Summary
This study shows that the loss of two leaf-expressed isoforms of sucrose-phosphate synthase limits sucrose export and nocturnal starch breakdown, but does not affect photosynthetic carbon partitioning.
Key words: Arabidopsis thaliana, enzyme family, starch metabolism, sucrose biosynthesis, sucrose-phosphate synthase.
Abstract
Sucrose (Suc)-phosphate synthase (SPS) catalyses one of the rate-limiting steps in the synthesis of Suc in plants. The Arabidopsis genome contains four annotated SPS genes which can be grouped into three different families (SPSA1, SPSA2, SPSB, and SPSC). However, the functional significance of this multiplicity of SPS genes is as yet only poorly understood. All four SPS isoforms show enzymatic activity when expressed in yeast although there is variation in sensitivity towards allosteric effectors. Promoter–reporter gene analyses and quantitative real-time reverse transcription–PCR studies indicate that no two SPS genes have the same expression pattern and that AtSPSA1 and AtSPSC represent the major isoforms expressed in leaves. An spsa1 knock-out mutant showed a 44% decrease in leaf SPS activity and a slight increase in leaf starch content at the end of the light period as well as at the end of the dark period. The spsc null mutant displayed reduced Suc contents towards the end of the photoperiod and a concomitant 25% reduction in SPS activity. In contrast, an spsa1/spsc double mutant was strongly impaired in growth and accumulated high levels of starch. This increase in starch was probably not due to an increased partitioning of carbon into starch, but was rather caused by an impaired starch mobilization during the night. Suc export from excised petioles harvested from spsa1/spsc double mutant plants was significantly reduced under illumination as well as during the dark period. It is concluded that loss of the two major SPS isoforms in leaves limits Suc synthesis without grossly changing carbon partitioning in favour of starch during the light period but limits starch degradation during the dark period.
Introduction
In plants, sucrose (Suc) plays a major role in growth and development. It is the main end-product of photosynthesis in leaves, and in the majority of higher plants it also serves as the mobile form of photoassimilate that is transported from source to sink organs. In addition to its role as a central metabolite, Suc can also serve as a signalling molecule in the control of gene expression (Rolland et al., 2006; Wind et al., 2010) and is often accumulated in response to environmental stresses such as cold, salinity, and drought (Yang et al., 2001; Strand et al., 2003; Niedzwiedz-Siegien et al., 2004). Carbon fixed during the day is either exported from the plastid as triose phosphates to serve for Suc synthesis within the cytosol, or it is retained within the chloroplast to fuel the synthesis of transitory starch. In the subsequent dark period, starch is mobilized and thereby provides a steady supply of substrates for Suc synthesis and export to sink organs (Zeeman and ap Rees, 1999). Suc is synthesized from the activated hexose pool by the consecutive action of two enzymes. Suc-phosphate synthase (SPS; EC 2.4.1.14) catalyses the formation of Suc-6-phosphate (Suc-6-P) from fructose-6-phosphate and UDP-glucose. Subsequently, Suc-6-P is hydrolysed by a specific Suc-phosphate phosphatase (SPP; EC 3.1.3.24) to release Suc. While SPP appears to exert only little control of flux through Suc biosynthesis (Chen et al., 2005b ), the reaction catalysed by SPS represents a major regulatory step in the pathway (Huber and Huber, 1996). Based on the analysis of plants with reduced SPS expression by antisense technology, the flux control coefficient for Suc synthesis of the enzyme was estimated to be 0.2 in Arabidopsis leaves (Strand et al., 2000; Stitt et al., 2010) and 0.5 in potato leaves (Geigenberger et al., 1995), demonstrating that SPS is an important regulator of the Suc biosynthetic pathway. SPS enzyme activity is controlled by different interconnected mechanisms including post-translational modification via phosphorylation, direct control by metabolic effectors, such as activation by glucose-6-phosphate (Glc-6-P) and inhibition by inorganic phosphate (Pi; Huber and Huber, 1996; Winter and Huber, 2000), and transcriptional regulation during development, in response to external cues, and during the diurnal cycle (Gibon et al., 2004; Privat et al., 2008; Roy Choudhury et al., 2008; Okamura et al., 2011).
In all species examined to date, SPS is encoded by a small gene family. Based on phylogenetic analyses, individual members have been classified into three major groups (A, B, and C) in dicots, while some monocots seem to comprise at least one additional group (D-group) (Langenkämper et al., 2002; Lunn and MacRae, 2003; Castleden et al., 2004; Lutfiyya et al., 2007). It has been predicted that all angiosperm genomes harbour at least one gene from each of the respective families and that at least one member of each family is expressed (Lunn and MacRae, 2003). The genome of Arabidopsis has four annotated SPS genes, referred to as AtSPSA1 (At5g20280), AtSPSA2 (At5g11110), AtSPSB (At1g04920), and AtSPSC (At4g10120) (Langenkämper et al., 2002; Lunn and MacRae, 2003; Sun et al., 2011).
The functional significance of this multiplicity of SPS genes is as yet only poorly understood. Comparative studies of SPS gene expression in Arabidopsis (Sun et al., 2011), wheat (Castleden et al., 2004), maize (Lutfiyya et al., 2007), tobacco (Chen et al., 2005a ), and rice (Okamura et al., 2011) revealed distinct, but partially overlapping spatial and temporal expression patterns for the SPS gene families. Although there is some overlap in gene expression on the tissue level, it is still unclear whether there is co-expression of different isoforms in the same cell, as most tissue samples that have been analysed would contain several different cell types. Thus, it remains difficult to assign specific functions to any of the SPS gene families as no consistent expression patterns have been observed across species. A functional analysis of SPS isoforms in tobacco revealed that silencing of NtSPSC, an isoform that was shown to be exclusively expressed in source leaves, led to a massive increase in leaf starch content, an effect that could not be observed upon silencing of NtSPSA (Chen et al., 2005a ). Physiological and molecular data suggest that in tobacco leaves SPSC is specifically involved in sucrose synthesis during starch mobilization in the dark (Chen et al., 2005a ). The analysis of single knock-outs for SPSA1 and SPSC in Arabidopsis did not reveal a similar effect on starch metabolism (Sun et al., 2011), and thus functional specification of individual SPS isoforms probably differs on the species level.
In the case of other enzymes of central carbon metabolism, such as hexokinase (Karve et al., 2008), fructokinase (Arsova et al., 2010), or trehalose-6-phosphate synthase (Vandesteene et al., 2010), it has been shown that distinct isoforms appear to lack the predicted catalytic activity and thus might fulfil regulatory rather than metabolic functions. For SPS, it is currently not known whether all annotated Arabidopsis isoforms are catalytically active.
To shed further light on a functional specification of SPS isoforms in Arabidopsis, all enzymes have been produced recombinantly in yeast and their activity has been demonstrated. Based on promoter–reporter gene studies, it is concluded that SPSA1 and SPSC are the major leaf isoforms in Arabidopsis, and the analysis of an spsa1/spsc double knock-out mutant revealed profound effects on leaf carbohydrate metabolism and growth in these plants, indicating that these two isoforms have overlapping functions in leaf carbohydrate metabolism.
Materials and methods
Plasmid construction and expression in yeast
Arabidopsis SPS expression constructs were generated using Gateway® cloning technology (Invitrogen, Karlsruhe, Germany). To this end, the entire coding region of each SPS isoform was PCR amplified from Arabidopis cDNA using the primers listed in Supplementary Table S1 available at JXB online. Fragments were inserted into the pENTR-D/TOPO vector and entirely sequenced. No differences from the annotated genes were found. Subsequently, the fragments were recombined via L/R-Clonase (Invitrogen) into a Gateway®-compatible derivative of the yeast expression vector pYES-2 (Invitrogen) generating an N-terminal triple myc tag.
Yeast SPS expression constructs were transformed into the protease-deficient Saccharomyces cerevisiae strain CY1905 (kindly provided by C. Koch, FAU Erlangen-Nürnberg) using the lithium acetate method (Gietz et al., 1995). Transformants were selected and maintained on SC-ura medium with 2% glucose. Expression of the different SPS isoforms was induced in SC-ura medium with 2% galactose, and cultures were incubated at 30 °C on a rotary shaker for 24h. Subsequently, the cells were harvested by centrifugation and kept at –80 °C until further use.
Purification of recombinant SPS isoforms
Yeast cells equivalent to ~2g wet weight were re-suspended in 5ml of extraction buffer [25mM HEPES (pH 7.5), 12mM MgCl2, 0,5mM EDTA, 1ml of dithiothreitol (DTT), Roche protease inhibitor cocktail complete tablet (Roche)] and disrupted by several rounds of shaking with glass beads. Cell debris was removed by centrifugation for 10min at 25 000 g and subsequently by 60min at 100 000 g. The clear supernatant was further fractionated by precipitation with ammonium sulphate in steps of 20, 40, 60, and 80%. By western blot analysis, SPS proteins were detected in the 60% fraction. After diluting this fraction in 15ml of extraction buffer, proteins were further separated by DEAE anion exchange chromatography with a linear gradient of buffer B (extraction buffer containing 1M NaCl) from 0 to 100% over 60min at a flow rate of 1ml min–1 using an Äkta purifier (GE Healthcare). SPS-containing fractions between 250mM and 350mM NaCl were pooled and then diluted with 45ml of extraction buffer for a final chromatography using a gradient as above and a MonoQ HR5/5 column. Fractions containing the highest SPS activity were used for further analysis.
SPS activity measurement
SPS activity was assayed by quantifying the fructosyl moiety of Suc using the anthrone test exactly as described by Baxter et al. (2003).
Plant growth conditions
Wild-type Arabidopsis thaliana of ecotype Col-0 and T-DNA insertion lines in this background were sown on soil and stratified at 4 °C in the dark for 3 d. Subsequently, plants were grown in an 8h light (22 °C)/16h dark (20 °C) photoperiod at 50% relative humidity and a photon flux density (PFD) of 100 μmol m–2 s–1. All plant material was immediately frozen in liquid nitrogen after harvesting and stored at –80 °C prior to analysis.
Generation and analysis of GUS reporter lines
Promoter fragments comprising either 1.5kb upstream of the start codon for AtSPSA, AtSPSB, and AtSPSC, or, in case of AtSPSA2, the entire intergenic region of 800bp were amplified by PCR using genomic DNA from Arabidopsis Col-0 as a template and the primers listed in Supplementary Table S1 available at JXB online. The resulting fragments were cloned into the vector pGEM-T (Promega) and sequence verified. Subsequently, the fragments were fused with the β-glucuronidase (GUS) reporter gene in the intermediary vector pAKK1431. The resulting constructs were digested with PacI and the promoter–GUS fragment was inserted into the PacI site of the binary vector pAKK1437B (kindly provided by U. Hammes, FAU Erlangen-Nürnberg).
Arabidopsis thaliana Col-0 plants were transformed using standard procedures (Clough and Bent, 1998). After selection of transformed plants using BASTA treatment, the presence of the transgene was verified by PCR using gus-specific primers. Expression of the reporter gene was monitored in T0, T1, and T2 and/or T3 transgenic plants, using histochemical staining according to Jefferson et al. (1987).
qRT-PCR
For quantitative real-time reverse transcription–PCR (qRT-PCR) analysis, total RNA was isolated from frozen plant material using the method described by Logemann et al. (1987). cDNA was synthesized and qRT-PCR was performed as previously described (Arsova et al., 2010) on a Mx3000P Q-PCR system (Stratagene) in combination with the Brilliant II SYBR Green Q-PCR Master Mix Kit (Stratagene). Oligonucleotides used for determination of relative mRNA abundance of the different SPS genes are summarized in Supplementary Table S1 available at JXB online.
Isolation of T-DNA lines
The T-DNA Arabidopsis insertion lines for AtSPSA1 and AtSPSC were obtained from the Nottingham Arabidopsis Stock Center (Nottingham University) and confirmed by PCR-based genotyping using the primers listed in Supplementary Table S1 available at JXB online. Two independent alleles were obtained for each isoform, namely SALK_148643 (spsa1), SAIL_764_B04 (spsa1-2), SALK_037958 (spsc), and SALK_020179 (spsc-2). The PCR products obtained using T-DNA left border primers and gene-specific primers were sequenced to confirm the location of the inserts. Double knock-out lines for spsa1 and spsc were obtained by crossing spsa1 and spsc single mutant lines, and offspring homozygous for both insertions were identified by PCR. The knock-out status of the T-DNA mutants was confirmed by RT-PCR. To this end, total RNA from leaves of T-DNA lines and wild-type plants was extracted according to Logemann et al. (1987) and 2 μg were subjected to DNase digestion to remove any contaminating genomic DNA. RNA was reverse transcribed using RevertAid reverse transcriptase (Fermentas) according to the manufacturer’s instructions. PCR was done with Taq DNA polymerase (New England Biolabs) in a 25 μl reaction volume with primers specific to individual isoforms and 35 cycles (see Supplementary Table S1 available at JXB online).
Carbohydrate determination
Soluble sugar and starch levels were determined in leaf samples extracted with 80% (v/v) enthanol/20mM HEPES, pH 7.5, as described (Stitt et al., 1989), adapted for determination in a plate reader by direct downscaling of the assay to a volume of 200 μl. Maltose was measured from the same extracts with high-performance liquid chromatography (HPLC) using the conditions as described by Schneider et al. (2008).
Measurement of phosphorylated intermediates
Phosphorylated intermediates were extracted from 50mg of ground Arabidopsis leaf tissue powder with 1ml of 1M ice-cold perchloric acid as described (Horst et al., 2010). A 300 μl aliquot of the extract was filtrated through 10kDa filter plates (AcroPrep TM96–PALL Life Science). A 10 μl aliquot of the filtrates was analysed on an ICS3000 HPLC System (Dionex) connected to a QTrap 3200 Triple-Quadrupole mass spectrometer as described (Horst et al., 2010). Spike-in experiments were perfomed by adding standard solution of all analysed phosphorylated intermediates to Arabidopsis tissue powder prior to extraction to analyse matrix effects.
CO2 partitioning
The incorporation of 14CO2 in Arabidopsis leaves and partitioning analysis of 14C-labelled metabolites were performed as previously described by Lee et al. (2008) with minor modifications. Leaves of 6-week-old dark-adapted Arabidopsis plants were randomly distributed in a sealed custom-built Perspex chamber with a total gas volume of 0.19 litres, containing two layers of Whatman paper (Whatman, Maidstone, UK) soaked with 15ml of 1M NaHCO3 solution (pH 9.1). Pre-illumination was conducted for 10min at a PFD of 100 μmol m–2 s–1 Then, the atmosphere inside the chamber was enriched with NaH14CO3 (specific activity 29.3 kBq mmol–1) by injection of 12.5 μCi of NaH14CO3 through a septum, leading to a concentration of 5% 14CO2 in the gas phase. Labelling was performed for 40min at a PFD of 100 μmol m–2 s–1, and afterwards the leaves were extracted and further processed as described in Lee et al. (2008). Per extract, three leaves of the spsa1/spsc double knock-out mutants were pooled, while only one leaf of the other genotypes was used. Due the loss of a small proportion of labelled material during the separation, the relative values of the individual fractions do not add up to 100%.
Determination of Suc efflux from source leaves
Collection of phloem exudates for Suc efflux analysis was performed with plants grown for 5 weeks under the conditions as described above. Two (three in the case of the double knock-out plants) mature source leaves per plant were detached by cutting petioles close to the stem. Excised petioles were immediately placed in a 1.5ml test tube containing 200 μl of 5mM EDTA, and exudates were collected beginning after 1h of recovery for 3h. For the measurement of the light exudation rate, petioles were harvested 4h before the end of the light period and kept under conditions of 150 μmol m−2 s−1 supplementary light and water-saturated atmosphere for the duration of the experiment. For the assessment of dark exudation, petioles were harvested at the beginning of the dark period and kept in darkness for the duration of sampling. Aliquots were sampled from the exudates after 1, 2, and 3h, and the Suc content was determined as described above.
Results
All four annotated Arabidopsis SPS isoforms display enzymatic activity when expressed in yeast but display different allosteric properties
Full-length cDNAs encoding each of the four annotated Arabidopsis SPS isoforms were cloned into a yeast expression vector for galactose-inducible expression of the entire protein. An N-terminal myc-tag was introduced into each PCR product to facilitate immunological detection of the recombinant enzyme. Western blot analysis using anti-myc antibodies revealed that the highest expression of all four isoforms was achieved 24h after induction with galactose (data not shown). The recombinant proteins were then purified from yeast crude extracts by a combination of ammonium sulphate precipitation and two rounds of anion exchange chromatography on DEAE-cellulose and Mono-Q columns, respectively. All four recombinant proteins displayed enzymatic activity when assayed under non-limiting substrate conditions in the presence of the allosteric activator Glc-6-P, although the specific activity varied between isoforms. The specific activity in the presence of 20mM Glc-6-P and in the absence of Pi was highest for AtSPSB (131.2±4 mmol Suc-6-P μg protein–1 h–1) followed by AtSPSA2 (71.2±7 mmol Suc-6-P μg protein–1 h–1), while isoforms AtSPSA1 and AtSPSC displayed considerably lower specific activity (30.2±0.6 mmol Suc-6-P μg protein–1 h–1 and 33.3±3 mmol Suc-6-P μg protein–1 h–1, respectively) under these conditions.
The recombinant Arabidopsis SPS isoforms differed markedly in their response towards the allosteric effectors Glc-6-P and Pi (Fig. 1). AtSPSB showed a striking stimulation by Glc-6-P. At Glc-6-P concentrations >20mM its activity was 6.8-fold higher as compared with the activity without the activator. The other three recombinant SPS isoforms did not display significant changes in activity when tested in the presence of increasing Glc-6-P concentrations. When assayed for their sensitivity towards Pi inhibition at suboptimal activator concentrations (10mM Glc-6-P), AtSPSB showed a sharp decline in activity at increasing concentrations of Pi, with an ~4-fold reduction above 10mM Pi. The activity of the other three isoforms was not prone to Pi inhibition (Fig. 1).
Fig. 1.
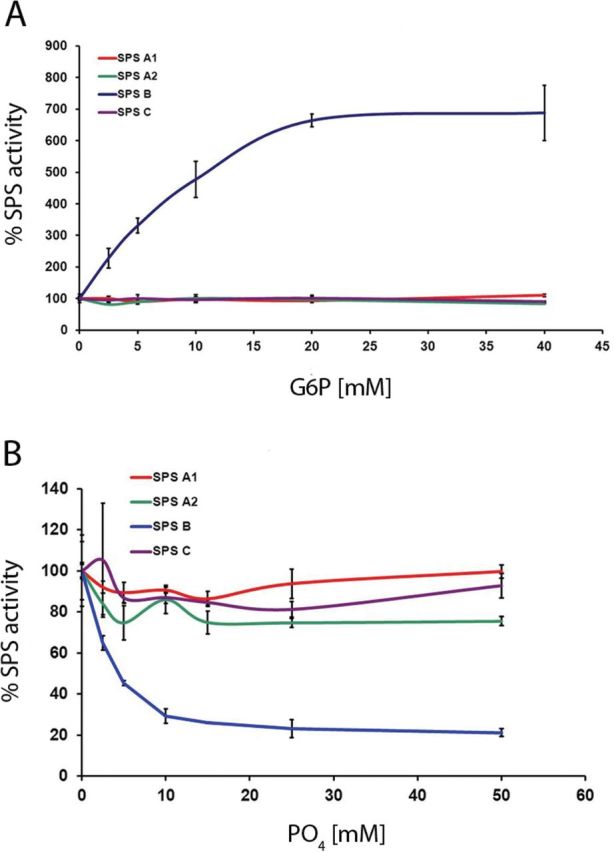
Allosteric regulation of purified recombinant Arabidopsis SPS isoforms expressed in S. cerevisiae. Graphs represent relative changes in SPS activity in the presence of different Glc-6-P (A) and Pi (B) concentrations, respectively. Each value is the mean ±SD of three measurements.
Taken together, these results demonstrate that all four recombinant Arabidopsis SPS isoforms display enzymatic activity when expressed in yeast, although their specific activity might vary, and that under the conditions applied they respond differently to allosteric regulation by Glc-6-P and Pi.
SPS isoforms have overlapping but distinct expression patterns
To characterize the in planta expression pattern of the four Arabidopsis SPS genes at high spatial resolution, wild-type Arabidopsis plants were transformed with constructs containing the GUS reporter gene on one of the SPS gene promoter regions. The resulting transgenic plants were stained for GUS activity in different tissues and at various developmental stages. Detailed examination revealed considerable, specific localization of SPS isoform expression in particular cell types and tissues. The following observations have been made on multiple lines of the relevant transformants. In 11-day-old seedlings, strong GUS staining was observed in plants harbouring the AtSPSA1 and AtSPSC promoter–GUS constructs, while the same tissue was weakly stained in AtSPSB–GUS seedlings (Fig. 2A–D). The roots remained unstained in these three lines. In contrast, when seedlings harbouring the AtSPSA2–GUS construct were analysed, strong staining was observed in roots, but no GUS expression could be detected in cotyledons and the hypocotyl (Fig. 2B). Expression of AtSPSA1 and AtSPSC was also detectable in leaves of 6-week-old plants (Fig. 2E, F), while no GUS staining was observed in leaves for AtSPSB and AtSPSA2 (data not shown). From the GUS staining pattern, it appeared that AtSPSA1 is expressed throughout the leaf lamina including the vasculature, whereas AtSPSC expression was confined to the intercostal regions (Fig. 2E, F).
Fig. 2.
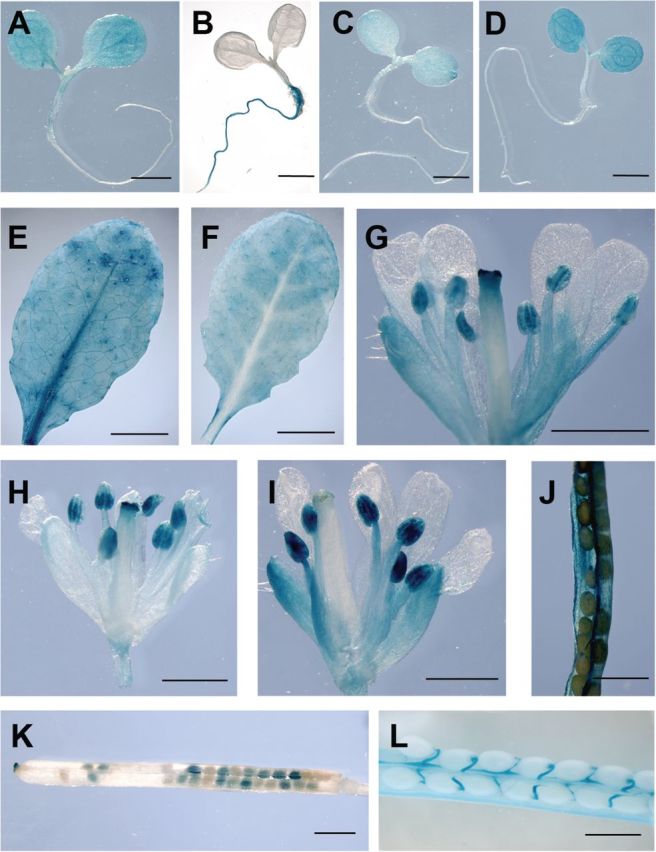
Expression pattern of Arabidopsis SPS genes as revealed by the analysis of SPS promoter–GUS plants. (A–D) GUS histochemical staining of 11-day-old seedlings grown in vitro (A, AtSPSA1–GUS; B, AtSPSA2–GUS; C, AtSPSB–GUS; D, AtSPSC–GUS). (E) GUS staining of a mature source leaf of an AtSPSA1–GUS plant. (F) Histochemical GUS staining of a mature source leaf of an AtSPSC–GUS plant. (G–I) GUS staining of inflorescence tissue from AtSPSA1–GUS (G), AtSPSB–GUS (H), and AtSPSC–GUS (I) transgenic plants. (J–L) Histochemical GUS staining of siliques sampled from AtSPSA1–GUS (J), AtSPSB–GUS (K), and AtSPSC–GUS (L) plants. The scale bar represents 1mm, with the exception of E and F where the bar is equivalent to 5mm. (This figure is available in colour at JXB online.)
GUS staining of flowers of AtSPSA2–GUS plants showed no reporter gene expression (data not shown). In contrast, AtSPSA1–, AtSPSB–, and AtSPSC–GUS plants all showed GUS activity in flowers, although the detailed pattern of expression differed from one isoform to another (Fig. 2G–I). All three promoters show expression in anthers, suggesting that they might be expressed in pollen. AtSPSA1 and AtSPSC were expressed in flower petals (Fig, 2G, I). AtSPSA1 and AtSPSB also displayed promoter activity in the stigma, while AtSPSC did not, suggesting that staining of the stigma in AtSPSA1 and AtSPSB is not just caused by germinating pollen.
GUS histochemical analysis of siliques from transgenic lines harbouring the SPS promoter–GUS reporter constructs indicated that expression of AtSPSA1 was primarily in the silique sheaths, while AtSPSB strongly stained the seeds but also faintly stained the stigma region and the receptacle (Fig. 2J, K). The AtSPSC promoter region conferred GUS expression only in the vascular system of the siliques, including the funiculi (Fig. 2L).
To provide further information on the expression patterns of SPS genes, qRT-PCR analysis was performed using isoform-specific primers on total RNA extracted from samples taken from a range of organs 6h after the onset of light (Fig. 3). The expression values of each isoform in a given organ are expressed as the percentage of the mRNA level of the ubiquitin gene. In accordance with the GUS expression data, the qRT-PCR analysis revealed that no two isoforms have the same expression pattern. AtSPSA1 is most abundant in all tissues tested and, together with AtSPSC, constitutes the major SPS gene expressed in leaves. However, even in leaves, AtSPSC expression was ~75% lower than that of AtSPSA1 (Fig. 3). AtSPSB showed by far the lowest expression in all organs analysed (1.8×10–5 in mature leaves to 6.5×10–4 in buds when expressed as a percentage of ubiquitin expression). Given the fact that samples for RNA isolation were taken 6h into the light period, it currently cannot be excluded that expression of individual SPS isoforms relative to each other changes over the diurnal cycle.
Fig. 3.
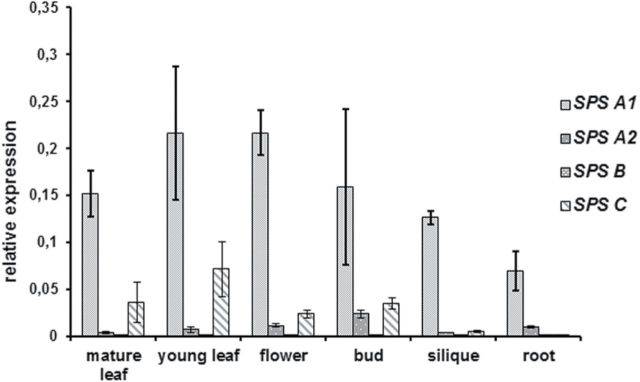
Real-time quantitative PCR analysis of SPS isoform expression in different tissues of Arabidopsis plants. Transcript levels are expressed as a percentage of the transcript level of ubiquitin 10 (At4g05320) in the same organ. Values are the means ±SD and of three replicates. All samples were taken from plants grown in soil under short-day conditions for 3–6 weeks, except those representing reproductive tissues which were taken from plants grown under long-day condtions (14h light/10h dark) for an additional 3–5 weeks. Silique samples contained seeds and represented a full range of developmental stages. All samples were taken in the middle of the photoperiod.
Taken together, it appears that more than one isoform is expressed in each tissue examined, although relative expression levels can vary considerably.
Loss of the two major leaf SPS isoforms reduces plant growth
Based on the expression analyses presented above, it can be concluded that AtSPSA1 and AtSPSC constitute the major leaf isoforms in Arabidopsis. To investigate the role of SPS in photosynthetic carbon metabolism in more detail, efforts were initially concentrated on the functional analysis of these two isoforms. To gain insight into the in planta function of the SPSA1 and SPSC isoform in Arabidopsis, T-DNA insertion lines were identified from the SALK collection (Salk_148643, spsa1; Salk_037958, spsc).
The T-DNA insertions in spsa1 and spsc are in the 11th exon (3769 nucleotides downstream of the ATG codon) and in the 10th intron at position – 3024, respectively (Fig. 4). Plants homozygous for the T-DNA insertion were generated by self-pollination (Fig. 4). RT-PCR analysis showed that the respective SPS transcript was absent in both the spsa1 and the spsc line, indicating a complete knock-out (Fig. 4). In addition to the knock-out in individual SPS isoforms, a double knock-out line lacking both SPSA1 and SPSC expression was generated by crossing the corresponding single knock-outs, self-pollinating the resulting heterozygous plants, and PCR screening for homozygous progeny. Homozygous double mutants, spsa1/spsc, were identified in the F3 generation by PCR on genomic DNA. Loss of SPSA1 and SPSC gene expression in the double mutant was verified by RT-PCR (Fig. 4C).
Fig. 4.
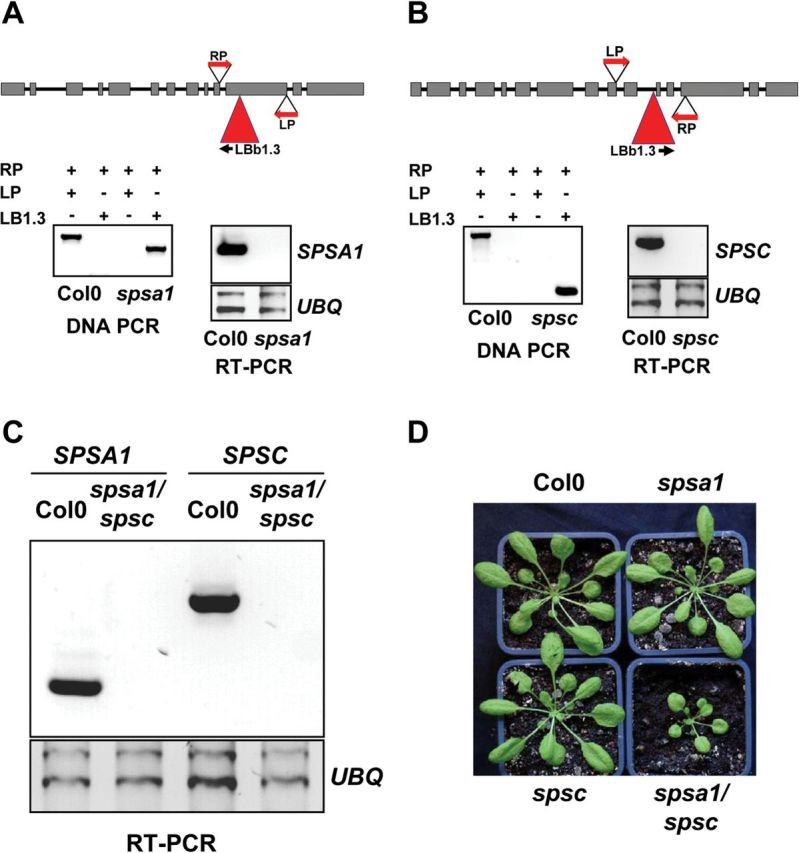
Molecular analysis of the SPS Arabidopsis knock-out and the mutants analysed in this study. (A and B) spsa1 and spsc single knock-outs. The T-DNA insertion sites and primer (LP, RP, and LBb1.3) locations are indicated. Expression of AtSPSA1 and AtSPSC transcripts was shown by RT-PCR with gene-specific primer sets. UBQ serves as an internal control. (C) RT-PCR analysis of SPSA1 and SPSC expression in spsa1/spsc double knock-out plants with UBQ serving as an internal control. (D) Growth phenotype of 6-week-old SPS knock-out mutants compared with the Col-0 wild-type control. Plants were cultivated under short-day conditions with 8h light/16h dark. (This figure is available in colour at JXB online.)
When grown on soil for 7 weeks, both the spsa1 and the spsc mutant were phenotypically indistinguishable from the wild-type control (Fig. 4D). However, growth of the spsa1/spsc double mutant was drastically reduced compared with the wild type (Fig. 4D). A reduction of 50% in rosette diameter was observed after 6 weeks of cultivation under short-day conditions (Supplementary Fig. S1 available at JXB online).
Taken together, it appears that in leaves of Arabidopsis SPSA1 and SPSC have at least overlapping functions since only a double knock-out of both isoforms affected plant growth.
SPS enzyme activity and carbohydrate levels in SPS knock-out Arabidopsis plants
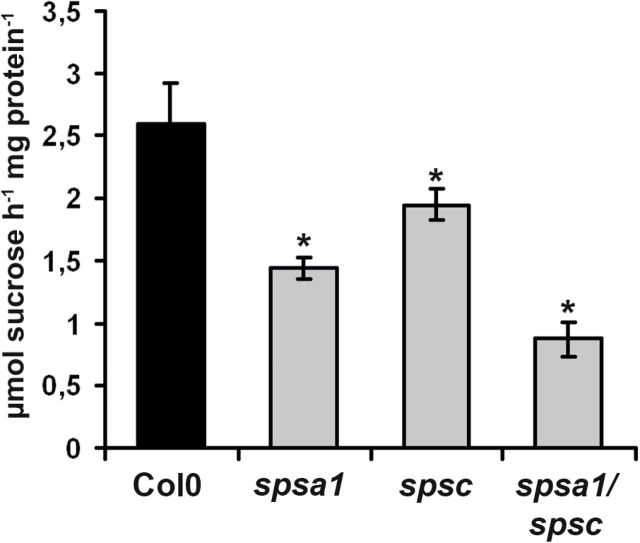
The maximal extractable SPS activity from mature source leaves in spsa1 plants was reduced by ~44% as compared with the wild type, while spsc mutants showed a reduction by ~25% (Fig. 5). These results could be confirmed in a set of independent spsa1 (spsa1-2; SAIL_764_B04) and spsc (spsc-2; Salk_020179) alleles, respectively (Supplementary Fig. S2 available at JXB online).
Fig. 5.
SPS enzymatic activity in the different SPS knock-out genotypes compared with the Col0 wild-type control. Samples were taken from leaves of 6-week-old plants cultivated under short-day conditions. Samples were harvested 6h into the photoperiod. Values represent the mean ±SD (n=4). An asterisk indicate a significant difference at P<0.05 as analysed by t-test.
In contrast, spsa1/spsc double mutants showed an additive reduction in maximal SPS activity by approximately two-thirds relative to the wild type (Fig. 5). To determine how loss of SPSA1 and SPSC affects leaf carbohydrate metabolism in single and double mutants, the levels of non-structural carbohydrates were determined in all three genotypes both at the end of the light period and at the end of the dark period. At the end of the light period, glucose contents were significantly reduced only in mature leaves of the spsa1 mutant (Table 1). In the double mutant as well as in spsc single mutant plants, Suc levels were significantly reduced after 8h of illumination. Towards the end of the dark period, soluble sugar levels were not grossly different between mutant plants and the wild type despite an ~3-fold increase in glucose in spsa1/spsc double mutant plants (Table 1). Compared with control plants, single mutants of spsa1 displayed a significant increase in leaf starch content at the end of the light period, while in the spsa1/spsc double mutants starch content increased by a factor of three at this time point (Table 1). The effect on leaf starch content was even more pronounced at the end of the dark period. While starch was almost completely degraded in wild-type and spsc single mutant plants, the levels in spsa1 plants were significantly increased by a factor of two while starch levels in spsa1/spsc double mutants remained as high as at the end of the light period (Table 1).
Table 1.
Changes in carbohydrate contents in leaf material harvested from the different SPS knock-out genotypes compared ith the Col-0 wild-type controlPlants were 6 weeks old. Values represent the mean ±SD of at least four independent samples. Samples were taken at the end of the 16h dark period (ED) and at the end of the 8h light period (EL). Each set of samples has its corresponding wild type.
| Plant line | Glucose | Fructose | Sucrose | Starch |
|---|---|---|---|---|
| Metabolite contents in μmol g–1 FW–1 | ||||
| Col-0/ED | 0.95±0.22 | ND | 0.60±0.08 | 1.57±0.13 |
| spsa1/ED | 1.00±0.13 | ND | 0.53±0.05 | 2.41±0.32** |
| spsc/ED | 0.80±0.11 | ND | 0.57±0.11 | 1.95±0.48 |
| spsa1/spsc/ED | 3.08±0.31** | 0.39±0.12 | 0.65±0.09 | 29.07±1.74** |
| Col-0/EL | 1.89±0.27 | 0.19±0.08 | 1.11±0.07 | 12.76±1.11 |
| spsa /EL | 0.97±0.12* | ND | 1.03±0.05 | 14.69±1.07* |
| spsc/EL | 1.44±0.08 | ND | 0.90±0.03 ** | 12.91±0.44 |
| spsa1/spsc/EL | 3.03±0.50 ** | 0.28±0.13 | 0.76±0.07 ** | 31.15±5.22** |
Asterisks indicate a significant difference (*P<0.05; **P<0.001) according to t-test. ND, not detectable.
Next, the levels of intermediates of Suc biosynthesis were determined in leaves harvested 5h after the onset of light. While there were no significant changes in hexose-phosphates or UDP-glucose between the wild type and sps single mutants, the levels of these intermediates were at least 2-fold increased in spsa1/spsc double mutants (Table 2). Suc-6-P levels in spsa1/spsc double knock-out plants were below the detection level, which is in keeping with the reaction catalysed by the enzymes.
Table 2.
Contents of hexose-phosphates, UDP-glucose, and Suc-6-P in leaves of different SPS knock-out genotypes compared with the Col-0 wild-type controlSamples were taken from 6-week-old plants in the light 5h into the photoperiod and immediately snap-frozen in liquid N2.
| Plant line | Glc-6-P | Glc-1-P | Fru-6-P | UDP-Glc | Suc-6-P |
|---|---|---|---|---|---|
| Metabolite content in nmol g–1 FW–1 | |||||
| Col-0 | 129.2±25.8 | 30.6±2.3 | 99.5±21.4 | 27.5±11.2 | 1.3±0.3 |
| spsa1 | 163.5±29.1 | 33.3±4.8 | 81.6±18.4 | 37.6±22.6 | 1.0±0.1 |
| spsc | 139.5±19.4 | 29.3±0.1 | 102.3±7.7 | 36.4±24.1 | 1.3±0.2 |
| spsa1/spsc | 411.7±26.1* | 62.7±1.8* | 209.9±23.8* | 77.3±17.1* | ND |
Values represent the mean (±SD) of six individual plants.
An asterisk indicate a significant difference at P<0.05 according to t-test. ND, not detectable.
In order to investigate whether a loss of SPS expression could affect the leaf’s ability to export photoassimilates, Suc efflux rates from detached petioles were determined over a period of 3h during the light as well as during the dark period. In the light, efflux rates of petioles taken from spsa1 and spsc single knock-out plants were similar to those of wild-type petioles, indicating that the loss of one SPS isoform in leaves does not grossly affect Suc export rates (Fig. 6A). In contrast, spsa1/spsc double knock-out plants showed considerably reduced Suc concentrations in phloem exudates after the 3h collection period that reached only 25% of the respective wild-type controls and SPS single knock-out plants (Fig. 6A). During the dark, when no CO2 fixation takes place and Suc synthesis is fuelled from starch degradation, Suc efflux from spsa1/spsc double knock-out plants was reduced by ~60% at 3h, while petioles from SPS single knock-out plants did not display significantly different Suc efflux rates as compared with the wild-type control (Fig. 6B).
Fig. 6.
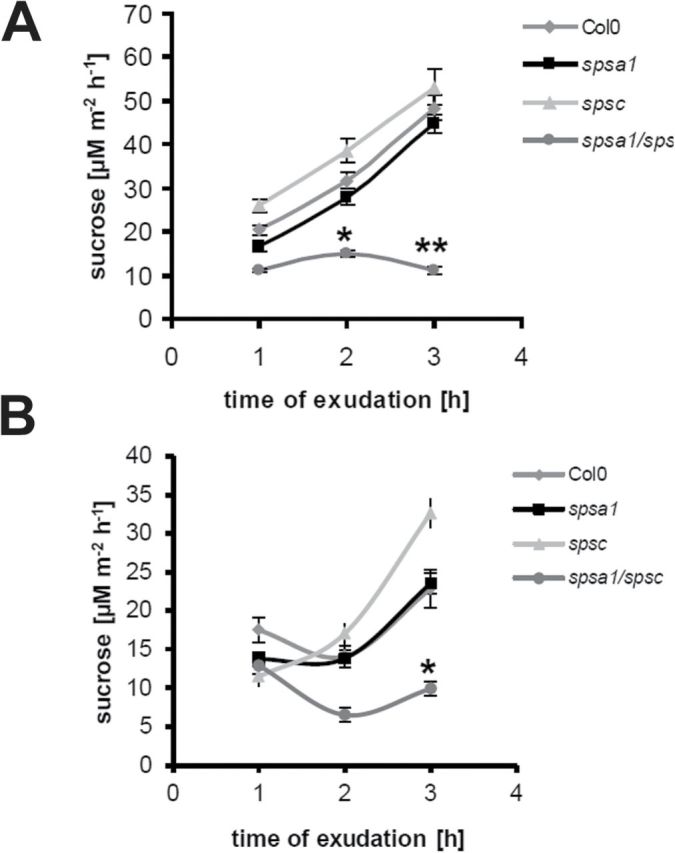
Suc efflux from source leaves of Arabidopsis sps mutant lines during vegetative growth. Phloem exudates were collected from two mature source leaves (three in the case of spsa1/spsc double mutants) per line after a growth period of 6 weeks under short-day conditions. Suc content in the exudates was determined 1, 2, and 3h after excision. (A) Suc efflux from petioles that were excised 4h before the end of the light period and that were kept under the same light conditions. (B) Suc efflux from petioles during the dark. Leaves were excised immediately at the beginning of the dark period. Data are presented as means ±SD of six individual plants per line. Asterisks indicate a significant difference (*P<0.05; **P<0.001) as analysed by t-test.
These data indicate that SPS enzymatic activity in spsa1/spsc double mutants becomes limiting for sucrose biosynthesis and export from source leaves during the light as well as during the dark period.
The accumulation of starch in leaves of spsa1/spsc double knock-out plants is due to a reduced rate of starch mobilization during the night
Steady-state levels of leaf carbohydrates reflect the balance between synthesis and degradation. Therefore, the starch accumulation observed in spsa1/spsc double knock-out plants could either be due to an increased partitioning of newly fixed carbon into starch synthesis, or due to a reduced rate of starch mobilization during the night, or both. To address the question of whether alterations in carbon partitioning during photosynthesis cause the accumulation of starch in spsa1 single and spsa1/spsc double mutants, the fate of recently assimilated CO2 was investigated. To this end, fully expanded leaves taken from 6-week-old plants were placed in a sealed chamber at a PAR similar to the growth conditions (100 μmol m–2 s–1). Leaves were incubated in air enriched with ~5% 14CO2 for 40min. Following ethanol extraction, radiolabel was determined in the insoluble and soluble fraction. As shown in Table 3, total incorporation of 14C was not significantly different between genotypes. Incorporation of 14C into the insoluble fraction (mainly representing starch and a minor proportion of cell wall material and proteins, respectively) was unchanged in all three knock-out genotypes as compared with the control, indicating that neither a loss of single SPS isoforms nor a combined knock-out in spsa1/spsc double mutants had an influence on photosynthetic carbon partitioning into starch under the conditions applied. The soluble fraction was further divided into a neutral (mainly sugars), cationic (amino acids), and two anionic fractions representing phosphorylated intermediates and carboxylic acids, respectively. The only significant change was observed in the fraction representing phosphorylated intermediates where incorporation of label was reduced by ~40% in the case of the spsa1/spsc double mutant (Table 3). Taken together, these results imply that the elevated starch levels found in spsa1/spsc double knock-out plants probably arise from a reduced capacity to mobilize starch during the dark period and are not mainly due to changes in carbon partitioning during photosynthesis.
Table 3.
14CO2 partitioningDetached, fully expanded leaves from 7-week-old dark-adapted Arabidopsis plants were enclosed in an incubation chamber under saturated 14CO2 and light for 40min. Three leaves per genotype harvested from independent plants were used and randomly distributed within the chamber to compensate for possible CO2 concentration gradients. The incorporation of 14C into soluble and insoluble fractions was determined after hot ethanol extraction. The soluble extract was further fractionated using ion exchange chromatography into neutral (predominantly free sugars), anionic (phosphorylated intermediates in fraction anionic I and carboxylic acids in fraction anionic II), and cationic (free amino acids) fractions. The values are given as a percentage of total 14C incorporation. The experiment was repeated twice with similar results.
| Col-0 | spsa1 | spsc | spsa1/spsc | |
|---|---|---|---|---|
| Total incorporation (μmol 14 C g FW–1) | 365±114 | 305±81 | 349±43 | 323±89 |
| Soluble | 46.5±2.5 | 46.1±3.4 | 49.7±1.8 | 41.7±4.3 |
| Insoluble | 53.5±2.5 | 53.9±3.4 | 50.3±1.8 | 58.3±4.3 |
| Neutral | 13.0±1.2 | 11.8±1.5 | 14.6±1.3 | 10.0±1.6 |
| Anionic I | 1.13±0.11 | 0.99±0.16 | 1.08±0.09 | 0.62±0.08* |
| Anionic II | 0.80±0.10 | 0.89±0.08 | 0.65±0.08 | 0.72±0.05 |
| Cationic | 0.15±0.01 | 0.20±0.02 | 0.14±0.03 | 0.19±0.03 |
Values represent the mean (± SE) from five different plants.
An asterisk indicate a significant difference at P<0.05 from the wild type according to t-test.
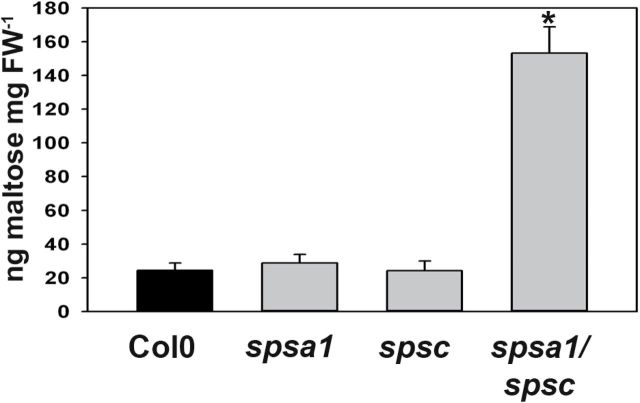
To investigate this possibility further, the levels of maltose, which is the major product of starch degradation in leaves (Zeeman et al., 2007), were measured. Maltose levels were determined in leaves from 6-week-old plants harvested at the end of the dark period. Maltose levels in spsa1 and spsc single knock-out lines did not differ significantly from those in the wild type (Fig. 7). In contrast, the maltose content in spsa1/spsc double knock-out plants was elevated ~6-fold as compared with the control (Fig. 7). This finding is consistent with a reduced capacity to use carbohydrates from starch breakdown for the synthesis of sucrose in these plants.
Fig. 7.
Maltose content in leaves of the different SPS knock-out genotypes compared with the Col0 wild-type control. Samples were harvested at the end of the dark period. Values represent the mean ±SE (n=6). The asterisk indicates a significant difference at P<0.01 as analysed by t-test.
Discussion
The basic framework for understanding SPS regulation and its role in carbon partitioning between Suc and starch was elaborated during the 1980s, and the first nucleotide sequences became available in the early 1990s. However, before the Arabidopsis genome was sequenced in 2000 (Arabidopsis Genome Initiative, 2000) and expressed sequence tag (EST) data from other species accumulated, it was not clear that apparently in all plants SPS would be encoded by a small gene family giving rise to different SPS isoforms. Consequently, the contribution of different SPS isoforms, which might possess different regulatory and biochemical properties, to overall regulation of Suc biosynthesis remained largely unconsidered. Here it is shown that all four annotated SPS isoforms from Arabidopsis display enzymatic activity when heterologously expressed in yeast. This probably reflects the position of Suc as a central metabolite in plants which is likely to require fine-tuned control of its synthesis to maintain carbon homeostasis on a tissue and cellular level. This could be brought about by different SPS isoforms displaying subtle differences in their regulatory properties. The general picture of SPS regulation implies that SPS activity is regulated by the allosteric effectors, Glc-6-P (activator) and Pi (inhibitor), and effector sensitivity is altered by protein phosphorylation (Huber and Huber, 1996; Winter and Huber, 2000). It is thought that this regulatory mechanism serves to balance Suc synthesis within the cytosol with the momentary rate of photosynthesis (Stitt et al., 1987). However, three of the four Arabidopsis SPS isoforms were not prone to allosteric regulation by Glc-6-P and Pi when recombinantly expressed in yeast, including the two major leaf isoforms AtSPSA1 and AtSPSC, which appear to control Suc synthesis in photosynthetic tissue. This indicates that either Arabidopsis leaf SPS is not regulated in the same way as SPS from spinach and a range of other plants (Huber and Huber, 1996), or that rendering these SPS isoforms susceptible to allosteric regulation requires a post-translational modification such as phosphorylation. The lack of allosteric regulation has previously been reported to occur in soybean leaf SPS (Nielsen and Huber, 1989), and another study reported on the purification of an SPS isoform that was not affected by either effector from rice leaves (Salerno et al., 1998). The spinach SPS A isoform and the maize SPS B isoform were both expressed in Escherichia coli and shown to have catalytic activity while lacking Pi sensitivity (Worrell et al., 1991; Sonnewald et al., 1993). When the spinach SPS protein was heterologously expressed in tobacco leaves, the enzyme was highly sensitive to Pi. This indicates that for spinach SPSA a plant-specific factor, probably a post-translational modification, is necessary to render the enzyme prone to allosteric regulation. Whether this also holds true for the Arabidopsis A1, A2, and C SPS isoforms is currently not known. Remarkably, AtSPSB is highly sensitive to allosteric regulation when expressed in yeast. It appears unlikely that only this particular isoform is correctly post-translationally modified in this system to result in effector sensitivity while the others are not. Thus, the differences in regulation of the recombinant enzymes might reflect different regulatory requirements in vivo. It would be interesting to determine which structural differences are responsible for the different regulation of AtSPSB as opposed to the other Arabidopsis SPS isoforms. As a cautionary note, in addition to the full-length proteins, all SPS protein purifications contained proteolytic degradation products that might retain enzymatic activity, albeit with different kinetic properties as compared with the intact protein. In addition, it cannot be excluded that the enzyme preparations contain yeast proteins that could interfere with the SPS activity measurements. The presence of impurities can also lead to an underestimation of the specific activity of individual isoforms. The values measured for the recombinant proteins are considerably lower than those reported for SPS purified from plants (e.g. 80 μmol min–1 mg protein–1 for spinach leaf SPS; Sonnewald et al., 1993). Thus, future experiments addressing illumination of the biochemical properties of individual SPS isoforms will require enzyme preparations of improved purity.
The notion that different enzymatic properties of SPS isoforms reflect at least a certain degree of functional specialization is further supported by their distinct expression pattern. The tissue specificity of SPS isoform expression as determined by GUS reporter gene assays and qRT-PCR is in good agreement with previous in silico analysis of SPS expression patterns in Arabidopsis derived from large-scale expression data (Sun et al., 2011). However, the GUS data indicate subtle differences in cell type-specific expression on the organ level. This is particularly true for siliques, where GUS expression for AtSPSA1, AtSPSB, and AtSPSC reporter constructs is detectable although each stains a different cell type in this organ. In agreement with previous observations of SPS isoform expression in tobacco (Chen et al., 2005a ), it appears that the AtSPSB gene is predominantly expressed in seeds and reproductive organs, while it is absent from leaves. Whether or not this isoform is required for sucrose metabolism in flower tissues remains to be determined. Thus far it has not been possible to identify a clear AtSPSB Arabidopsis knock-out mutant from the available T-DNA collections; however, RNA interference (RNAi) of NtSPSB in tobacco results in male sterility (S. Chen and F. Börnke, unpublished data), implying a specific role for the B isoform in flower development at least in this species.
Based on the promoter–GUS analyses and qRT-PCR measurements, AtSPSA1 and AtSPSC are the only isoforms clearly expressed in leaves, although the spatial distribution within the leaf tissue might vary. In accordance with previous observations (Sun et al., 2011), AtSPSA1 is the major leaf isoform based on expression and the reduction in enzyme activity in spsa1 mutant lines. However, a reduction of SPS enzymatic activity by 44% in spsa1 single mutants did not limit plant growth under controlled growth conditions, indicating that AtSPSC activity could compensate for most of the loss in SPSA1. A striking difference between spsa1 and spsc single mutants is that the former contains significantly more starch at the end of the light period as well as at the end of the dark phase, while the latter shows reduced Suc levels only at the end of the light period. Thus, although both isoforms have overlapping functions there seem to be subtle differences with respect to their role in leaf carbohydrate metabolism. Expression of SPSC is under diurnal control in Arabidopsis, showing the highest expression levels towards the end of the dark period with a steady decline over the day (Harmer et al., 2000; Gibon et al., 2004). This could argue for a role for this isoform in starch to Suc conversion during the night as has previously been suggested for the SPSC isoform of Nicotiana tabacum (Chen et al., 2005a ). However, measurement of SPS protein levels using mass spectrometry revealed higher SPSC protein amounts during the day compared with those during the night (Lehmann et al., 2008). This could indicate that the protein level follows a time shift in the circadian rhythm, as observed for other proteins (Gibon et al., 2004), and it would also lead to an underestimation of the relative contribution of this isoform to photosynthetic Suc metabolism in leaves. The mass spectrometric data on SPS protein abundance in leaves suggest that SPSA2 is the dominant isoform in leaves with respect to protein levels (Lehmann et al., 2008). This is in stark contrast to the expression and activity data presented here. Neither the promoter–GUS analysis and qRT-PCR nor previously published microarray data (Gibon et al., 2004) provide evidence for strong SPSA2 expression in leaves. Even if mRNA levels are not reflecting the actual protein amount, as might be the case for SPSC, the reduction of enzyme activity in leaves of the spsa1/spsc double mutant by 67% does not support the proposal of SPSA2 being the major leaf SPS isoform. A possible explanation for this discrepancy might be the fact that SPSA2 expression is strongly induced under stress conditions such as cold (Lehmann et al., 2008), and thus mRNA levels could depend on the actual growth conditions of the plants. A different physiological status of the plants might also explain the discrepancy in SPSA2 expression between the promoter–GUS analysis (main expression in roots) and the qRT-PCR measurements (main expression in floral tissues) that was observed in the present experiments.
An at least overlapping function of SPSA1 and SPSC in leaf Suc synthesis is further supported by the phenotype of spsa1/spsc double knock-out plants which show a 67% reduction in leaf SPS activity, severely reduced growth, and massive accumulation of starch in their leaves. Although these results are in agreement with previous findings suggesting that AtSPSA1 is the major contributor to total SPS activity in Arabidopsis leaves, they differ in the proportion of enzyme activity that can be attributed to the individual isoforms. Sun et al. (2011) reported a decrease in SPS activity in spsa1 mutant plants of 80%, and of 10% in spsc plants. Differences between the observed reduction in SPS activity in the present studies and those reported by Sun et al. (2011), despite the the fact same T-DNA insertion lines were analysed, cannot be accounted for. However, it is difficult to understand why a reduction of 80% in SPS activity would not affect plant growth as reported by Sun et al. (2011), although antisense repression of SPS in transgenic Arabidopsis plants was shown to reduce SPS activity to a similar extent with a concomitant decrease in biomass accumulation of 50–60% (Strand et al., 2000). In contrast to the study by Sun et al. (2011), a significant reduction in Suc levels towards the end of the light period in spsc single knock-out plants was observed in the present study. However, the conditions under which the plants were cultivated differ substantially between the two studies. While Sun and colleagues grew their plants in a 10h photoperiod and a PFD of 200 μmol m–2 s–1, the plants analysed here were grown in a 8h photoperiod with a PFD of 100 μmol m–2 s–1. The shorter daylength and the lower light intensity could limit photosynthetic carbon assimilation to an extent that worsens the effect of reduced SPS enzyme activity on metabolism.
Obviously, SPS activity and Suc biosynthetic capacity in the spsa1/spsc double knock-out plants becomes limiting for growth, which is also manifested by the accumulation of phosphorylated intermediates in leaves of spsa1/spsc double knock-out plants and by the reduction in Suc efflux from petioles. A similar reduction in SPS activity as well as in growth to that in the spsa1/spsc double knock-out plants has been reported to occur in transgenic Arabidopsis lines upon antisense repression using a construct directed against AtSPSA1 (Strand et al., 2000). However, on the metabolite level, SPS antisense plants were quite distinct from the SPS knock-out plants reported here. In the transgenics, leaf starch was reduced rather than increased, and there was also no increase in phosphorylated intermediates (Strand et al., 2000). The reason for this difference between the two approaches is currently unknown, as the extent to which other SPS genes may have been affected by the antisense construct or, alternatively, may have been up-regulated to compensate was not investigated (Strand et al., 2000).
In a previous study, the increase in starch content in spsa1 single mutants was interpreted as a shift in photosynthetic carbon allocation to compensate for a reduced Suc biosynthetic capacity (Sun et al., 2011). However, no changes in photosynthetic carbon partitioning under ambient light and saturated CO2 conditions were observed in SPS single knock-outs or in the spsa1/spsc double knock-out plants as compared with the wild type. Thus, the increase in leaf starch content in spsa1 and spsa1/spsc double knock-out plants does not appear to be solely the result of a shift in carbon partitioning during photosynthesis. However, it needs to be taken into account that CO2 partitioning can be greatly affected by the CO2 concentration, and thus partitioning under ambient growth conditions could be different from that observed under saturated CO2 levels. An alternative explanation for the changes in starch content would be a reduced mobilization rate during the dark period. Indeed, while wild-type and spsc plants consumed ~90% of their leaf starch during the dark period, spsa1 mutant plants mobilized only 80% of transitory leaf starch. This slight difference in starch mobilization between wild-type and spsa1 mutant plants can be explained either by a specific functional role for AtSPSA1 in Suc synthesis during the dark period or simply by the higher contribution of this isoform to overall SPS activity. The spsa1/spsc double knock-out plants were only able to use ~10% of their starch during the dark period, indicating that nocturnal starch mobilization is strongly impaired in these plants. In addition, the spsa1/spsc double knock-out plants accumulated high levels of maltose and glucose at the end of the night. Maltose is the primary end-product of starch degradation in Arabidopsis leaves, and plants unable to metabolize maltose display a starch excess phenotype (Chia et al., 2004; Lu and Sharkey, 2004).
In essence, the present data indicate that starch mobilization is operating down to the level of maltose generation in sps double mutants, but the subsequent metabolization of its breakdown products is presumably impaired. The fact that Suc exudation of petioles harvested from spsa1/spsc double knock-out plants is strongly reduced in the dark argues for a limitation in Suc biosynthetic capacity also during phases of starch mobilization, which then leads to a feedback inhibition of starch breakdown. A similar observation has been made in transgenic tobacco plants silenced for NtSPSC expression, which is the major SPS isoform involved in nocturnal Suc biosynthesis. As a result of a reduced starch breakdown during the dark period, these plants also accumulate high amounts of starch in their leaves, a phenomenon that was not observed upon silencing of the SPS A isoform (Chen et al., 2005a ). The results obtained from Arabidopsis suggest that in this species no such strict functional separation in nocturnal Suc synthesis between the SPSA1 and C isoform exists, but rather both enzymes act jointly in providing a steady supply of Suc during the night.
To summarize, it could be shown that all SPS isoforms in Arabidopsis possess catalytic activity and, in accordance with a previous study (Sun et al., 2011), AtSPSA1 probably represents the major leaf SPS isoform contributing almost half of the overall enzyme activity. Under normal growth conditions, AtSPSA1 and AtSPSC have partially overlapping functions in leaf carbohydrate metabolism but also display some functional specialization. Loss of SPSA1 leads to slightly but significantly elevated starch levels while loss of SPSC reduces Suc content in leaves at the end of the light period. A loss of both isoforms limits Suc synthetic capacity during the day as well as during the dark period, and leads to starch accumulation in leaves. Taken together, the data suggest that this is a result of a reduced starch mobilization during the night rather than a shift in carbon partitioning.
Future studies will need to reveal the structural differences underlying the observed biochemical differences of the recombinant SPS proteins as well as the functional analysis of AtSPSA2 and AtSPSB in planta.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Rosette diameter as a proxy for plant growth of Arabidopsis SPS mutant plants as compared with the Col-0 wild type.
Figure S2. Analysis of an independent set of Arabidopsis spsa1 and spsc T-DNA alleles.
Table S1. Oligonucleotides used in this study.
Acknowledgements
We are grateful to the Arabidopsis Biological Resource Center for providing Arabidopsis mutant seeds. No conflict of interest is declared. This work was supported by a grant from the Deutsche Forschungsgemeinschaft to FB [grant no. BO 1916/2-1].
References
- Arabidopsis Genome Iniative. 2000. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408, 796–815. [DOI] [PubMed] [Google Scholar]
- Arsova B, Hoja U, Wimmelbacher M, Greiner E, Üstün S, Melzer M, Petersen K, Lein W, Börnke F. 2010. Plastidial thioredoxin z interacts with two fructokinase-like proteins in a thiol-dependent manner: evidence for an essential role in chloroplast development in Arabidopsis and Nicotiana benthamiana. The Plant Cell 22, 1498–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter CJ, Foyer CH, Turner J, Rolfe SA, Quick WP. 2003. Elevated sucrose-phosphate synthase activity in transgenic tobacco sustains photosynthesis in older leaves and alters development. Journal of Experimental Botany 54, 1813–1820. [DOI] [PubMed] [Google Scholar]
- Castleden CK, Aoki N, Gillespie VJ, MacRae EA, Quick WP, Buchner P, Foyer CH, Furbank RT, Lunn JE. 2004. Evolution and function of the sucrose-phosphate synthase gene families in wheat and other grasses. Plant Physiology 135, 1753–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Hajirezaei M, Börnke F. 2005. a Differential expression of sucrose-phosphate synthase isoenzymes in tobacco reflects their functional specialization during dark-governed starch mobilization in source leaves. Plant Physiology 139, 1163–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Hajirezaei M, Peisker M, Tschiersch H, Sonnewald U, Börnke F. 2005. b Decreased sucrose-6-phosphate phosphatase level in transgenic tobacco inhibits photosynthesis, alters carbohydrate partitioning, and reduces growth. Planta 221, 479–492. [DOI] [PubMed] [Google Scholar]
- Chia T, Thorneycroft D, Chapple A, Messerli G, Chen J, Zeeman SC, Smith SM, Smith AM. 2004. A cytosolic glucosyltransferase is required for conversion of starch to sucrose in Arabidopsis leaves at night. The Plant Journal 37, 853–863. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method forAgrobacterium-mediated transformation of Arabidopsis thaliana . The Plant Journal 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Krause HP, Hill LM, Reimholz R, MacRae E, Quick P, Sonnewald U, Stitt M. 1995. The regulation of sucrose synthesis in the leaves and tubers of potato plants. In: Pontis HG, Salerno GL, Echeverria E, eds. Sucrose metabolism, biochemistry, physiology and molecular biology , Vol. 14 Rockville, MD: American Society of Plant Physiologists, 14–24. [Google Scholar]
- Gibon Y, Blaesing OE, Hannemann J, Carillo P, Hohne M, Hendriks JHM, Palacios N, Cross J, Selbig J, Stitt M. 2004. A robot-based platform to measure multiple enzyme activities in Arabidopsis using a set of cycling assays: comparison of changes of enzyme activities and transcript levels during diurnal cycles and in prolonged darkness. The Plant Cell 16, 3304–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz RD, Schiestl RH, Willems AR, Woods RA. 1995. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast 11, 355–360. [DOI] [PubMed] [Google Scholar]
- Harmer SL, Hogenesch JB, Straume M, Chang H-S, Han B, Zhu T, Wang X, Kreps JA, Kay SA. 2000. Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science 290, 2110–2113. [DOI] [PubMed] [Google Scholar]
- Horst RJ, Doehlemann G, Wahl R, Hofmann J, Schmiedl A, Kahmann R, Kämper J, Sonnewald U, Voll LM. 2010. Ustilago maydis infection strongly alters organic nitrogen allocation in maize and stimulates productivity of systemic source leaves. Plant Physiology 152, 293–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber SC, Huber JL. 1996. Role and regulation of sucrose-phosphate synthase in higher plants. Annual Review of Plant Physiology and Plant Molecular Biology 47, 431–444. [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. 1987. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO Journal 6, 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karve A, Rauh BL, Xia X, Kandasamy M, Meagher RB, Sheen J, Moore BD. 2008. Expression and evolutionary features of the hexokinase gene family in Arabidopsis. Planta 228, 411–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenkämper G, Fung RWM, Newcomb RD, Atkinson RG, Gardner RC, MacRae EA. 2002. Sucrose phosphate synthase genes in plants belong to three different families. Journal of Molecular Evolution 54, 322–332. [DOI] [PubMed] [Google Scholar]
- Lee SK, Jeon JS, Börnke F, et al. 2008. Loss of cytosolic fructose-1,6-bisphosphatase limits photosynthetic sucrose synthesis and causes severe growth retardations in rice (Oryza sativa). Plant, Cell and Environment 31, 1851–1863. [DOI] [PubMed] [Google Scholar]
- Lehmann U, Wienkoop S, Tschoep H, Weckwerth W. 2008. If the antibody fails—a mass Western approach. The Plant Journal 55, 1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logemann J, Schell J, Willmitzer L. 1987. Improved method for the isolation of RNA from plant tissues. Analytical Biochemistry 163, 16–20. [DOI] [PubMed] [Google Scholar]
- Lu Y, Sharkey TD. 2004. The role of amylomaltase in maltose metabolism in the cytosol of photosynthetic cells. Planta 218, 466–473. [DOI] [PubMed] [Google Scholar]
- Lunn JE, MacRae E. 2003. New complexities in the synthesis of sucrose. Current Opinion in Plant Biology 6, 208–214. [DOI] [PubMed] [Google Scholar]
- Lutfiyya LL, Xu N, D’Ordine RL, Morrell JA, Miller PW, Duff SM. 2007. Phylogenetic and expression analysis of sucrose phosphate synthase isozymes in plants. Journal of Plant Physiology 164, 923–933. [DOI] [PubMed] [Google Scholar]
- Niedzwiedz-Siegien I, Bogatek-Leszczynska R, Come D, Corbineau F. 2004. Effects of drying rate on dehydration sensitivity of excised wheat seedling shoots as related to sucrose metabolism and antioxidant enzyme activities. Plant Science 167, 879–888. [Google Scholar]
- Nielsen TH, Huber SC. 1989. Unusual regulatory properties of sucrose-phosphate synthase purified from soybean (Glycine max) leaves. Physiologia Plantarum 76, 309–314. [Google Scholar]
- Okamura M, Aoki N, Hirose T, Yonekura M, Ohto C, Ohsugi R. 2011. Tissue specificity and diurnal change in gene expression of the sucrose phosphate synthase gene family in rice. Plant Science 181, 159–166. [DOI] [PubMed] [Google Scholar]
- Privat I, Foucrier S, Prins A, et al. 2008. Differential regulation of grain sucrose accumulation and metabolism in Coffea arabica (Arabica) and Coffea canephora (Robusta) revealed through gene expression and enzyme activity analysis. New Phytologist 178, 781–797. [DOI] [PubMed] [Google Scholar]
- Rolland F, Baena-Gonzalez E, Sheen J. 2006. Sugar sensing and signaling in plants: conserved and novel mechanisms. Annual Review of Plant Biology 57, 675–709. [DOI] [PubMed] [Google Scholar]
- Roy Choudhury S, Roy S, Das R, Sengupta D. 2008. Differential transcriptional regulation of banana sucrose phosphate synthase gene in response to ethylene, auxin, wounding, low temperature and different photoperiods during fruit ripening and functional analysis of banana SPS gene promoter. Planta 229, 207–223. [DOI] [PubMed] [Google Scholar]
- Salerno GL, Pagnussat GC, Pontis HG. 1998. Studies on sucrose-phosphate synthase from rice leaves. Cellular and Molecular Biology 44, 407–416. [PubMed] [Google Scholar]
- Schneider S, Beyhl D, Hedrich R, Sauer N. 2008. Functional and physiological characterization of Arabidopsis INOSITOL TRANSPORTER1, a novel tonoplast-localized transporter for myo-inositol. The Plant Cell 20, 1073–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnewald U, Quick WP, Macrae E, Krause KP, Stitt M. 1993. Purification, cloning and expression of spinach leaf sucrose-phosphate synthase in Escherichia coli . Planta 189, 174–181. [DOI] [PubMed] [Google Scholar]
- Stitt M, Huber SC, Kerr PS. 1987. Control of photosynthetic sucrose formation. In: Hatch MD, Boardman NK, eds. The biochemistry of plants . New York: Academic Press, 327–409. [Google Scholar]
- Stitt M, Lilley RMC, Gerhardt R, Heldt HW. 1989. Determination of metabolite levels in specific cells and subcellular compartments of plant leaves. Methods in Enzymology 174, 518–552. [Google Scholar]
- Stitt M, Lunn J, Usadel B. 2010. Arabidopsis and primary photosynthetic metabolism—more than the icing on the cake. The Plant Journal 61, 1067–1091. [DOI] [PubMed] [Google Scholar]
- Strand A, Foyer CH, Gustafsson P, Gardestrom P, Hurry V. 2003. Altering flux through the sucrose biosynthesis pathway in transgenic Arabidopsis thaliana modifies photosynthetic acclimation at low temperatures and the development of freezing tolerance. Plant, Cell and Environment 26, 523–535. [Google Scholar]
- Strand A, Zrenner R, Trevanion S, Stitt M, Gustafsson P, Gardestrom P. 2000. Decreased expression of two key enzymes in the sucrose biosynthesis pathway, cytosolic fructose-1,6-bisphosphatase and sucrose phosphate synthase, has remarkably different consequences for photosynthetic carbon metabolism in transgenic Arabidopsis thaliana . The Plant Journal 23, 759–770. [DOI] [PubMed] [Google Scholar]
- Sun J, Zhang J, Larue CT, Huber SC. 2011. Decrease in leaf sucrose synthesis leads to increased leaf starch turnover and decreased RuBP regeneration-limited photosynthesis but not Rubisco-limited photosynthesis in Arabidopsis null mutants of SPSA1. Plant, Cell and Environment 34, 592–604. [DOI] [PubMed] [Google Scholar]
- Vandesteene L, Ramon M, Le Roy K, Van Dijck P, Rolland F. 2010. A single active trehalose-6-P synthase (TPS) and a family of putative regulatory TPS-like proteins in Arabidopsis . Molecular Plant 3, 406–419. [DOI] [PubMed] [Google Scholar]
- Wind J, Smeekens S, Hanson J. 2010. Sucrose: metabolite and signaling molecule. Phytochemistry 71, 1610–1614. [DOI] [PubMed] [Google Scholar]
- Winter H, Huber SC. 2000. Regulation of sucrose metabolism in higher plants: localization and regulation of activity of key enzymes. Critical Reviews in Plant Science 19, 31–67. [DOI] [PubMed] [Google Scholar]
- Worrell AC, Bruneau JM, Summerfelt K, Boersig M, Voelker TA. 1991. Expression of a maize sucrose phosphate synthase in tomato alters leaf carbohydrate partitioning. The Plant Cell 3, 1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Zhang J, Wang Z, Zhu Q. 2001. Activities of starch hydrolytic enzymes and sucrose-phosphate synthase in the stems of rice subjected to water stress during grain filling. Journal of Experimental Botany 52, 2169–2179. [DOI] [PubMed] [Google Scholar]
- Zeeman SC, ap Rees T. 1999. Changes in carbohydrate metabolism and assimilate partitioning in starch-excess mutants of Arabidopsis . Plant, Cell and Environment 22, 1445–1453. [Google Scholar]
- Zeeman SC, Smith SM, Smith AM. 2007. The diurnal metabolism of leaf starch. Biochemical Journal 401, 13–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.