Abstract
We present an interesting case of a woman with new onset hypertension and abdominal fullness who was found to have huge bilateral perinephric fluid collections. Extensive workup revealed that she had secondary polycythemia, extensive truncal and proximal extremities telangiectasia and IgA-lambda monoclonal gammopathy of underdetermined significance. We believe that this is one of the rare cases consistent with the recently described TEMPI syndrome.
Keywords: page kidney, perinephric cysts, polycythemia, telangiectasia
Background
Perinephric cystic fluid collections are more frequently seen due to the readily available imaging modalities. However, their origin is still debatable and represent a host of different pathological conditions. Both clinical and imaging characteristics are needed to diagnose these conditions. Recently, there was much discussion about TEMPI syndrome in the context of such perinephric fluid collections. This syndrome, which comprises of cutaneous telangiectasia, elevated erythropoietin level with erythrocytosis, monoclonal gammopathy, perinephric fluid collections and intrapulmonary shunting, has been described in six patients [1], and we believe our case is in the league of this interesting syndrome.
Case report
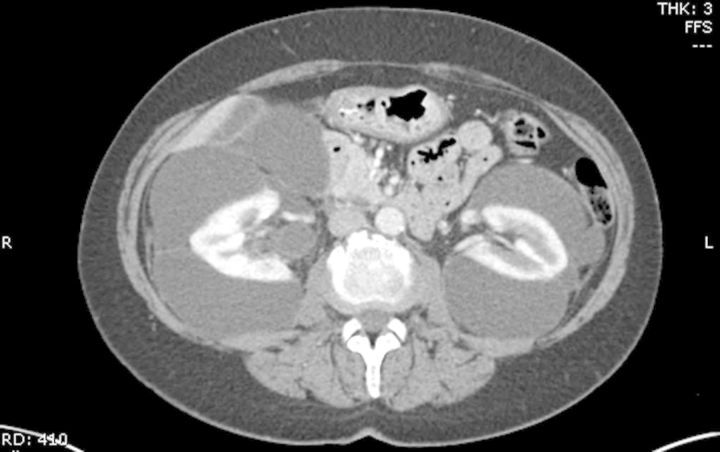
A 58-year-old woman was referred to our clinic for evaluation of uncontrolled hypertension and fluid collections surrounding the kidneys bilaterally. The patient had been doing well until ∼6 months prior to presentation, when she developed indigestion and bloating. She was diagnosed with worsening hypertension over the past 2–3 years. An ultrasound of the abdomen 2 months prior to presentation showed large cystic structures surrounding both the kidneys, dilation of common bile duct (1.2 cm), liver hemangioma and also non-obstructing gallstones. A computed tomography (CT) scan of the abdomen and pelvis revealed bilateral perinephric fluid collections compressing both the kidneys (Figure 1). The renal parenchyma appeared normal. There was also right hydronephrosis and proximal hydroureter along with enhanced liver lesions, most likely hemangiomas. There was mild extrahepatic biliary duct dilatation and colonic diverticulosis.
Fig. 1.
CT scan of abdomen shows multiple perinephric cystic fluid collections compressing both the kidneys.
During her first visit, she only complained of nausea. Her past medical history was significant for hyperlipidemia, diffuse non-obstructive coronary artery disease diagnosed 3 years earlier via a cardiac catheterization, congestive heart failure (NYHA-1) with left ventricular ejection fraction of 40%, thyroidectomy many years ago for a nodule, carpal tunnel surgery on the left hand and 30 pack-year history of smoking. Her family history was significant for hypertension and diabetes in a sibling and pulmonary embolism in her mother.
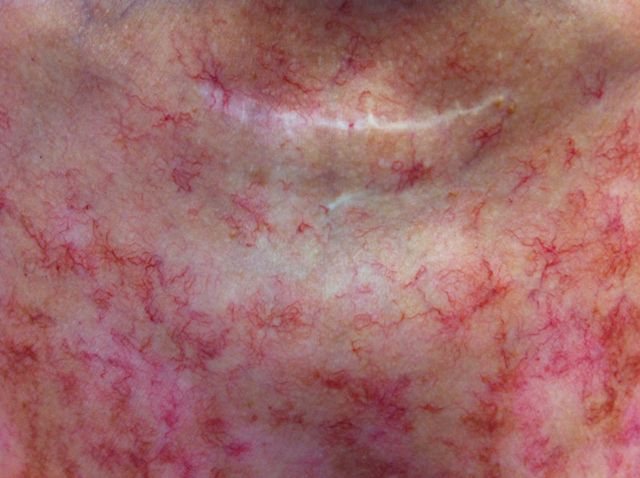
On physical examination, she appeared in no apparent distress and her vital signs included a heart rate of 87 b.p.m, respiratory rate of 16/min and blood pressure (BP) of 158/108 mmHg. She was afebrile with oxygen saturation of 96% on room air. Her cardiovascular and respiratory exam was within normal limits. Her abdominal exam was significant for bilateral flank fullness but no tenderness. She had vitiligo spots along with very prominent numerous skin telangiectasias located on the upper chest anteriorly and posteriorly (Figure 2) and to a lesser degree in the abdomen, base of neck, arms and thighs.
Fig. 2.
Extensive cutaneous telangiectasia on the anterior chest wall.
Her medications included levothyroxine 112 μg, nifedipine XL 30 mg, hydrochlorothiazide (HCTZ) 25 mg and niacin 500 mg daily. Her laboratory data were: blood urea nitrogen 7.85 mmol/L (11 mg/dL), creatinine 88.4 μmol/L (1.0 mg/dL), sodium 142 mmol/L, potassium 2.9 mmol/L, bicarbonate 30 mmol/L, calcium 2.025 mmol/L (8.1 mg/dL), total protein 73 g/L (7.3 g/dL), albumin 27 g/L (2.7 g/dL), alkaline phosphatase 119 U/L, liver enzymes, amylase and lipase were within normal limits. Her hemoglobin was 16.6 g/dL, hematocrit 51.7% with white blood cells (WBC) and platelets within normal limits. JAK2 mutation was negative. The erythropoietin level was 134 mU/mL. Uric acid was 321.2 μmol/L (5.4 mg/dL), intact parathyroid hormone 237 ng/L (pg/mL), 25-hydroxy Vit D 36.4 nmol/L (14.6 ng/mL), aldosterone 0.125 nmol/L (4.5 ng/dL), renin 33.65 μmol/mL/h (1.42 mg/mL/h) and sedimentation rate 24 mm/h, and ANA, c-ANCA and p-ANCA (perinuclear) were undetected. Urinalysis was unremarkable and the random urine protein/creatinine ratio was 1.1 g/g. Arterial blood gas revealed, pH 7.45, pCO2 36 mmHg, PO2 87 mmHg, O2 saturation 91.5% on room air and A-a gradient of 17.3 mmHg.
HCTZ was discontinued, nifedipine XL was increased to 60 mg and lisinopril 10 mg and potassium chloride 20 mEq daily were added to her regimen. The CT-guided aspiration of the perinephric fluid collections revealed multiple septations. Approximately 1000 mL of clear fluid was aspirated from the right side and 650 mL from the left side. Cell count showed WBC 56/mm3, red blood cells 60/mm3, lymphocytes 94% and monocytes 6%. Chemical analysis showed albumin <10 g/L (<1.0 g/dL), creatinine 61.9 μmol/L (0.7 mg/dL), lactate dehydrogenase 34 U/L, protein 3 g/L (0.3 g/dL). Cytology revealed a small number of lymphocytes, no epithelial or malignant cells.
Renal parenchyma expanded following the procedure. However, a repeat CT scan 24 h later showed reaccumulation of the cysts bilaterally. She subsequently underwent bilateral laparoscopic decortication of perinephric cysts. Post procedure, her BP improved and her medications were appropriately reduced.
Serum protein electrophoresis and immunofixation studies revealed 14 g/L (1.4 g/dL) of IgA-lambda monoclonal protein. Free serum kappa (k) was 4.6 mg/L and lambda (l) was 52.3 mg/L with the k/l ratio of 0.09. Urine protein electrophoresis showed a monoclonal band of IgA. Bone marrow biopsy revealed 10% plasma cells, hypocellular marrow with erythroid hyperplasia and absent iron stores. Her bone survey showed no lytic lesions. She was diagnosed as IgA monoclonal gammopathy of underdetermined significance (MGUS).
The repeat CT scan 5 months later showed interval decrease in bilateral perinephric fluid collections but hydronephrosis was unchanged on right and increased on left.
A follow-up trans-thoracic echocardiogram showed left ventricular ejection fraction of 20%, severe global hypokinesis and an apical thrombus. A cardiac catheterization revealed moderate diffuse coronary artery disease. She was started on anticoagulation with warfarin.
During follow-up, she reported 3 weeks of diarrhea, episodes of vomiting and increasing abdominal fullness. Clostridium difficile toxin and fecal leukocytes smear were both negative. An esophagoscopy revealed gastritis with Helicobacter pylori. Colonoscopy showed multiple small angiectasis and a diminutive polyp. Multiple biopsies of the gastrointestinal tract revealed no amyloid deposition but lymphocytic colitis. She was treated for H. pylori and was given loperamide for her colitis. A follow-up CT scan of abdomen revealed reappearance of two perinephric cysts bilaterally, although this time they were smaller in size. A CT-guided renal biopsy showed that 6 of 15 glomeruli were globally sclerosed, 10% interstitial fibrosis and hypertensive vascular changes but no evidence of light-chain deposition disease or amyloidosis. There was no interstitial edema or suggestion of intra-renal lymphangiectasia.
Her renal function has remained stable, and after treatment for diarrhea and H. pylori, her symptoms have improved. A repeat echocardiogram showed improvement in left ventricular ejection fraction to 36% and no thrombus.
Discussion
This patient presented with worsening BP control and abdominal fullness. Further investigation revealed very large bilateral perinephric cystic fluid collections compressing both the kidneys resulting in the Page physiology [2, 3]. Our patient's BP significantly improved following the un-roofing of bilateral perinephric cystic fluid collections.
Cases of such fluid collections surrounding the kidney have baffled many over the decades. These can be peri- (encasing the whole kidney) or paranephric (lie along the side of the kidney), unilateral or more frequently bilateral. They have been called perirenal hydronephrosis, hydrocele renis, hygroma renale, pararenal pseudohydronephrosis, renal lymphangiomatosis, renal lymphangiectasis, peripelvic lymphangiectasis and polycystic disease of the renal sinus [4]. Histologically, these perinephric cysts, which should be more accurately termed as ‘pseudocysts’, are locally extensive accumulations of fluid in fibrous sacs surrounding one or both the kidneys. The fluid collection might be extra-capsular but is often subcapsular [5]. Fluid analysis is transudate (uriniferous) or modified transudate (hematoma).
Diagnosis is made by imaging with either renal ultrasound, CT scan or magnetic resonance imaging [6]. Treatment is varied depending on the extensiveness of the disease. Some cases do respond to the aspiration of the cysts but most of the time the fluid re-accumulates necessitating marsupialization, or even nephrectomy [7–9]. Biopsy of the kidney is rarely performed in such cases except when nephrectomy is undertaken or after autopsy. Some biopsies have shown extensive interstitial edema and dilated intra-renal lymphatics, which made the authors conclude the diagnosis as renal lymphangiectasia [9–12]. Renal/retroperitoneal lymphangiectasia is a rare benign disease speculated to be due to malformative blockage of renal lymphatic draining resulting in large perinephric fluid collections with or without intra-abdominal cystic structures occasionally draping the retroperitoneal vessels [9–15].
The pathology of the cysts in our case showed fibrous cystic wall with no epithelium and chronic inflammation. This led us to believe that these were in fact pseudocysts. Subsequent renal biopsy was only significant for hypertensive changes and no interstitial edema or light chain or amyloid deposits. Recently, Sykes et al. [1] described a multisystem disease named TEMPI syndrome in six patients. The characteristics of this syndrome are cutaneous telangiectasia, erythrocytosis, monoclonal gammopathy, perinephric fluid collections and intrapulmonary shunting [1]. Our patient did have IgA-lambda MGUS, extensive cutaneous telangiectasia, secondary polycythemia and extensive perinephric fluid collections. Our patient differed from the previous cases of TEMPI in that all previous reports were with IgG MGUS, with the exception of a recent case of renal lymphangiectasis, polycythemia and IgA MGUS who lacked cutaneous telangiectasia [15]. We attributed the erythrocytosis to increased erythropoietin secretion in association with perinephric fluid collections as has been reported in patients with this condition [9, 16]. However, she was a chronic smoker, which could have also contributed to it. But, the arterial blood gas analysis did not show any evidence of hypoxemia. Her normal A-a gradient was against intrapulmonary shunting that has been reported in some of the cases of TEMPI syndrome [1]. Some of the cases of TEMPI syndrome had history of spontaneous venous thromboses as well as intracranial hemorrhages [16]. Our patient did not have history of such events. However, she developed non-ischemic cardiomyopathy and left ventricular apical thrombus, which has not been described as a part of TEMPI syndrome. We also had to consider POEMS syndrome in our differential diagnosis, which is characterized with polyneuropathy (sensory and motor), organomegaly (hepatomegaly, splenomegaly and/or lymphadenopathy), endocrinopathy (hypogonadism, hypothyroidism and adrenal insufficiency), monoclonal gammopathy (lambda monoclonal plasma cell dyscrasia), skin changes (hyperpigmentation, hypertrichosis, telangiectasia and hemangioma), and one or more of the following features: osteosclerotic myeloma, Castleman's disease, erythrocytosis, thrombocytosis, edema, pleural effusion, ascites, elevated serum vascular endothelial growth factor level and papilledema [17]. However, the prominent feature of our patient was perinephric fluid collections, which is not a feature of POEMS syndrome, and our patient lacked some of the hallmarks of the syndrome, e.g. polyneuropathy, organomegaly, endocrinopathy, osteosclerotic myeloma etc.
Our patient was not treated with any immunomodulatory drugs, but disappearance of cutaneous telangiectasia, normalization of serum erythropoietin and eradication of monoclonal gammopathy along with resolution of perinephric fluid collections and decreased intrapulmonary shunting has been described in two patients treated with bortezomib [1, 18]—a proteasome inhibitor, which has been approved for the treatment of multiple myeloma and mantle cell lymphoma. This suggests that plasma cells may play a key role in the pathogenesis of TEMPI syndrome. However, the theory of monoclonal paraprotein causation in TEMPI syndrome remains elusive. Further understanding of this syndrome and investigation of its pathogenesis are warranted.
Conflict of interest statement
None declared.
References
- 1.Sykes DB, Schroyens W, O'Connell C. The TEMPI syndrome—a novel multisystem disease. New Eng J Med. 2011;365:475–477. doi: 10.1056/NEJMc1106670. doi:10.1056/NEJMc1106670. [DOI] [PubMed] [Google Scholar]
- 2.Page IH. A method for producing persistent hypertension by cellophane. Science. 1939;89:273–274. doi: 10.1126/science.89.2308.273. doi:10.1126/science.89.2308.273. [DOI] [PubMed] [Google Scholar]
- 3.Engel WJ, Page IH. Hypertension due to renal compression resulting from subcapsular hematoma. J Urol. 1955;73:735–739. doi: 10.1016/S0022-5347(17)67466-4. [DOI] [PubMed] [Google Scholar]
- 4.Spriggs AI. Perinephric cysts. J Urol. 1952;67:414–432. doi: 10.1016/S0022-5347(17)68367-8. [DOI] [PubMed] [Google Scholar]
- 5.Puri A, Bajpai M, Gupta AK. Bilateral spontaneous perinephric urinomas: case report and review of the literature. Urology. 2004;64:590–591. doi: 10.1016/j.urology.2004.04.057. doi:10.1016/j.urology.2004.04.057. [DOI] [PubMed] [Google Scholar]
- 6.Balci NC, Akun E, Erturk M, et al. Renal-related perinephric fluid collections: MRI findings. Magn Reson Imaging. 2005;23:679–684. doi: 10.1016/j.mri.2005.04.003. doi:10.1016/j.mri.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Schwarz A, Lenz T, Klaen R, et al. Hygroma renale: pararenal lymphatic cysts associated with renin-dependent hypertension (Page kidney). Case report on bilateral cysts and successful therapy by marsupialization. J Urol. 1993;150:953–957. doi: 10.1016/s0022-5347(17)35660-4. [DOI] [PubMed] [Google Scholar]
- 8.Castle EP, Herrell SD. Laparoscopic management of page kidney. J Urol. 2002;168:673–674. doi:10.1016/S0022-5347(05)64720-9. [PubMed] [Google Scholar]
- 9.Chen Z, Qi L, Tang Z, et al. Renal lymphangiectasia. Scand J Urol Nephrol. 2009;43:428–430. doi: 10.3109/00365590902930857. doi:10.3109/00365590902930857. [DOI] [PubMed] [Google Scholar]
- 10.Bazari H, Attar EC, Dahl DM, et al. Case records of the Massachusetts General Hospital. Case 23–2010. A 49-year-old man with erythrocytosis, perinephric fluid collections, and renal failure. N Engl J Med. 2010;363:463–475. doi: 10.1056/NEJMcpc1004086. doi:10.1056/NEJMcpc1004086. [DOI] [PubMed] [Google Scholar]
- 11.Meredith WT, Levine E, Ahlstrom NG, et al. Exacerbation of familial renal lymphangiomatosis during pregnancy. Am J Roentgenol. 1988;151:965–966. doi: 10.2214/ajr.151.5.965. [DOI] [PubMed] [Google Scholar]
- 12.Ueda S, Yanagida H, Sugimoto K, et al. Chronic renal insufficiency in a boy with cystic renal lymphangiectasia: morphological findings and long-term follow-up. Clin Nephrol. 2007;68:416–421. doi: 10.5414/cnp68416. [DOI] [PubMed] [Google Scholar]
- 13.Upreti L, Dev A, Kumar Puri S. Imaging in renal lymphangiectasia: report of two cases and review of literature. Clin Radiol. 2008;63:1057–1062. doi: 10.1016/j.crad.2007.12.013. doi:10.1016/j.crad.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Bano S, Yadav SN, Chaturvedi S, et al. Retroperitoneal lymphangiectasia-radiologic appearances, complications and management alternatives: a case report. Abdom Imaging. 2010;35:372–375. doi: 10.1007/s00261-009-9528-5. doi:10.1007/s00261-009-9528-5. [DOI] [PubMed] [Google Scholar]
- 15.Viglietti D, Sverzut JM, Peraldi MN. Perirenal fluid collections and monoclonal gammopathy. Nephrol Dial Transplant. 2012;27:448–449. doi: 10.1093/ndt/gfr433. doi:10.1093/ndt/gfr433. [DOI] [PubMed] [Google Scholar]
- 16.Burton IE, Sambrook P, McWilliam LJ. Secondary polycythaemia associated with bilateral renal lymphocoeles. Postgrad Med J. 1994;70:515–517. doi: 10.1136/pgmj.70.825.515. doi:10.1136/pgmj.70.825.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dispenzieri A, Kyle RA, Lacy MQ, et al. POEMS syndrome: definitions and long-term outcome. Blood. 2003;101:2496–2506. doi: 10.1182/blood-2002-07-2299. doi:10.1182/blood-2002-07-2299. [DOI] [PubMed] [Google Scholar]
- 18.Kwok M, Korde N, Landgren O. Bortezomib to treat the TEMPI syndrome. N Engl J Med. 2012;366:1843–1845. doi: 10.1056/NEJMc1202649. doi:10.1056/NEJMc1202649. [DOI] [PMC free article] [PubMed] [Google Scholar]