Abstract
Background
Australian aboriginal people living in remote regions have extraordinary higher rates of mortality compared with other Australian ethnicities. Albuminuria marks the underlying renal disease. This study assessed the predictive value of albuminuria for nonrenal and renal deaths in a remote Australian aboriginal community over a follow-up period of >14 years.
Methods
From 1992 to 1997, 85% of community members participated in a health screen, which included measurement of urine albumin/creatinine (ACR) levels. Deaths and dialysis initiations were recorded until 30 November 2010. The rates of natural nonrenal and renal deaths were assessed over a mean of 14 years in the 956 participants aged 18 years and over at baseline, and mortality associated with baseline levels of albuminuria (ACR ≥ 2.7 mg/mmol) was estimated.
Results
There were 203 natural deaths; 70 were renal deaths and 133 were nonrenal deaths, including 60 cardiovascular disease (CVD) deaths. Higher baseline ACR predicted all categories of natural death, with no apparent lower threshold for effect. Baseline ACR ≥ 2.7 mg/mmol predicted a 3.3-fold increase in all natural deaths, a 2-fold increase in nonrenal deaths and a 1.7-fold increase in CVD deaths, after adjustment for other factors. Eighty-nine percent (62 out of 70) of renal deaths occurred in those with ACR ≥ 34 at baseline, with a 24-fold increase in risk. Albuminuria (ACR ≥ 2.7 mg/mmol) contributed to 66% of risk for all natural deaths over the interval.
Conclusions
Albuminuria was still a remarkable predictor for all-cause natural death over an average of 14 years follow-up interval in this aboriginal community.
Keywords: aboriginal people, ACR, albuminuria, nonrenal death, renal death
Introduction
Australian aboriginal people, especially those living in remote regions, have higher rates of all-cause mortality, cardiovascular deaths and end-stage renal disease (ESRD) [1, 2], compared with other Australian ethnicities. The disparities are greater for younger age groups. Adult Aborigines in the Northern Territory have mortality rates two to four times higher than non-aboriginal Australians, while the death rates of 25- to 44-year-olds are increased ∼15-fold [3].
The incidence of ESRD among Aborigines living in remote regions is increased up to 30 times the national incidence for all Australians [4, 5]. The high number of renal deaths and nonrenal deaths for aboriginal people is by no means explained solely by diabetes and its comorbidities [6, 7]. Albuminuria marks the underlying renal disease. In a study of one high-risk community, we showed that albuminuria, which was pervasive, not only marked all the future risk of renal deaths, but also predicted cardiovascular and nonrenal noncardiovascular deaths [8–10]. In that study, 825 adults, of a mean age of 33.6 years, were followed for a total of 4778 person-years and average of 5.8 years; they experienced 64 natural death end-points: of which 16 were renal and 36 were cardiovascular. Albuminuria [albumin/creatinine (ACR) ≥3.4 mg/mmol] was estimated to contribute to all the renal deaths and 75% of the risk for nonrenal natural deaths over that interval [1]. Studies in other populations and ethnic groups have also documented the association of albuminuria with nonrenal death particularly cardiovascular disease (CVD) mortality as well as with renal disease and renal death [6, 10, 11]. In one study of people aged ≥55 years, the exacerbation of risk for CVD started at a very low level of ACR [12]. A collaborative meta-analysis indicated that ACR ≥1.1 mg/mmol is an independent predictor of mortality risk in the general population [13].
A screening and treatment program for renal disease and hypertension was started in this community in November 1995 and was conducted with some vigor through mid-1999. It was associated with significant reduction of mortality in treated persons compared with historical controls matched for disease severity [8]. With a change in health service administration, the intervention faltered for a time, but in recent years has been taken up again, with protocols of systematic surveillance and treatment now embedded within primary care, although resources limit their optimal application. In a repeat community screen from 2004 to 2006, 30.4% of adults overall, of which 52% were aged ≥50 years, had prescriptions for antihypertensive agents (usually perindopril) and 18.1% overall, of which 34% were aged ≥50 years, had prescriptions for hypoglycemic agents [14]. This was the first aboriginal community in which the effects of angiotensin-converting enzyme inhibition in delaying renal and nonrenal deaths in people with albuminuria and hypertension were demonstrated [8, 15]. This has subsequently become a standard practice in all remote aboriginal health services, and has contributed to the reduction in indigenous death rates in the Northern Territory [16] and nationwide [5, 16].
We now describe terminal events in this cohort through a follow-up period of over 14 years in order to define whether albuminuria remains a significant predictor of mortality in the long-term, examine possible relationships with ACR over a continuum and probe for the predictive value of ACR below the traditional cutoff point for microalbuminuria.
Materials and methods
Subjects were adults in a remote aboriginal community who participated in the baseline health survey between 1992 and 1997 at ages ranging from 18 to 76 years. The profiles of this community have been described previously [6]. Minimum data for inclusion of 956 subjects were age, sex and urine ACR. The sample size was somewhat smaller when covariates such as weight, height, blood pressure, lipoprotein and blood glucose were included in analyses.
All natural deaths were documented until 30 November 2010, as recorded in community and hospital records. All death files were confirmed in the local Catholic Church Burial Records. Natural deaths were categorized by their primary or underlying cause into renal, nonrenal natural death and cardiovascular death, as described previously [17]. Renal death refers to participants who started dialysis for ESRD or who died with terminal renal failure without dialysis. The survival time of people who died of non-natural deaths, which included acute intoxications, accidents, drowning, suicide and homicide, was documented: they were censored from the analysis at time of death, and their deaths were not included in this analysis.
Height, weight, blood pressure and levels of glycemia were measured using standard procedures.
Some diagnostic criteria used in this study were as follows:
Diabetes: known to be diabetic before the survey and/or with fasting glucose ≥7.0 mmol/L (≥126.1 mg/dL) or 2-h glucose or random glucose level ≥11.1 mmol/L (200 mg/dL) at testing;
Obesity: body mass index ≥ 30 kg/m2;
Hypertension: systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg.
Urine albumin concentrations were measured simultaneously by both nephelometric and high-performance liquid chromatography techniques on baseline random urine samples after retrieval from −70°C storage. When examined over a Z-score continuum of their values, they were identical in defining clinical profiles and predicting death [17]. The results here are based on ACR levels derived from albumin measured by immunonephelometry (Beckman Instruments, Brea, CA, USA), and from urinary creatinine concentration measured using the modified kinetic Jaffe reaction (Olympus AU600 Autoanalyzer; inter-assay CV 2%). As the median value of ACR of this cohort was 2.7 mg/mmol, which is lower than the conventional microalbuminuria cutoff point, albuminuria was arbitrarily defined as urine ACR ≥2.7 mg/mmol in this study, while the conventional threshold of ACR ≥34 mg/mol was defined as overt proteinuria.
Quartiles of ACR level and log-transformed (log base 2) ACR were applied in the analyses. Baseline characteristics across progressive quartiles of ACR were compared. Cox regression analyses were employed to calculate hazard ratios (HRs, 95% CI) of all natural deaths, renal, nonrenal and CVD deaths predicted by ACR, and to allow adjustment for age, sex and other covariates. The Kaplan–Meier method was used to estimate fractional survivals according to different baseline ACR levels. The population attributable fraction (PAF) of mortality predicted by the presence of ACR ≥ 2.7 (mg/mmol) at baseline was calculated using Poisson regression adjusted for age, sex and time of follow-up year. All analyses were undertaken using Stata 11.1 (Stata Corp. Stata Statistical Software: Release 11.1, College Station. TX: StataCorp LP, 2009). Statistical significance was defined at the level of P < 0.05 (two-tailed).
This study was approved by the Ethics Committee of the Menzies School of Health Research and Territory Health Services and The Behavioral and Social Science Ethical Review Committee of the University of Queensland.
Results
Nine hundred and fifty-six participants were followed for a total of 13 714 person-years, range: 0–18 years, median: 16 years and mean: 14 years. ACR ranged from 0–675.5 (mg/mmol) with a median value of 2.7 (mg/mmol). Their characteristics by baseline quartiles of ACR are shown in Table 1. Age was progressively higher with higher ACR quartiles, and levels of cholesterol and triglycerides, and the percentage with obesity, hypertension and diabetes was also higher, either across quartiles on a continuum or with levels above the ACR median. Those in the highest quartile were more often female.
Table 1.
Characteristics of participants aged 18 years and over with ACR quartiles
| ACR Quartile 1 | ACR Quartile 2 | ACR Quartile 3 | ACR Quartile 4 | ||
|---|---|---|---|---|---|
| Characteristics | (ACR range 0–0.6) | (ACR range 0.7–2.7) | (ACR range 2.7–19.5) | (ACR range 19.6–675.5) | P value |
| Female % | 40.8 | 50.6 | 46.8 | 59.2 | 0.001 |
| Age (year) | 30.2 (10.4) | 32.3 (11.5) | 35.0 (11.8) | 42.0 (12.1) | <0.001 |
| Total cholesterol (mmol/L) | 4.37 (1.01) | 4.51 (1.01) | 4.71 (1.05) | 5.21 (1.28) | <0.001 |
| High density lipoprotein (HDL) cholesterol (mmol/L) | 1.15 (0.33) | 1.14 (0.28) | 1.10 (0.25) | 1.06 (0.23) | 0.0064 |
| Triglyceride (mmol/L) | 1.64 (1.29) | 1.84 (1.25) | 2.14 (1.24) | 3.12 (2.68) | <0.001 |
| Obesity% | 8.4 (4.9–12.0) | 7.6 (4.2–11.0) | 16.2 (11.4–20.9) | 24.6 (19.0–30.1) | <0.001 |
| Hypertension% | 13.5 (9.1–17.9) | 14.7 (10.1–19.3) | 23.9 (18.4–29.4) | 41.7 (35.4–48.1) | <0.001 |
| Diabetes % | 2.5 (0.5–4.5) | 7.1 (3.8–10.3) | 10.6 (6.7–14.5) | 31.8 (25.9–37.7) | <0.001 |
Data were mean (SD) for continuous variables and percentage (95% CI) for categorical variables.
A total of 203 natural deaths were documented, while 37 people died of misadventure (non-natural deaths). The natural deaths included 70 renal deaths and 133 nonrenal deaths of which 60 were CVD deaths. Table 2 shows deaths by gender: numbers and rates of renal deaths were more common in females, but there was no gender difference in other categories of death.
Table 2.
Natural death by sex for Tiwi people aged 18 years and over
| Men | Women | All | P value | |
|---|---|---|---|---|
| Number of participants | 485 | 471 | 956 | |
| Follow-up years | 6930 | 6785 | 13 714 | |
| All natural deaths | ||||
| Number | 100 | 103 | 203 | |
| Incidence | 14.4 (11.8–17.5) | 15.2 (12.4–18.4) | 14.8 (12.8–17.0) | 0.7159 |
| Renal death | ||||
| Number | 26 | 44 | 70 | |
| Incidence | 3.8 (2.5–5.5) | 6.5 (4.7–8.7) | 5.1 (4.0–6.4) | 0.0247 |
| Nonrenal death | ||||
| Number | 74 | 59 | 133 | |
| Incidence | 10.7 (8.4–13.4) | 8.7 (6.6–11.2) | 9.7 (8.1–11.5) | 0.2362 |
| CVD death | ||||
| Number | 35 | 25 | 60 | |
| Incidence | 5.1 (3.5–7.0) | 3.7 (2.4–5.4) | 4.4 (3.3–5.6) | 0.2256 |
The P value refers to comparison between men and women; incidence refers to death per 1000 person-years (95% CI).
Table 3 shows the numbers and rates of natural death by quartile of baseline ACR level. The incidence of all natural deaths, nonrenal and CVD deaths rose significantly with increasing ACR quartiles. However, all renal deaths were confined to people in Quartiles 3 and 4 of baseline ACR levels, i.e. ACR ≥ 2.7 mg/mmol. Figure 1A–C shows the Kaplan–Meier survival curves for all natural deaths, renal and nonrenal deaths by baseline ACR quartiles. After 14 years, only 51.4% of people in the highest baseline ACR quartile had avoided all natural deaths: 70.3% had avoided renal deaths and 73.1% had avoided nonrenal deaths. The survival curve of renal death for people within the category of the highest ACR quartile was significantly different from people in lower ACR quartile, as was for nonrenal death.
Table 3.
Natural death by ACR quartile for Tiwi people aged 18 years and over
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P trend | |
|---|---|---|---|---|---|
| ACR range (mg/mmol) | 0–0.6 | 0.7–2.7 | 2.72–19.6 | 20.2–675.5 | |
| Number of participants | 240 | 241 | 236 | 239 | |
| Follow-up years | 3766 | 3736 | 3465 | 2749 | |
| All natural deaths | |||||
| Number | 11 | 25 | 42 | 125 | |
| Incidence | 2.9 (1.5–5.2) | 6.7 (4.3–9.9) | 12.1 (8.7–16.3) | 45.5 (38.0–53.9) | <0.001 |
| Renal death | |||||
| Number | 0 | 0 | 5 | 65 | |
| Incidence | 1.4 (0.5–3.4) | 23.6 (18.3–33.3) | <0.001 | ||
| Nonrenal death | |||||
| Number | 11 | 25 | 37 | 60 | |
| Incidence | 2.9 (1.5–5.2) | 6.7 (4.3–9.9) | 10.7 (7.5–14.7) | 21.8 (16.7–28.1) | <0.001 |
| CVD death | |||||
| Number | 4 | 15 | 11 | 30 | |
| Incidence | 1.1 (0.3–2.7) | 4.0 (2.2–6.6) | 3.2 (1.6–5.7) | 10.9 (7.4–15.5) | <0.001 |
Incidence per 1000 person-years (95% CI).
Fig. 1.
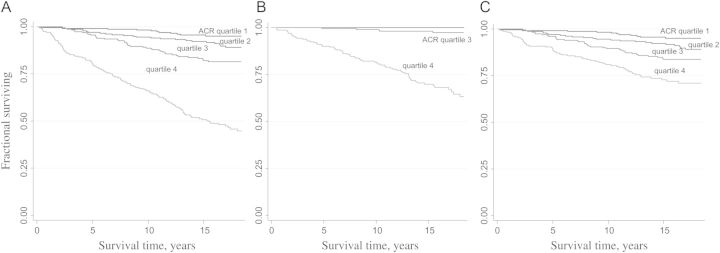
(A) Survival of all natural deaths by baseline ACR quartiles. (B) Survival of renal death by baseline ACR quartiles. (C) Survival of nonrenal death by baseline ACR quartiles.
Table 4 lists HRs (95% CI) of different endpoints according to quartiles of baseline ACR and log-transformed (Base 2) ACR. The risks of all natural deaths and non-renal deaths rose with increasing ACR quartiles, and with each doubling of ACR, with similar levels of risk exacerbation for females and males. For example, the HRs (95%CI) of log-transformed (Base 2) ACR for all natural deaths were 1.4 (1.3–1.6) for females and 1.3 (1.1–1.4) for males; and the HRs for nonrenal deaths were 1.2 (1.0–1.3) for females and 1.1 (1.0–1.3) for males. Compared with those with the first ACR quartile, the risks of CVD death were significantly and comparably raised in those with ACR in the second and third quartiles, and strikingly elevated in those in the highest ACR quartiles. The trends persisted with adjustment for age, sex and other factors, including blood pressures and glucose levels (Table 4).
Table 4.
HRs (95% CI) of outcomes by ACR quartile and log-transformed (Base 2) ACR
| ACR quartiles |
||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | P trend | Log 2ACR | |
| ACR range (mg/mmol) | 0–0.6 | 0.7–2.7 | 2.72–19.6 | 20.2–675.5 | 0–675.5 | |
| All natural deaths | ||||||
| Crude HR (95% CI) | 1.0 | 2.3 (1.1–4.7) | 4.2 (2.1–8.1) | 15.7 (8.5–29.1) | <0.001 | 1.4 (1.4–1.5) |
| HR1 (95% CI) | 1.0 | 2.0 (1.0–4.2) | 3.3 (1.7–6.5) | 9.5 (5.1–17.6) | <0.001 | 1.3 (1.3–1.4) |
| HR2 (95% CI) | 1.0 | 1.5 (0.7–3.3) | 2.6 (1.3–5.4) | 6.2 (3.1–12.4) | <0.001 | 1.3 (1.2–1.4) |
| HR3 (95% CI) | 1.0 | 1.7 (0.7–3.9). | 3.2 (1.5–7.0) | 7.8 (3.7–16.6) | <0.001 | 1.4 (1.3–1.5) |
| HR4 (95% CI) | 1.0 | 1.4 (0.6–3.0) | 2.5 (1.2–5.1) | 5.9 (3.0–11.9) | <0.001 | 1.3 (1.2–1.4) |
| HR5 (95% CI) | 1.0 | 1.0 (0.4–2.5) | 2.2 (1.0–4.7) | 5.1 (2.4–10.6) | <0.001 | 1.3 (1.2–1.4) |
| Renal deatha | ||||||
| Crude HR (95% CI) | 2.4 (2.0–2.8) | |||||
| HR1 (95% CI) | 2.4 (2.0–2.8) | |||||
| HR2 (95% CI) | 2.7 (2.1–3.4) | |||||
| HR3 (95% CI) | 2.8 (2.3–3.6) | |||||
| HR4 (95% CI) | 2.7 (2.1–3.4) | |||||
| HR5 (95% CI) | 3.0 (2.2–4.0) | |||||
| Non-renal death | ||||||
| Crude HR(95% CI) | 1.0 | 2.3 (1.1–4.7) | 3.7 (1.9–7.2) | 7.5 (3.9–14.2) | <0.001 | 1.2 (1.2–1.3) |
| HR1 (95% CI) | 1.0 | 2.0 (1.0–4.1) | 2.8 (1.4–5.5) | 4.0 (2.1–7.8) | <0.001 | 1.1 (1.1–1.2) |
| HR2 (95% CI) | 1.0 | 1.5 (0.7–3.2) | 2.3 (1.1–4.7) | 2.9 (1.4–6.1) | 0.001 | 1.1 (1.0–1.2) |
| HR3 (95% CI) | 1.0 | 1.6 (0.7–3.8) | 2.7 (1.2–6.0) | 3.3 (1.5–7.4) | 0.001 | 1.1 (1.1–1.3) |
| HR4 (95% CI) | 1.0 | 1.2 (0.5–2.7) | 1.9 (0.9–3.9) | 2.4 (1.1–5.2) | 0.006 | 1.1 (1.0–1.2) |
| HR5 (95% CI) | 1.0 | 1.1 (0.5–2.9) | 1.9 (0.9–4.2) | 2.2 (1.0–4.9) | 0.023 | 1.1 (1.0–2.2) |
| CVD death | ||||||
| Crude HR (95% CI) | 1.0 | 3.8 (1.3–11.4) | 3.0 (1.0–9.4) | 10.1 (3.5–28.7) | <0.001 | 1.2 (1.1–1.4) |
| HR1 (95% CI) | 1.0 | 3.5 (1.1–10.4) | 2.4 (0.8–7.6) | 6.1 (2.1–17.7) | 0.001 | 1.2 (1.1–1.3) |
| HR2 (95% CI) | 1.0 | 2.5 (0.8–8.0) | 1.9 (0.6–6.0) | 3.7 (1.2–11.5) | 0.033 | 1.1 (1.0–1.3) |
| HR3 (95% CI) | 1.0 | 3.4 (0.9–12.5) | 2.6 (0.7–9.7) | 4.8 (1.3–17.0) | 0.023 | 1.1 (1.0–1.3) |
| HR4 (95% CI) | 1.0 | 2.2 (0.7–7.0) | 1.6 (0.5–5.1) | 3.2 (10–10.0) | 0.067 | 1.1 (1.0–1.3) |
| HR5 (95% CI) | 1.0 | 2.1 (0.6–7.2) | 1.5 (0.5–5.1) | 2.7 (0.8–8.6) | 0.135 | 1.1 (1.0–1.3) |
aThe HR of renal death by log-transformed ACR; HR 1: adjusted for sex and age; HR 2: adjusted for sex, age, diabetes, hypertension, obesity, total cholesterol, HDL and triglycerides; HR 3: adjusted for sex, age, systolic blood pressure, plasma glucose, obesity, total cholesterol, HDL and triglycerides; HR 4: adjusted for sex, age, diabetes, hypertension, obesity, smoker, total cholesterol, HDL and triglycerides; HR 5: adjusted for sex, age, diabetes, hypertension, obesity, CRP, total cholesterol, HDL and triglycerides.
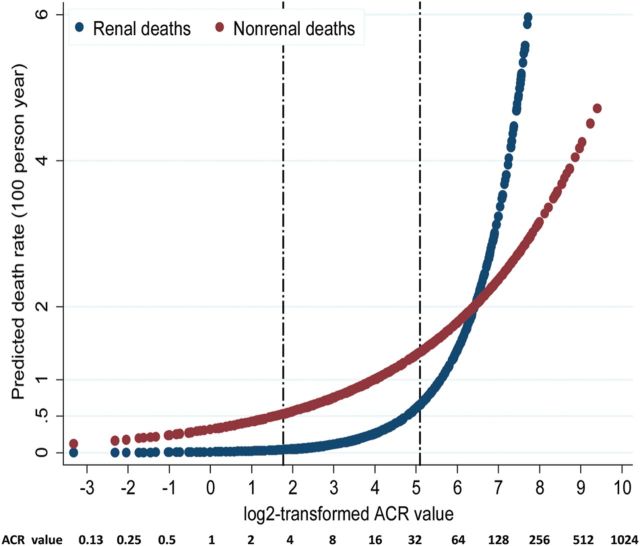
Figure 2 shows predicted renal and nonrenal deaths by log-transformed (Base 2) ACR for people aged ≥18 years. The statistical significance between the two curves has been calculated by comparing their relative operating characteristic (ROC) curves. It indicated that the ROC curve for renal death predicted by ACR is significantly greater than for nonrenal death. The rate of increase of predictions of nonrenal death rate speeds up at ACR level <3.4 mg/mmol, while the threshold for renal death was at a higher ACR level (Figure 2). Table 5 shows that those with an ACR ≥ 2.7 mg/mmol (median) are predicted to have a 3.3-fold increased risk of all natural deaths, and 2.2-fold and 1.9-fold increased risk of nonrenal and CVD deaths, respectively, compared with those with an ACR < 2.7 mg/mmol after adjusting for sex and age. As no renal deaths occurred in those with ACR < 2.7 mg/mmol, the HR of ACR ≥ 2.7 versus ACR < 2.7 could not be calculated (Table 5). However, 89% (62 out of 70) of renal deaths occurred in those with ACR ≥ 34 at baseline, with a HR (95% CI) of 23.8 (8.0–71.2) for females and 23.7 (4.1–135.0) for males after adjustment for a number of risk factors. It also shows that the PAF of natural deaths associated with ACR levels at or above the median value of 2.7 mg/mmol was 66% for all natural deaths, 45% for nonrenal deaths and 34% for CVD death (Table 5).
Fig. 2.
Predicted renal and nonrenal death rates (100 person-years) by ACR. Note: the two etched lines stand for actual ACR values equal to 3.4 and 34 mg/mmol, respectively.
Table 5.
Risk of mortality by ACR ≥ 2.7 and PAF for Tiwi people aged 18 years and over
| ACR < 2.7 |
ACR ≥ 2.7 |
||||||
|---|---|---|---|---|---|---|---|
| events | HR | Events | Crude HR (95% CI) | HR1 (95% CI) | HR 2 (95% CI) | PAF (95%CI)b | |
| All natural deaths | 34 | 1.0 | 169 | 5.9 (4.1–8.5) | 4.3 (2.9–6.2) | 3.3 (2.1–5.2) | 0.66 (0.53–0.75) |
| Renal deatha | 0 | 1.0 | 70 | See notes | See notes | See notes | 1.00 |
| Nonrenal death | 34 | 1.0 | 99 | 3.4 (2.3–5.1) | 2.4 (1.6–3.5) | 2.1 (1.3–3.4) | 0.45 (0.26–0.60) |
| CVD death | 18 | 1.0 | 42 | 2.7 (1.6–4.7) | 2.0 (1.1–3.4) | 1.7 (0.8–3.3) | 0.34 (0.01–0.57) |
HR1 adjusted for sex and age; HR 2 adjusted for sex, age, diabetes, hypertension, obesity, total cholesterol, high density lipoprotein and triglycerides.
aHR of renal death for ACR ≥ 2.7 versus <2.7 could not be calculated as there were no events in the reference group.
bPAF of death predicted by ACR ≥ 2.7 mg/mmol and adjusted for age, sex and time of follow-up.
When analyzed around the ‘traditional’ microalbuminuria cutoff of ACR ≥ 3.4 mg/mmol, the PAFs were 64% for all natural deaths, 41% for nonrenal death and 32% for CVD death. These compare with those of 84%, 65% and 75% for the same categories of deaths calculated by Hoy et al. in the first 5 years of the observation in people before systematic treatment was initiated [6].
Discussion
In this aboriginal community, albuminuria (ACR ≥ 2.7 mg/mmol) predicted nonrenal death, while overt proteinuria (ACR ≥ 34 mg/mmol) predicted renal death over an average 14 years follow-up (median follow-up of 16 years). This confirms our previous observation of the predictive value of albuminuria for natural death over a much shorter interval in people who were followed without or before the introduction of systematic treatment [8]. The relationship still applies, over almost 3-fold increase in average duration of follow-up, almost three times the number of person-years follow-up, and >3 times the number of deaths. Among these deaths, the number of renal deaths was increased >3-fold, and of CVD deaths more than doubled. Despite advancing age, the mortality of those without pathologic albuminuria at baseline remains small, and that those with the highest baseline levels continue to do poorly even after 10–15 years.
The relationships also remained strong against a changing background of events, which include a fluctuating level of surveillance and treatment, and against a background of falling mortality rates overall. Falling mortality rates, which were first detected after introduction of systematic treatment, are now evident from overall community deaths and census data, and were echoed in remote Australian aboriginal people more generally (report in preparation).
To our knowledge, this is the longest follow-up study of the predictive values of albuminuria over natural death although many population-based studies in this patient group have been published in the last few decades [13, 18–26]. For example, Drury et al. reported that reduced estimated glomerular filtration rate (eGFR) and albuminuria are independent risk factors for CVD events and mortality rate in a 5-year follow-up diabetic cohort [22]. In a 12.9-year follow-up for a cohort of 1113 elderly men, the urinary albumin excretion rate and eGFR increased the risk of CVD death [24].
The PAF calculations suggest that 66% of all natural deaths including 100% of renal deaths and 45% of nonrenal deaths would have been avoided if the baseline ACR levels had been uniformly less than the population median of 2.7 mg/mmol. Alternatively, with all baseline levels <3.4 mg/mmol, the ‘traditional’ microalbuminuria threshold, the avoided proportions would have been 64% for all natural deaths; 100% for renal deaths and 41% for nonrenal deaths, respectively.
The substantial reduction in PAFs of ACR ≥ 3.4 for all natural deaths and nonrenal deaths over the longer follow-up in relation to that in our first report is probably due to culling of people at highest risk of death from the cohort in the early years, as well as mitigation of outcomes due to more systematic treatment of those with disease. This should also have improved the outcomes in people who were ‘normal’ at baseline, who subsequently developed overt albuminuria or hypertension, if they were detected and treated. Despite these considerations, albuminuria remained a powerful predictor of all-cause natural death over a 14-year period. These provide powerful arguments for unflagging efforts to prevent the development of pathologic levels of albuminuria and to reduce its progression.
In our study, the predictions of renal death by baseline ACR levels were incontrovertible, but with a threshold level of baseline ACR that was clearly already abnormal (i.e. ACR ≥ 2.7 mg/mmol). However, it also showed the prediction of nonrenal death by baseline ACR levels starting below the traditional microalbuminuria cutoff (3.4 mg/mmol). This supports the 2001 report of Gerstein et al. in a group of subjects aged ≥55 years, with or without diabetes in the context of cardiovascular risk prediction [12]. The potential explanations for these important associations remain speculative.
In this study, the eGFR (Modification of Diet in Renal Disease formula, without adjustment for race) was also a significant predictor of renal deaths in the presence of other factors, including ACR. However, the predictive value of the terminal outcome by ACR was not significantly affected by adding GFR in the modeling. Previous reports also suggest that the predictive value of microalbuminuria on mortality is independent of eGFR [27], and albuminuria and eGFR were multiplicatively associated with all-cause mortality without evidence for interaction [13, 21, 26].
The rates of renal death in this aboriginal cohort are astounding compared with those in Western populations. Females had a significantly higher renal death rate than males, which is also seen in other remote aboriginal populations [14]. This is consistent with the higher prevalence of albuminuria in females than in males, which in turn might be related to their lower birth weights and higher rates of diabetes, as well as their failure to show usual female-related relative protection from childhood poststreptococcal glomerulonephritis [6, 14, 28, 29].
Strengths of this study are >80% community participation in the baseline screen, the excellent ascertainment of outcomes and the long duration of follow-up. Potential weaknesses include the fact that only one baseline random urine sample was taken and that ACR was not confirmed with a morning urine sample, and the necessary ‘lumping’ of categories of death into mutually exclusive categories by primary or underlying cause, whereas most natural deaths actually had more than one underlying morbidity or contributory condition. However, demonstration of the relationship of albuminuria to the distinct and accurate category of all-cause natural death is clear. Furthermore, the main predictions are unchanged when deaths are grouped by both underlying and associated causes. The changing background of underlying health profiles and of disease management in the follow-up period poses an additional challenge to mechanistic interpretation, but it represents the real world of health service delivery, against which the phenomenon described here remains very powerful. Finally, nearly one-third of the variances for all natural deaths remain unexplained by these simplistic models. We anticipate that these findings will apply to other remote aboriginal communities with similar renal failure and mortality profiles [5]. However, they need to be tested in the context of less remote-living indigenous Australians, who have lower rates of both nonrenal and renal deaths.
Acknowledgements
The authors thank the residents of the participating community, councils, health services and many other people who assisted with field work. Data were collected by the renal research team at the Menzies School of Health Research, Darwin, NT.
Funding. The funding for this study was provided by grants from the National Health & Medical Research Council (NHMRC) of Australia (No. 921134, 951342 and 320860) and from Territory Health Services, WEH's NHMRC Australia Research Fellowship (#511081), Kidney Health Australia, Rio Tinto, the Colonial Foundation of Australia, Janssen Cilag and Amgen. Conflict of interest statement. None declared.
References
- 1.Spencer J, Silva D, Hoy W. An epidemic of renal failure among Australian aboriginal. Med J Aus. 1998;168:537–541. doi: 10.5694/j.1326-5377.1998.tb139080.x. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham J, Condon JR. Premature mortality in aboriginal adults in the Northern Territory. Med J Aus. 1996;165:309–312. doi: 10.5694/j.1326-5377.1996.tb124987.x. [DOI] [PubMed] [Google Scholar]
- 3.Veroni M, Gracey M, Rouse I. Patterns of mortality in Western Australian aboriginals, 1983–89. Int J Epidemiol. 1994;23:73–81. doi: 10.1093/ije/23.1.73. doi:10.1093/ije/23.1.73. [DOI] [PubMed] [Google Scholar]
- 4.Cass A, Cunningham J, Wang Z, et al. Regional variation in the incidence of end-stage renal disease in Indigenous Australians. Med J Aust. 2001;175:24–27. doi: 10.5694/j.1326-5377.2001.tb143507.x. [DOI] [PubMed] [Google Scholar]
- 5.Australian Institute of Health and Welfare. Chronic kidney disease in Aboriginal and Torres Strait islander people 2011. Canberra: AIHW; 2011. Cat. no. PHE 151. [Google Scholar]
- 6.Hoy WE, Wang Z, VanBuynder P, et al. The natural history of renal disease in Australian Aborigines. Part 2 Albuminuria predicts natural death and renal failure. Kidney Int. 2001;60:249–256. doi: 10.1046/j.1523-1755.2001.00793.x. doi:10.1046/j.1523-1755.2001.00793.x. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z, Hoy WE. Cardiovascular risk among urban aboriginal people [Letters] Med J Aus. 2003;179:557. doi: 10.5694/j.1326-5377.2003.tb05688.x. [DOI] [PubMed] [Google Scholar]
- 8.Hoy WE, Wang Z, Baker PR, et al. Reduction in natural death and renal failure from a systematic screening and treatment program in an Australian aboriginal community. Kidney Int. 2003;83(suppl):S66–S73. doi: 10.1046/j.1523-1755.63.s83.14.x. doi:10.1046/j.1523-1755.63.s83.14.x. [DOI] [PubMed] [Google Scholar]
- 9.Hoy W, McDonald SP. Albuminuria: marker or target in indigenous populations. Kidney Int. 2004;92(suppl):S25–S31. doi: 10.1111/j.1523-1755.2004.09207.x. doi:10.1111/j.1523-1755.2004.09207.x. [DOI] [PubMed] [Google Scholar]
- 10.Hoy WE. Renal disease in aboriginal Australians. Med J Aus. 1996;165:126–127. doi: 10.5694/j.1326-5377.1996.tb124882.x. [DOI] [PubMed] [Google Scholar]
- 11.Ryu S, Chang Y, Kim D-I, et al. Gamma-glutamyltransferase as a predictor of chronic kidney disease in nonhypertensive and nondiabetic Korean men. Clin Chem. 2007;53:71–77. doi: 10.1373/clinchem.2006.078980. doi:10.1373/clinchem.2006.078980. [DOI] [PubMed] [Google Scholar]
- 12.Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. doi: 10.1001/jama.286.4.421. doi:10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 13.Chronic Kidney Disease Prognosis Consortium. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. doi:10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott JA, Sharma SK, Bloomfield H, et al. Chronic disease profiles in a high risk aboriginal community over a 10-year interval. Nephrology. 2008;13(suppl.3):A121. [Google Scholar]
- 15.Hoy WE, Baker P, Kelly A, et al. Reducing premature death and renal failure in Australian Aborigines: a community-based cardiovascular and renal program. Med J Aust. 2000;172:473–478. [PubMed] [Google Scholar]
- 16.Andreasyan K, Hoy WE. Patterns of mortality in indigenous adults in the Northern Territory, 1998–2003: are people living in more remote areas worse off? Med J Aust. 2009;190:307–311. doi: 10.5694/j.1326-5377.2009.tb02418.x. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z, Hoy W, Nicol JL, et al. Predictive value of nephelometric and high-performance liquid chromatography assays of urine albumin for mortality in a high-risk aboriginal population. Am J Kidney Dis. 2008;52:672–682. doi: 10.1053/j.ajkd.2008.03.007. doi:10.1053/j.ajkd.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Pavkov ME, Knowler WC, Hanson RL, et al. Predictive power of sequential measures of albuminuria for progression to ESRD or death in pima indians with type 2 diabetes. Am J Kidney Dis. 2008;51:759–766. doi: 10.1053/j.ajkd.2008.01.011. doi:10.1053/j.ajkd.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Astor BC, Hallan SI, Miller ER, et al. Glomerular filtration rate, albuminuria, and risk of cardiovascular and all-cause mortality in the US population. Am J Epidemiol. 2008;167:1226–1234. doi: 10.1093/aje/kwn033. doi:10.1093/aje/kwn033. [DOI] [PubMed] [Google Scholar]
- 20.de Boer IH, Katz R, Cao JJ, et al. Cystatin c, albuminuria, and mortality among older adults with diabetes. Diabetes Care. 2009;32:1833–1838. doi: 10.2337/dc09-0191. doi:10.2337/dc09-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011;79:1331–1340. doi: 10.1038/ki.2010.550. doi:10.1038/ki.2010.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drury PL, Ting R, Zannino D, et al. Estimated glomerular filtration rate and albuminuria are independent predictors of cardiovascular events and death in type 2 diabetes mellitus: the fenofibrate intervention and event lowering in diabetes (field) study. Diabetologia. 2011;54:32–43. doi: 10.1007/s00125-010-1854-1. doi:10.1007/s00125-010-1854-1. [DOI] [PubMed] [Google Scholar]
- 23.Muntner P, Bowling CB, Gao L, et al. Age-specific association of reduced estimated glomerular filtration rate and albuminuria with all-cause mortality. Clin J Am Soc Nephrol. 2011;6:2200–2207. doi: 10.2215/CJN.02030311. doi:10.2215/CJN.02030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nerpin E, Ingelsson E, Riserus U, et al. The combined contribution of albuminuria and glomerular filtration rate to the prediction of cardiovascular mortality in elderly men. Nephrol Dial Transplant. 2011;26:2820–2827. doi: 10.1093/ndt/gfq848. doi:10.1093/ndt/gfq848. [DOI] [PubMed] [Google Scholar]
- 25.Schmieder RE, Mann JF, Schumacher H, et al. Investigators O. Changes in albuminuria predict mortality and morbidity in patients with vascular disease. J Am Soc Nephrol. 2011;22:1353–1364. doi: 10.1681/ASN.2010091001. doi:10.1681/ASN.2010091001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79:1341–1352. doi: 10.1038/ki.2010.536. doi:10.1038/ki.2010.536. [DOI] [PubMed] [Google Scholar]
- 27.Bruno G, Merletti F, Bargero G, et al. Estimated glomerular filtration rate, albuminuria and mortality in type 2 diabetes: the casale monferrato study. Diabetologia. 2007;50:941–948. doi: 10.1007/s00125-007-0616-1. doi:10.1007/s00125-007-0616-1. [DOI] [PubMed] [Google Scholar]
- 28.Hoy WE, Rees M, Kile E, et al. A new dimension to the Barker hypothesis: low birth weight and susceptibility to renal disease. Kidney Int. 1999;56:1072–1077. doi: 10.1046/j.1523-1755.1999.00633.x. doi:10.1046/j.1523-1755.1999.00633.x. [DOI] [PubMed] [Google Scholar]
- 29.Hoy WE, White AV, Dowling A, et al. Post-streptococcal glomerulonephritis is a strong risk factor for chronic kidney disease in later life. Kidney Int. 2012;81:1026–1032. doi: 10.1038/ki.2011.478. doi:10.1038/ki.2011.478. [DOI] [PubMed] [Google Scholar]