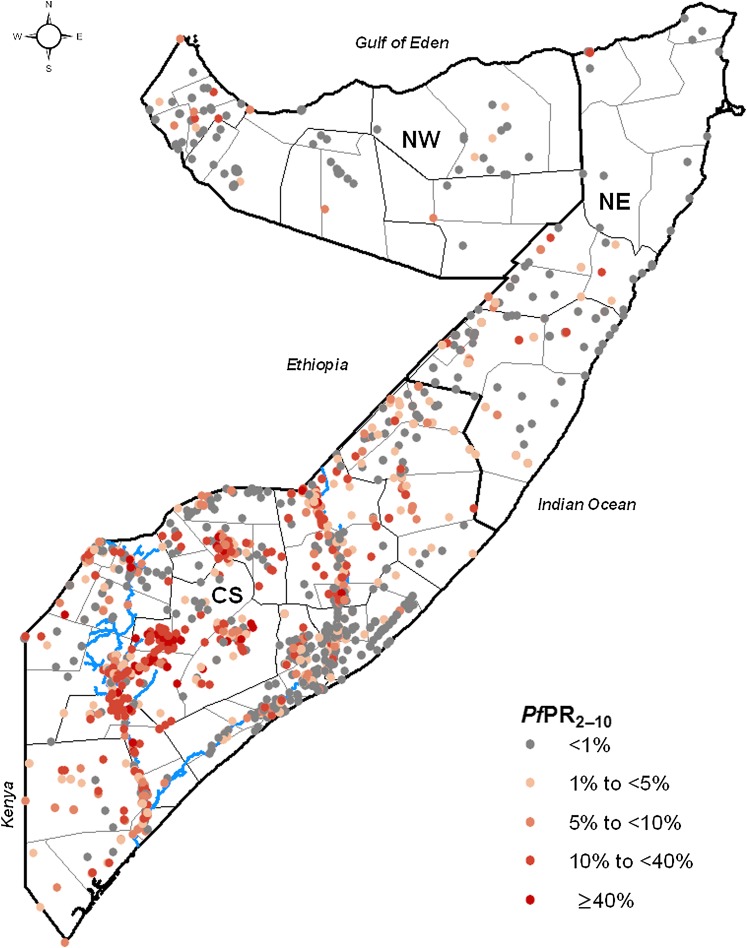
Somalia is divided into the three zones of North West (Somaliland), North East (Puntland) and Central South (figure 1). The northern zones are generally dry and hot, whereas the Central South zone has subtropical climate and is where the two major rivers of the country, the Shabelle and the Juba, are located.10 11 Anopheles arabiensis is the dominant malaria transmitting vector throughout the country, although Anopheles funestus is reported in Central South.12 13 P falciparum is the dominant species of the malaria parasite.14–16 The presence of Plasmodium vivax cases have also been reported with studies in Somaliland showing relatively high vivax antibody responses.17 The failure of the long rains in Somalia in 2010 combined with the below average rainfalls in previous two seasons have resulted in a severe drought.11
Figure 1.
Zone, regional and district maps of Somalia showing the distribution of the age-standardised community Plasmodium falciparum parasite rate (PfPR2–10) data (n=1558) assembled during the period 2007–2010 (including 54 surveys undertaken in 2011). The zones are CS, Central South; NE, North East; NW, North West. The thick black line show the zone boundaries, the thin black lines show the regional boundaries and the thin grey lines show the district boundaries. The blues lines show the location of the Juba (lower) and Shabelle (upper) Rivers.
Since 1991, Somalia has had no effective central government and has experienced frequent internal armed conflicts resulting in the breakdown of public services and political disintegration.18 Although the transitional federal government, formed in February 2004, is internationally recognised as the official government of Somalia,19 in reality the three zones currently function as semi-independent states. Consequently, there are three ministries of health, but the majority of healthcare is provided by international and national non-government organisations that have come together under the umbrella of Somalia Aid Coordinating Board, which was later reformed as the Somalia Support Secretariat.20
The main funder of malaria control in Somalia is the Global Fund to Fight Aids TB and Malaria with the United Nations Children Fund (Unicef) as the principal recipient and the WHO as subrecipient.21 22 In 2010, the second national malaria strategy was launched with universal scale-up of vector control, parasitological diagnosis, effective antimalarials, improved surveillance and epidemic preparedness and response as the main strategic approaches.22 23 Since 2004, over US$77 million has been approved to Somalia by the Global Fund to Fight Aids TB and Malaria for malaria control resulting in the distribution of almost 1 million long-lasting insecticidal nets (LLINs).24
Community survey data
The community P falciparum parasite rate (PfPR) is the most commonly used indicator for mapping malaria transmission.7 This is because it is easy to measure, has a historical legacy and a predictable relationship with other measures of transmission intensity, such as entomological inoculation rate and the basic reproductive rate.25 26 The PfPR data used for the present study were assembled through the Food and Agriculture Organization–Food Security and Nutrition Analysis Unit (FAO-FSNAU) surveys undertaken regularly in Somalia.16 27 These surveys were initially established to monitor the nutritional status of children <5 years of age and began in 2000.27 Investigations of malaria prevalence covering persons of all ages were only included from 200716 and have been undertaken annually since covering most regions of Somalia. A detailed description of the sampling design is provided elsewhere.16 During the survey, respondents provided a finger prick blood sample that was examined for the presence of P falciparum infection using a rapid diagnostic test (Paracheck Pf, Orchid Biomedical Systems, Goa, India). Consent was obtained for all individuals before interview and separately for the malaria testing. Additional information was recorded on the date of survey and age and sex of participants. After all survey data were assembled, each surveyed community was geo-coded using combinations of global positioning systems, electronic gazetteers (Google Earth, Encarta and Alexandria) and other sources of longitude and latitude such as a settlement database collated by FAO-SWALIM.11
Assessment of ecological and climatic predictors of malaria risk
The transmission intensity of malaria is influenced by climatic and ecological factors through their independent or combined effects on the survival of the Anopheline vectors and the Plasmodium parasites within the vector.28 The ecological and climatic factors used commonly to improve the precision of empirical malaria mapping are urbanisation, rainfall, temperature and distance to potential mosquito larva breeding sites.
Data at 1×1 km spatial resolution on urbanisation,29 annual mean precipitation30 31 and enhanced vegetation index32 were assembled. Maps of rivers, flood plains, reservoirs and coastal wetlands were assembled from the Global Wetlands and Lakes Database33 and Euclidean distances to these proximates of breeding sites were computed in ArcGIS 10 (ESRI Inc., New York, USA). As a metric for the effect of temperature on malaria transmission, a temperature suitability index (TSI) at a spatial resolution of 1×1 km was used. TSI was constructed using monthly temperature time series within a biological modelling framework to quantify the effect of ambient temperature on sporogony and vector survivorship and determine the suitability of an area to support transmission globally.34 The values of the underlying ecological and climatic covariates were extracted to each survey location using ArcGIS 10 Spatial Analyst tool. Distance to potential breeding sites was log-transformed before analysis because of its high positive skew. The covariates were then included in total-sets analysis, which is an automatic model selection process based on a generalised linear regression model and implemented using the bestglm package in R.35 36 This approach selects the best combination of the covariates based on the value of the Bayesian Information Criteria statistic,37 which selects the lowest Bayesian Information Criteria as the best model fit. Details of the ecological and climatic predictors of PfPR and the results of the total-set analysis are provided in supplementary information 1.
The space–time Bayesian geostatistical model for predicting P falciparum distribution in Somalia
Space–time MBG methods offer the flexibility of predicting an outcome to any given year and location in a time series by harnessing fully both the spatial and temporal relationships of the data and generate uncertainties of the predictions from the full posterior distributions.38
In this study, the assembled PfPR data were standardised to the classical age range of 2 to <10 years using an algorithm based on modified catalytic conversion models.39 The continuous surfaces of the age-standardised data (PfPR2–10) were generated using a space–time MBG framework8 40 whereby Bayesian inference was implemented using the Markov Chain Monte Carlo algorithm. Details of model code40 and statistical procedures8 are provided in supplementary information 2. In brief, the value of PfPR2–10 was modelled as a transformation of a spatiotemporally structured field superimposed with unstructured (random) variation on a regular 1×1 km grid from 2007 to 2010. The number of P falciparum-positive responses from the total sample at each survey location was modelled as a conditionally independent binomial variate given the unobserved underlying age-standardised PfPR2–10 value39 and a linear function of the climatic and environmental predictors. The unstructured component was represented as Gaussian distribution with zero mean. The spatiotemporal component was represented by a stationary Gaussian process41 with covariance defined by a spatially anisotropic version of the space–time covariance function proposed by Stein (2005).42 To partly model seasonality, the covariance function was modified to allow the time-marginal model to include a periodic component of wavelength 12 months in the temporal covariance structure. Each survey was referenced temporally using the midpoint (in decimal years) between the recorded start and end months. For each grid location, samples of the annual mean of the full posterior distribution of PfPR2–10 for each year were generated. These PfPR2–10 samples were then used to generate continuous maps of the annual mean. To determine the probable maximal malaria risk, the highest value of predicted mean annual PfPR2–10 value at each 1×1 km grid location over the period 2007–2010 were extracted. These were then combined to generate a single map of maximum mean PfPR2–10.
Assessing uncertainty of model predictions
As a first step to understanding the uncertainty around the predictions of PfPR2–10 using the Bayesian geostatistical model, the continuous mean maps were accompanied by estimates of the posterior standard deviation (SD). For the maximum mean PfPR2–10 map, the posterior SDs associated with the selected mean value was used. To allow for a scaled comparison of the uncertainty of the 2010 PfPR2–10 map and the 2010 annual mean PfPR2–10, the coefficient of variation, which is a measure of dispersal around the mean,43 was computed as the ratio of the SD to the mean. Higher values of the coefficient of variation suggest increasing uncertainty of model predictions. In addition, a spatially representative validation set of PfPR2–10 survey data were also selected using a spatially declustered sampling algorithm.40 The annual predictions were then repeated in full using the remaining data to predict mean PfPR2–10 at the validation locations. The ability of the model to predict point values of PfPR at unsampled locations was quantified using two simple summary statistics: the mean prediction error (MPE) and the mean absolute prediction error (MAPE). The MPE provides a measure of the model bias, while the MAPE is a measure of the average accuracy of individual predictions.
Defining district-level malaria endemicity and population at risk
A 2010 population surface for Somalia at 100×100 m spatial resolution was provided by the AfriPop project.29 44 Using this map, estimates of the total population of each 1×1 km pixel to which mean PfPR2–10 was predicted was computed in ArcGIS 10. The 1×1 km grid squares with attached population estimates were further classified separately by the mean PfPR2–10 2010 and the maximum mean PfPR2–10. To weight endemicity for population distribution, only those grid squares with population were retained and from these the mean PfPR2–10 was computed for each district. Based on the aggregate mean PfPR2–10, districts were then classified using a modification of the classical malaria endemicity classification.45 The hypoendemic class (≤10% PfPR2–10) was split into <1% PfPR2–10; 1% to <5% PfPR2–10 and 5%–10% PfPR2–10. The endemicity class of <1% PfPR2–10 represents the threshold at which an area is considered to be under low stable endemic control and a decision for sustaining control or aiming for elimination can be made.3 6 The hyperendemic (>50%–75% PfPR2–10) and holoendemic (>75% PfPR2–10) classes were also combined, while the mesoendemic class (>10%–50% PfPR2–10) remained unchanged. The number of districts and population by these endemicity classes were then summarised based on both the 2010 mean PfPR2–10 (contemporary risk) map and the maximum mean PfPR2–10 (receptive risk) map.
Results
Predictions of mean annual PfPR2–10 to 2010 and maximum mean posterior PfPR2–10
A total of 1558 P falciparum community surveys (figure 1) in which 103 593 persons were examined were assembled for the period 2007–2011 in Somalia. The majority of the data were located in the Central South zone where most of the population live. Survey data were collected across nine different months over the 5 years, with the majority of data (76%) assembled in the months of November, December, May and June corresponding to the peak malaria seasons in Somalia. The results of the total-set analysis showed that the model with urbanisation, precipitation, enhanced vegetation index and distance to main water bodies and flood plains as the best fit in predicting PfPR, and these variables were subsequently included in the malaria prediction model (supplementary information 1). TSI was not selected as a statistically strong predictor of P falciparum prevalence in Somalia.
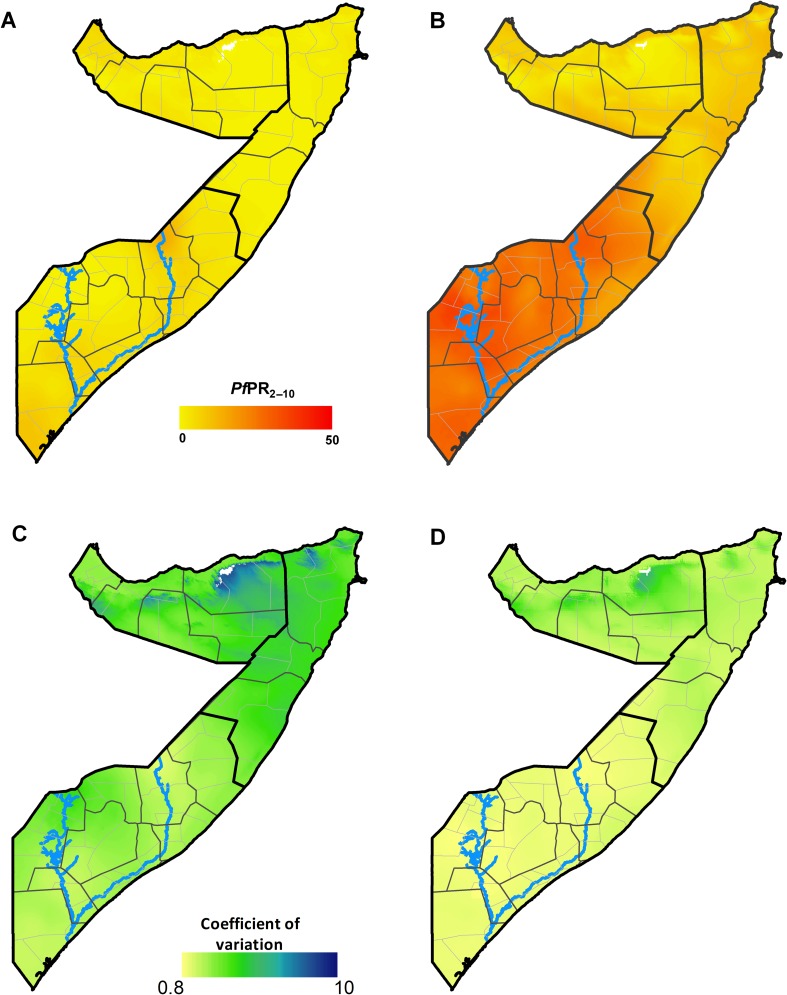
The continuous 2010 malaria endemicity map for Somalia PfPR2–10 showed the majority of locations were predicted to have parasite prevalence of <5% indicating largely hypoendemic transmission (figure 2A). The majority of areas in North East and North West zones were predicted to be under PfPR2–10 <1%. In contrast, the maximum annual mean PfPR2–10 map showed a substantially different risk landscape, with the majority of the Central South zone having PfPR2–10 of >10% and an a maximum predicted mean of 38%, suggesting that peak malaria transmission in all of Central South zone and southern parts of North East zone is mesoendemic (figure 2B). In the northern zones, maximum mean risks were predicted to be predominantly between 5% and <10% PfPR2–10.
Figure 2.
(A) Map of the posterior annual mean PfPR2–10 prediction to 2010 (contemporary) at 1×1 km grid location in Somalia. (B) Map of the maximum mean PfPR2–10 prediction (receptive) at 1×1 km grid location as computed from the posterior annual mean PfPR2–10 prediction for each year from 2007 to 2010. (C) Map of the coefficient of variation (the SD/the mean PfPR2–10 prediction) of the contemporary prediction at 1×1 km grid location. (D) Map of the coefficient of variation at 1×1 km grid location of the receptive prediction. The thick black lines show the zone boundaries, the thin black lines show the regional boundaries and the thin grey lines show the district boundaries. Higher coefficient of variation of the predictions suggests higher uncertainties of the PfPR2–10 predictions. The scale bar for the continuous PfPR2–10 ends at 50, which is the upper limit of mesoendemic transmission. The blues lines show the location of the Juba (lower) and Shabelle (upper) Rivers.
The MPE and MAPE associated with the full space–time geostatistical model was 4.8% and 0.2%, respectively. The 2010 annual mean PfPR2–10 predictions were associated with higher coefficients of variation compared with the maximum mean PfPR2–10 predictions, although the difference was moderate (figure 2C,D). In both maps, uncertainty appeared highest in northern zones where data in space and time were fewest.
District estimates of contemporary and receptive malaria risk
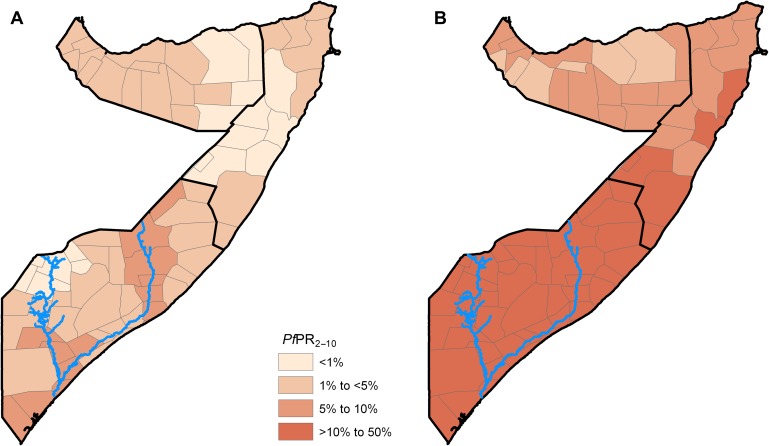
According to the contemporary district malaria endemicity map based on the annual mean PfPR2–10 map of 2010, all districts in Somalia were under hypoendemic transmission (figure 3A). Of the 74 districts, an estimated 17 (23%) districts covering about 1.1 million people (13%) were in the <1% PfPR2–10 risk class (table 1 and figure 3A). The majority of the districts (58%) and population (55%) were in the risk class of 1%–<5% PfPR2–10 and the rest were under risks of 5%–10% PfPR2–10.
Figure 3.
District (n=74) maps of Somalia classified by endemicity using a population-weighted: (A) posterior aggregate annual mean PfPR2–10 (contemporary) prediction to 2010 and (B) the maximum annual mean PfPR2–10 (receptive) predictions over the period 2007–2010. The blues lines show the location of the Juba (lower) and Shabelle (upper) Rivers.
Table 1.
A summary of districts (N=74) and population in 2010 (N=8.4 million) in Somalia classified by malaria endemicity
| Endemicity classification based on the 2010 annual mean PfPR2–10 (contemporary) predictions | Endemicity classification based on the maximum mean PfPR2–10 (receptive) predictions over the period 2007–2010 | |||
| Number (%) of districts | Population at risk, million, (%) | Number (%) of districts | Population at risk, million, (%) | |
| Population-weighted mean PfPR2–10 | ||||
| Hypoendemic | ||||
| <1% PfPR2–10 | 17 (23) | 1.1 (13) | 0 (0) | 0 (0) |
| 1% to <5% PfPR2–10 | 43 (58) | 4.6 (55) | 6 (8) | 1.2 (14) |
| 5%–10% PfPR2–10 | 14 (19) | 2.6 (31) | 20 (27) | 1.4 (17) |
| Mesoendemic (>10%–50% PfPR2–10) | 0 (0) | 0.0 (0) | 48 (65) | 5.8 (69) |
| Hyperendemic and holoendemic (>50% PfPR2–10) | 0 (0) | 0.0 (0) | 0 (0) | 0.0 (0) |
District classifications of endemicity were computed from population-weighted posterior annual mean PfPR2–10 predicted to 2010 (contemporary) and the maximum annual mean PfPR2–10 (receptive) predictions over the period 2007–2010.
In contrast, the receptive risk map showed that there were no district under low stable endemic control (<1% PfPR2–10), and the majority of the districts (65%) and population (69%) were under the mesoendemic class (table 1 and figure 3B), with an upper district maximum mean estimate of 35% PfPR2–10. About 27% of the districts and 17% of the population were in the upper range of hypoendemicity (5%–10% PfPR2–10). The rest of the districts and population were in the intermediate hypoendemic class of 1%–<5% PfPR2–10.
Discussion
The malaria risk maps that are commonly available to countries in Africa to support malaria control planning are those that represent the contemporary distribution of risk.8 16 46–50 They have been developed primarily from geo-coded parasite rate survey data7 usually to predict risk to the most recent data year and therefore reflect transmission under scaled interventions during the era of the RBM partnership.51 52 In this study, we argue that, in addition to contemporary maps of malaria risks, low stable endemic control and unstable transmission countries require maps of receptivity to assess the risks of rebound and epidemics and decide on where to scale-up and/or sustain intervention coverage. To demonstrate this, we used community PfPR survey data from the period 2007–2010 within a space–time MBG framework to generate two continuous malaria risk maps for Somalia. One is a contemporary map of annual mean PfPR2–10 predicted to 2010 (figure 2A) and the other is the maximum annual mean PfPR2–10 map derived from the highest mean PfPR2–10 value predicted to a location in any year over the study period to approximate receptivity (figure 2B). We resolved these maps to the district, which is the malaria decision-making unit in Somalia, and classified them by endemicity using population-weighted mean PfPR2–10 (figure 3).
The efficacy and impact of malaria interventions on disease in an area are dependent on its intrinsic transmission potential.53–55 This is the theoretical basis upon which international guidelines for malaria control are formulated.1 56 One of the most important applications of malaria risk maps for control planning, therefore, is to inform the spatial targeting of the appropriate mixes of interventions.57 For Somalia, the results of the comparison of the contemporary and the receptive risk maps represents two very different transmission scenarios (table 1 and figure 3). The contemporary malaria risk map predicted that all of Somalia was under conditions of hypoendemic transmission (≤10% PfPR2–10) in 2010 with a fifth of the districts under risks of <1% PfPR2–10, while the majority of the districts and population were in the intermediate hypoendemic transmission class of 1% to <5% PfPR2–10. Under these transmission conditions, targeted distribution of LLINs and indoor residual spraying aimed at control of residual foci are recommended, while intermittent presumptive treatment in pregnancy is not.56 Instead of being part of the broader monitoring and evaluation process, disease surveillance is also regarded as an intervention in of itself1 comprising high-quality passive case detection, case notification and active case detection in which all febrile cases within proximity of the index case are tested and those positive for malaria infection are radically treated.58 In the districts where transmission is <1% PfPR2–10, malaria elimination is considered to be technically feasible3 6 presenting an opportunity to re-orient the subnational strategy here towards elimination and undertake an assessment of its operational feasibility.5 In contrast, the receptive risk map predicted that over 65% of the districts and population were under mesoendemic transmission (>10%–50% PfPR2–10) with the rest exposed to hypoendemic transmission. Using this map, in the hypoendemic districts LLINs and indoor residual spraying would be better targeted to foci of risk and in preparation for possible epidemics as universal coverage with these interventions is unlikely to be the most cost-effective. In those areas of receptive mesoendemic transmission, which comprise 65% of the districts, universal coverage with LLINs should be the sustained ambition.1 56 The two divergent potential national malaria strategies emanating from the two different descriptions of risk highlight the danger of relying on contemporary risk maps to make decisions that require the understanding of the intrinsic transmission potential of malaria.
Available maps that describe pre-RBM distribution of risks are either expert opinion maps59 or climate-based deterministic transmission suitability models60 and not driven by empirical data. Even where empirical data may be available, in countries with unstable malaria transmission susceptible to seasonal and anomalous climatic variations such as the recent drought in Somalia, the risks measured at one time point may not be representative of the possible peak risk levels for that point. Therefore, spatially nationally representative data over several years are required to capture these variations and estimate the highest possible transmission. In this study, PfPR data for Somalia that is available over four consecutive years has provided a unique opportunity to develop a novel way of selecting the maximum predicted risks within the time series. The resolution of risk levels at the malaria resource decision-making unit also represents a product that is likely to be of more policy relevance to the national programme managers compared with the more common pixel-level predictions of risks.
The study has some limitations. Although Somalia represents one of the few African countries with ubiquitous PfPR data in space and time there are gaps in the distribution of the data and uncertainty of the predictions are partly a function of these. The validation tests, however, show overall good predictive model performance with overall bias of MPE of <5% and a slight average overprediction of about MAPE of 0.2%. The coefficient of variation, which is the ratio of SD to mean PfPR2–10, appeared similar for both the 2010 mean and the maximum mean maps with uncertainty highest in northern zones where data in space and time were fewest (figure 2C,D). In selecting the maximal mean risk as predicted to a location in any year over the 4-year period, we make assumptions as if the modelled predictions were part of 4-year repeat ‘observations’ of PfPR2–10, in that location. The basis for this is that if the mean estimate at any 1×1 km location from the full posterior distribution of the space–time model is a robust estimate to the given time and location, then selected maximum mean estimate is equally so. We suggest that this is a plausible assumption but further efforts need to be invested in probabilistically selecting the maximum mean predictions from the series of usually spatially and temporally uneven data. Any uncertainties in the approach used to select the maximum annual mean PfPR2–10 are, however, unlikely to be the source of the major differences in endemicities when compared to the annual mean PfPR2–10 for 2010. The PfPR data used in this analysis were assembled during a period when access to control interventions had increased in Somalia with donor funding support. Although coverage of main vector control interventions remain modest,22 it is likely that some of observed data were influenced by these interventions and in parts of the country true receptivity may even be higher than estimated. Land-use changes due to urbanisation, large-scale agricultural schemes and hydroelectric power dam projects also act as modifiers of transmission and in mapping malaria risk these factors must be adjusted for. Urbanisation, which has been shown to reduce malaria transmission, was included in the analysis of receptivity for Somalia. The maps of water bodies and vegetation used in the analysis will to some degree capture any aquatic or agricultural land-use changes. However, due to the long civilwar and the lack of a functioning central government, such changes have been limited.
To compute a single estimate of risk for a relatively large area, such as districts in Somalia, will always obscure some of the heterogeneity in malaria distribution within that area regardless of the methods used. Any decision to do so is therefore a compromise between the practical applications of such a classification and the potential loss of precision in risk estimation. Approaches that directly adopt the heterogeneous properties of the prevalence data to make statistically robust single estimates of mean PfPR to an administrative unit are computationally and methodologically intensive61 but have the advantage of estimating the area-level uncertainty classification through joint simulation. In this study, we have used simpler approaches to partly capture the within-district heterogeneity in malaria risk when classifying them into a single endemicity class by first assigning pixel-level population to an endemicity class before aggregating to the district. Future efforts should explore approaches such as joint simulation61 and small area estimation62 techniques to describe the uncertainties around area-level estimates of risks robustly. Such measures of uncertainties are not only quantitative estimates of model validity but also help determine where future data assembly must be prioritised to improve precision.
In conclusion, the aim of this study was to demonstrate the need for malaria receptivity maps for optimal malaria resource planning in countries, which have either achieved low stable endemic control or are of unstable transmission and therefore susceptible to seasonality or climatic anomalies. We have used approaches that derive maps of contemporary malaria risk and approximations of receptivity within the same space–time MBG framework resolved at the district level in Somalia. The two maps show significantly divergent transmission scenarios in which the contemporary map describes the majority of Somalia as hypoendemic and while the other shows a largely mesoendemic transmission profile. These disparities have far-reaching consequences on decisions regarding the design and scale-up of interventions in Somalia. The results have important control implications for several low transmission countries in Africa. Urgent efforts must therefore be invested in assembling detailed historical data on parasite prevalence to allow for a better understanding of receptivity and equip national programmes with reliable estimates of receptivity that will enhance better decision-making.
Acknowledgments
The community survey parasite prevalence data were provided by the Food and Agricultural Organization–Food Security and Nutritional Analysis Unit (FAO-FSNAU) for Somalia. The authors are grateful for the support and diligence of all the FSNAU field staff who did a great job under difficult conditions. We are grateful to all household members who participated in the survey and agreed to the malaria testing. We thank Jacob Ouko and Damaris Kinyoki for their help with assembly of ancillary data. The authors are also grateful for comments on earlier drafts of the manuscript from Dr Emelda Okiro. This paper is published with permission from the Director of KEMRI.
Footnotes
To cite: Noor AM, Alegana VA, Patil AP, et al. Mapping the receptivity of malaria risk to plan the future of control in Somalia. BMJ Open 2012;2:e001160. doi:10.1136/bmjopen-2012-001160
Contributors: AMN was responsible for overall scientific management, study design, data cleaning, analysis, interpretation, drafting and production of the final manuscript. VAA was responsible for data cleaning, geo-coding, analysis and contributed to the final manuscript. APP was responsible for the coding of the MBG framework, developed the supplementary information on model specifications and contributed to final manuscript. GM and MB contributed to the survey design, data assembly and cleaning and contributed to final manuscript. FY and JA contributed to survey design, interpretation of results and contributed to final manuscript. RWS provided scientific guidance and contributed to the analysis, interpretation and preparation of the final manuscript. All authors read and approved the final manuscript.
Funding: Cross-sectional survey was funded by the FAO-FSNAU and partners. AMN is supported by the Wellcome Trust as an Intermediate Research Fellow (095127). RWS is supported by the Wellcome Trust as Principal Research Fellow (079080) that also funded support to APP. Programmatic support for this study was also provided through a Wellcome Trust Major Overseas Programme grant to the KEMRI/Wellcome Trust Research Programme (092654).
Competing interests: None.
Ethics approval: Ethical approval was provided through permission by the Ministry of Health Somalia, Transitional Federal government of Somalia Republic, Ref: MOH/WC/XA/146./07, dated 2 February 2007. Informed verbal consent was sought from all participating households and individuals. Participants who were positive for Plasmodium falciparum infection was treated with the correct dosages of the nationally recommended antimalarials.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Data from this study are not in the public domain.
References
- 1. World Health Organization. Malaria Elimination: a Field Manual For Low And Moderate Endemic Countries. Geneva: World Health Organization, 2007. [Google Scholar]
- 2. Feachem RGA, Phillips AA, Hwang J, et al. Shrinking the malaria map: progress and prospects. Lancet 2010;376:1566–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cohen JM, Moonen B, Snow RW, et al. How absolute is zero? An evaluation of historical and current definitions of malaria elimination. Malar J 2010;9:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tatem AJ, Qiu Y, Smith DL, et al. The use of mobile phone data for the estimation of the travel patterns and imported Plasmodium falciparum rates among Zanzibar residents. Malar J 2009;8:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tatem AJ, Smith DL. International population movements and regional Plasmodium falciparum malaria elimination strategies. Proc Nat Acad Sci USA 2010;107:12222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Snow RW, Marsh K. Malaria in Africa: progress and prospects in the decade since the Abuja Declaration. Lancet 2010;376:137–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hay SI, Snow RW. The Malaria Atlas Project: developing global maps of malaria risk. PLoS Med 2006;3:e473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gething PW, Patil AP, Smith DL, et al. A new world malaria map: Plasmodium falciparum endemicity in 2010. Malar J 2011;10:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Snow RW, Amratia P, Kabaria CW, et al. The changing limits and incidence of malaria in Africa: 1939-2009. Adv Parasitol 2012;78:169–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hadden RL. The Geology of Somalia: a selected Bibliography of Somalian Geology, Geography and Earth Science. Engineer Research and Development Laboratories, Topographic Engineering Centre. http://www.dtic.mil/cgi-bin/GetTRDoc?AD=ADA464006&Location=U2&doc=GetTRDoc.pdf (accessed 12 Dec 2011).
- 11. FAO-SWALIM Databases. http://geonetwork.faoswalim.org/geonetwork/srv/en/main.home (accessed 10 Dec 2011).
- 12. Choumara R. Notes sur le paludisme au Somaliland. Riv di Malariol 1961;40:9–34. [PubMed] [Google Scholar]
- 13. Kamal M. Entomological Surveillance in Somalia. Consultancy Report for World Health Organization Somalia. Cairo, Egypt: World Health Organization Eastern Mediterranean Office, 2007. [Google Scholar]
- 14. Ilardi I, Sebastian A, Leone F, et al. Epidemiological study of parasitic infections in Somali nomads. Trans R Soc Trop Med Hyg 1987;81:771–2. [DOI] [PubMed] [Google Scholar]
- 15. Warsame M, Perlmann H, Ali S, et al. The sero-reactivity against Pf155 (RESA) antigen in villagers from a meso-endemic area in Somalia. Trop Med & Parasitol 1989;40:412–14. [PubMed] [Google Scholar]
- 16. Noor AM, Clements ACA, Gething PW, et al. Spatial prediction of Plasmodium falciparum prevalence in Somalia. Malar J 2008;7:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bousema T, Jackie C, Patrick C, et al. Serological markers for low exposure to malaria. Emerg Infect Dis 2009;16:392–9.20202412 [Google Scholar]
- 18. The Crisis Group. Somalia. http://www.crisisgroup.org/en/regions/africa/horn-of-africa/somalia.aspx (accessed 12 Nov 2011).
- 19. The Transitional Federal Government of Somalia. http://en.wikipedia.org/wiki/History_of_the_Transitional_Federal_Government_of_the_Republic_of_Somalia (accessed 12 Dec 2011).
- 20. UNOPS 2010. The Somali Support Secretariat Project Board Terms of Reference as Promulgated at the 10 May 2010 Project Board Meeting. http://www.coordinate4somalis.info/images/stories/pdf/Somali%20Support%20Secretariat%20Project%20Board%20Terms%20of%20Reference.pdf (accessed 7 Mar 2012).
- 21. GFATM Project proposal. http://www.theglobalfund.org/search/docs/6SOMM_1418_0_full.pdf (accessed 10 Dec 2007).
- 22. World Health Organization-Roll Back Malaria. Somalia National Strategic Plan for Malaria 2011—2015. Somalia: World Health Organization Eastern Mediterranean Regional Office, 2010. [Google Scholar]
- 23. World Health Organization-Roll Back Malaria. National Malaria Prevention and Control Monitoring and Evaluation Plan 2011—2015. 2010. [Google Scholar]
- 24. GFATM Progress Report. http://www.theglobalfund.org/en/library/publications/progressreports/ (accessed 15 Dec 2011).
- 25. Smith DL, McKenzie FE, Snow RW, et al. Revisiting the basic reproductive number for malaria and its implications for malaria control. PLoS Biol 2007;5:e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smith DL, Dushoff J, Snow RW, et al. The entomological inoculation rate and Plasmodium falciparum infection in African children. Nature 2005;438:492–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. FSNAU Analytical Systems. http://www.fsausomali.org/200510112504_baselines.php (accessed 11 Jan 2012).
- 28. Molineaux L, Muir DA, Spencer HC, et al. The Epidemiology of Malaria and its Measurement. In: Wernsdorfer WH, McGregor I, eds. Malaria: Principles and Practice of Malariology. London: Churchill Livingstone, 1988;2:999–1089. [Google Scholar]
- 29. The AfriPop Project. http://www.clas.ufl.edu/users/atatem/index_files/Details.htm (accessed 15 Jan 2011).
- 30. Hijmans RJ, Cameron SE, Parra JL, et al. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 2005;25:1965–78. [Google Scholar]
- 31. WorldClim-Global Climate Database. http://www.wordlclim.org (accessed 10 Feb 2012).
- 32. MODIS- EVI Data Archives. ftp://n4ftl01u.ecs.nasa.gov/SAN/MOST/MOD10A2.005/ (accessed 12 Sep 2011).
- 33. Global Lakes and Wetlands Database Request. https://secure.worldwildlife.org/science/data/item1877.html (accessed 10 Sep 2011).
- 34. Gething PW, Van Boeckel T, Smith DL, et al. Modelling the global constraints of temperature on transmission of Plasmodium falciparum and P vivax . Parasite Vector 2011;4:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Miller A. Subset Selection in Regression. Boca Raton, FL: Chapman & Hall, 2002:238. [Google Scholar]
- 36. Lumley T. Leaps: Regression Subset Selection. R package version 2.7. http://cran.r-project.org/web/packages/leaps/index.html. (accessed 11 Apr 2012). [Google Scholar]
- 37. Schwarz G. Estimating dimensions of a model. Ann Stat 1978;6:461–4. [Google Scholar]
- 38. Diggle PJ. Spatio-temporal point processes, partial likelihood, foot and mouth disease. Stat Methods Med Res 2006;15:325–36. [DOI] [PubMed] [Google Scholar]
- 39. Smith DL, Guerra CA, Snow RW, et al. Standardizing estimates of the Plasmodium falciparum parasite rate. Malar J 2007;6:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Malaria Atlas project P falciparum Cartographic Code. https://github.com/malaria-atlas-project/mbgw-clean (accessed Oct 2011).
- 41. Banerjee S, Carlin BP, Gelfand AE. Hierarchical Modeling And Analysis For Spatial Data. Monographs on Statistics and Applied Probability 101. Boca Raton, Florida, U.S.A: Chapman & Hall/CRC Press LLC, 2004. [Google Scholar]
- 42. Stein ML. Space-time covariance functions. J Am Stat Assoc 2005;100:310–21. [Google Scholar]
- 43. Kirkwood TBL. Geometric means and measures of dispersion. Biometrics 1979;35:908–9. [Google Scholar]
- 44. Linard C, Alegana VA, Noor AM, et al. A high resolution spatial population database of Somalia for disease burden estimation. Intl J Health Geogr 2010;9:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Metselaar D, van Thiel PH. Classification of malaria. Trop Geogr Med 1959;11:157–61. [Google Scholar]
- 46. Noor AM, Gething PW, Alegana VA, et al. The risks of malaria infection in Kenya in BMC. Infect Dis 2009;9:e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gosoniu L, Veta AM, Vounatsou P. Bayesian geostatistical modeling of malaria indicator survey data in Angola. PLoS One 2010;5:e9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Riedel N, Vounatsou P, Miller JM, et al. Geographical patterns and predictors of malaria risk in Zambia: Bayesian geostatistical modelling of the 2006 Zambia national malaria indicator survey (ZMIS). Malar J 2010;9:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Taylor SM, Messina JP, Hand CC, et al. Molecular malaria epidemiology: mapping and burden estimates for the Democratic Republic of the Congo, 2007. PLoS One 2011;6:e16420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stensgaard A-S, Vounatsou P, Onapa AW, et al. Bayesian geostatistical modelling of malaria and lymphatic filariasis infections in Uganda: predictors of risk and geographical patterns of co-endemicity. Malar J 2011;10:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Roll Back Malaria. Progress And Impact Series Number 2. http://www.rbm.who.int/ProgressImpactSeries/docs/RBMMalariaFinancingReport-en.pdf (accessed 16 Feb 2012).
- 52. Roll Back Malaria. Progress And Impact Series Number 2. http://www.rbm.who.int/ProgressImpactSeries/docs/wmd2010report-en.pdf (accessed 16 Feb 2012).
- 53. Snow RW, Marsh K. The consequences of reducing transmission of Plasmodium falciparum in Africa. Adv Parasitol 2002;52:235–64. [DOI] [PubMed] [Google Scholar]
- 54. Killeen GF, Smith TA, Ferguson HM, et al. Preventing childhood malaria in Africa by protecting adults from mosquitoes with insecticide-treated nets. PLoS Med 2007;4:e229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Smith DL, Noor AM, Hay SI, et al. Predicting changing malaria risk following expanded insecticide treated net coverage in Africa. Trends Parasitol 2009;25:511–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. World Health Organization. Manual For Developing A National Malaria Strategic Plan. Geneva: WHO, 2011. [Google Scholar]
- 57. Noor AM, Alegana VA, Patil AP, et al. Predicting the unmet need for biologically targeted coverage of insecticide treated nets in Kenya. Am J Trop Med Hyg 2010;83:854–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Moonen B, Cohen JM, Snow RW, et al. Operational strategies to achieve and maintain malaria elimination. Lancet 2010;376:1592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lysenko AJ, Semashko IN. Geography of malaria. A medico-geographic profile of an ancient disease (In Russian). In: Lebedew AW, ed. Itogi Nauki: Medicinskaja Geografija. Moscow: Acad Sci, USSR, 1968:25–146. [Google Scholar]
- 60. Craig MH, Snow RW, le Sueur D. A climate-based distribution model of malaria transmission in Sub-Saharan Africa. Parasitol Today 1999;15:105–11. [DOI] [PubMed] [Google Scholar]
- 61. Gething PW, Patil AP, Hay SI. Quantifying aggregated uncertainty in Plasmodium falciparum malaria prevalence and populations at risk via efficient space-time geostatistical joint simulation. PLoS Comput Biol 2010;6:e1000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bayesian Methods For Combining Multiple Individual And Aggregate Data Sources In Observational Studies. http://www.bias-project.org.uk/software/#sae (accessed 15 Feb 2011).