Abstract
Background
Renal disease is an emerging problem in patients living with human immunodeficiency virus (HIV), as illustrated by an increased incidence of acute kidney injury and chronic kidney disease (CKD) from HIV, its associated treatment and comorbidities such as diabetes and vascular disease. We have established a combined HIV-renal clinic to manage such patients, enhance their treatment and minimize outpatient visits.
Methods
We have analysed the outcomes of the first 99 patients seen in the clinic using electronic patient records. These ninety-nine patients were referred to the service from HIV physicians in West London and all the patients were seen jointly by an HIV and a renal consultant.
Results
Sixty-five percent of the patients were referred with reduced renal function or proteinuria [mean creatinine at presentation 136 mcmol/L, estimated glomerular filtration rate (eGFR) 57 mL/min/1.73 m2]. The majority (53%) had risk factors predisposing to vascular disease including diabetes, hypertension, previous stroke or myocardial infarction. Overall, 27% of patients had a renal diagnosis directly associated with HIV (HIVAN, immune complex nephritis, tenofovir toxicity, Fanconi syndrome), 73% had an alternative possible cause. Twenty-seven percent of patients had low-level proteinuria (urine protein:creatinine ratio abnormal but <100 mg/mmol) or mildly reduced eGFR (40–66 mL/min/1.73 m2) without a clear underlying cause. Ten percent of patients were thought to have tenofovir-induced renal damage all of whom improved on cessation of this agent. Following the review in the combined clinic, 64% of patients had a change in treatment or management, with 50% improving their renal parameters as a result. Most patients were discharged back to their main HIV teams for ongoing follow-up.
Conclusions
A combined HIV-renal clinic can enhance patient care with reduced outpatient visits.
Keywords: chronic kidney disease, HIV, proteinuria, tenofovir
Introduction
Renal disease is very common in individuals living with human immunodeficiency virus (HIV) [1, 2] with just under a third having evidence of proteinuria [3] and a quarter a reduced glomerular filtration rate (GFR) [4]. Acute kidney injury and chronic kidney disease (CKD) are more common in HIV cohorts than in the general population. The causes of renal damage are multifactorial but the improved life expectancy of individuals living with HIV in the era of highly active anti-retroviral therapy (HAART) has also highlighted the increased prevalence of renal disease observed in an ageing population.
Patients with HIV are vulnerable to renal insults from a multitude of pathologies. In the pre-HAART era, opportunistic infections and the use of nephrotoxic therapies were commonplace. In the HAART era, individuals continue to experience problems from direct HIV infection, drug toxicity and immunocompromise (especially in the context of late presentation) but also from renal dysfunction secondary to complications that would be equally common in an ageing population. These complications include hypertension, type 2 diabetes, atherosclerosis and vascular disease and the perils of polypharmacy. Furthermore, certain renal diseases are seen more commonly in individuals living with HIV. These include membranous glomerulonephritis, [5, 6] focal segmental glomerulosclerosis, immune complex glomerulonephritis [7] and HIV-associated nephropathy (HIVAN). In addition, numerous antiretroviral therapy-associated renal complications are recognized. Thus, the differential diagnosis of renal dysfunction in patients with HIV has become very broad, and its management complex, given the multitude of anti-retroviral therapies in use and their risk of interaction.
In 2008, a new initiative was developed in our centre in the form of a consultant-led combined HIV/renal clinic in an attempt to improve the management of this emerging burden. We have reviewed the patient case-mix, interventions and clinical outcomes of its attendees in order to evaluate the service.
Method
The combined clinic was delivered once a month with all patients being seen jointly and simultaneously by an HIV and renal consultant within the West London Centre for Sexual Health. Patients were referred predominantly from a variety of HIV services in West London. Patients were referred back to their HIV team with advice for future management and follow-up, if no further specific renal input was required. Those requiring urgent review that were unable to wait for the next combined clinic were seen within a week in the routine renal outpatient clinic or as an inpatient if necessary. Complex decisions regarding anti-retroviral therapy which could not be made immediately in the outpatient setting were brought to a ‘virtual’ HIV clinic within the HIV service. Specialty trainees in HIV or renal medicine were invited to attend for a training experience.
Electronic patient records were used to collate clinical, demographic and laboratory data of all patients who attended the combined clinic from February 2008 to December 2010. Excluded from the data were patients requiring urgent review when seen on the wards if inpatients or in the routine renal outpatient clinic. Renal outcomes and diagnoses were identified from the patient record or, if undertaken, from renal biopsy.
Results
A total of 99 patients were seen in the combined clinic. Thirteen (13%) were female. The median age was 48 years (range 27–79) and seventeen (17%) were over 60 years old. Almost three quarters (73%) were white European, mean age 49.3 years (range 27–79), eighteen (18%) were black African, mean age 47.7 years (range 34–73) and five (5%) were Asian. There was no difference in the age profile by ethnicity.
Renal diagnoses
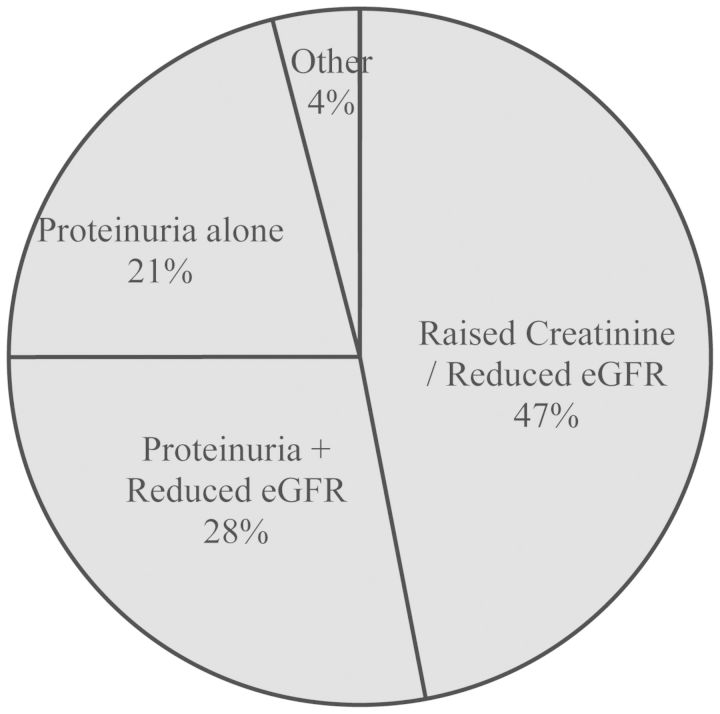
Twenty-five patients (25%) had a pre-existing renal diagnosis. The majority of patients (65%) were referred due to raised serum creatinine or reduced estimated glomerular filtration rate (eGFR). Thirty-nine patients (39%) were found to have proteinuria as well as reduced eGFR. Twenty-nine patients (29%) presented with proteinuria alone (Figure 1). The mean creatinine at presentation was 136 mcmol/L (sd 54), eGFR 57 mL/min/1.73 m2 (SD 19) and the urine protein:creatinine ratio was 90 mg/mmol (SD 123) (Table 1). Fifty-three (53%) patients had risk factors predisposing to vascular disease. Ten (10%) patients were diabetic: five of these presented with proteinuria and five with worsening eGFR. Eight out of these ten were given a clinical diagnosis of diabetic nephropathy or hypertensive nephropathy, one labelled as CKD-4 with no clear diagnosis and one labelled low-level stable proteinuria. Thirty-nine (39%) were hypertensive: 13 presented with proteinuria and 26 with worsening eGFR. Twenty of the known hypertensive patients were given a clinical diagnosis of hypertensive nephropathy and two had a renal biopsy: both showing HIVAN. The remainder had clinical diagnoses which were mostly low-level stable proteinuria (4) or non-progressive mild CKD (4). Four (4%) had confirmed overt cardiovascular disease (previous stroke or myocardial infarction).
Fig. 1.
Indication for referral.
Table 1.
Renal function (eGFR) and proteinuria in patients at presentation to a HIV–renal clinic
| Mean creatinine (mcmol/L) at presentation (SD) | Mean eGFR (mL/min/1.73 m2) at presentation (SD) | Mean urine PCR (mg/mmol) at presentation (SD) | |
|---|---|---|---|
| Overall | 136 (54) | 55 (19) | 90 (123) |
| White | 136 (55) | 57 (18) | 83 (119) |
| Black | 123 (47) | 66 (24) | 99 (106) |
| Asian | 146 (31) | 47 (9) | 76 (66) |
| Under 60 years | 133 (43) | 58 (19) | 88 (120) |
| Over 60 years | 150 (92) | 51 (22) | 96 (144) |
| On tenofovir | 124 (25) | 59 (15) | 66 (53) |
| Not on tenofovir | 148 (71) | 55 (22) | 114 (166) |
All differences non-significant.
Ten patients (10%) were thought to require a renal biopsy as a result of their initial review. A further eight patients with HIV had a renal biopsy performed in West London during the same period after referral via other routes. The biopsy-proven diagnoses included drug-induced renal damage, extensive renal scarring, granulomatous disease as well as HIVAN (Table 2). Only six patients (6%) were anti-retroviral naive at the time of referral and the renal diagnoses in these patients were one case of biopsy-proven granulomatous acute tubulointerstitial nephrititis (ATIN), chronic scarring, a previously diagnosed IgA nephropathy and use of creatine and whey protein supplements. Forty-nine patients already on HAART were taking a tenofovir disoproxil fumerate (tenofovir)-containing regimen, and in 10 patients tenofovir was thought to be at least in part responsible for renal damage. The renal function of all of these patients improved on cessation of the drug.
Table 2.
Renal histological and clinical diagnoses
| Number of patients (%) | Mean duration of HIV (months) (SD) | Mean CD4 count at presentation (cells/mL3) (SD) | |
|---|---|---|---|
| Biopsy-proven diagnoses | |||
| HIV-associated nephropathy (HIVAN) | 3 (3%) | 94 (90) | 667 (523) |
| Membranous glomerulopathy | 2 (2%) | 28 | 468 |
| ATIN alone | 1 (1%) | 16 | 772 |
| Granulomatous ATIN | 1 (1%) | 2 | 204 |
| Glomerulosclerosis (non-HIVAN) | 1 (1%) | 142 | 449 |
| Tubular scarring | 1 (1%) | 24 | 547 |
| Tenofovir-associated nephropathy | 1 (1%) | 59 | 578 |
| Clinical diagnoses | |||
| Hypertensive nephropathy | 19 (19%) | 143 (87) | 467 (223) |
| Non-progressive mild CKD (eGFR 40–66 mL/min/1.73 m2) | 14 (14%) | 121 (83) | 495 (202) |
| Low-level stable proteinuria (urine PCR <100 mg/mmol) | 12 (12%) | 166 (78) | 546 (446) |
| Likely tenofovir-associated nephropathy | 9 (9%) | 127 (84) | 447 (225) |
| Diabetic nephropathy | 5 (5%) | 138 (68) | 675 (263) |
| No kidney disease | 4 (4%) | 136 (90) | 479 (228) |
| HAART adverse drug reaction (non-tenofovir) | 3 (3%) | 151 (28) | 369 (260) |
| Chronic scarring | 3 (3%) | 50 (42) | 472 (189) |
| Creatine supplements | 3 (3%) | 133 (132) | 353 (228) |
| Proteinuria (PCR > 100 mg/mmol) unknown cause (no biopsy) | 2 (2%) | 168 (15) | 751 (413) |
| CKD (stage 4) unknown cause | 2 (2%) | 119 (137) | 554 (15) |
| Fanconi's syndrome | 2 (2%) | 222 (63) | 352 (139) |
| IgA/thin membrane disease | 2 (2%) | 83 (92) | 393 (25) |
| Acute tubular necrosis | 1 (1%) | 13 | 376 |
| Renal artery stenosis | 1 (1%) | 109 | 165 |
| Miscellaneousa | 7 (7%) | 84 (67) | 473 (152) |
aRenal cell carcinoma, renal tubular acidosis, congenital atrophic kidney and whey supplements.
Overall, only 27% of the patients had a renal diagnosis clearly associated with HIV infection or anti-retroviral toxicity (HIVAN, immune complex nephritis, tenofovir toxicity/Fanconi syndrome) (Table 2). However, 16 of the remaining patients without an obvious HIV-associated cause of their renal failure had non-progressive CKD with no confirmed cause and 12 patients had low-level proteinuria but no defined underlying diagnosis. It is not possible to identify whether these renal problems were truly unrelated to HIV or its treatment, or in fact a manifestation of underlying HIV infection. Two additional patients had heavy proteinuria but declined a renal biopsy. In all these cases, no other cause was identified from imaging and blood and serological testing.
Finally, in four patients (4%) no renal disease was identified and patients could be re-assured and referred back to their HIV physician. These patients had diagnoses including transient renal dysfunction now reversed, misinterpretation of urinary protein:creatinine ratios and normal renal function confirmed by isotopic GFR measurement despite reduced laboratory reported eGFR.
Six (6%) of the patients had co-infection with hepatitis B virus. Of these, two presented with proteinuria and four with a reduced eGFR. The clinical diagnoses included tenofovir toxicity (2), hypertensive nephropathy (2), Fanconi's syndrome (1) and low-level stable proteinuria (1). All were clinical diagnoses and unrelated to hepatitis B infection. Four (4%) had co-infection with hepatitis C virus. Two presented with proteinuria and two with a worsening eGFR. One had a biopsy-proven diagnosis of membranous glomerulopathy, and the remainder were labelled as having hypertensive nephropathy (1), non-progressive mild CKD and IgA nephropathy (previous biopsy-proven diagnosis). One additional (1%) patient had concomitant hepatitis B and C co-infection and presented with proteinuria (urine PCR 124 mg/mmol). Imaging suggested chronic renal scarring and proteinuria improved, with no biopsy having been undertaken.
The individuals included in this study were at various stages of HIV infection (Table 2). The mean duration of HIV was 129.2 (SD 88) months (10.7 years). The mean CD4 count at presentation was 495 (SD 262). The mean nadir CD4 count was 225 (SD 162); however, this number is unreliable as the electronic database used for data collection only had readings from 1990 and 57 (57%) patients had a diagnosis before this year. There were no significant differences in renal diagnoses based on the longevity of HIV diagnosis, although a suggestion that those with biopsy-proven interstitial nephritis had HIV for a shorter period. Anti-retroviral regimens were variable in this cohort. Six were naive to HIV anti-retroviral therapy, two were prescribed protease inhibitor monotherapy and one was taking double-boosted protease inhibitor therapy, thus the vast majority were prescribed a HAART regimen comprising at least three active anti-retroviral agents. There were no specific associations of individual therapies with renal diagnoses other than for tenofovir as described above.
Sixty-two patients (62%) acquired HIV through homosexual sex or intravenous drug use, and 19% through heterosexual sex and 2% through blood products. The remaining 16 (16%) were unknown. There was no association between presentation and diagnosis between these sub-groups.
Clinical outcomes
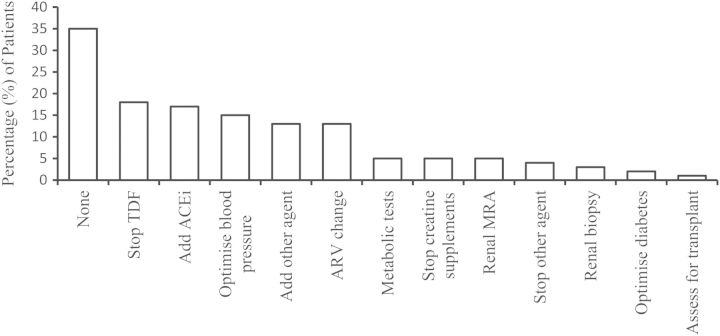
No specific intervention was needed in thirty-five (35%) patients and no change in therapy instituted. In the remaining sixty-four (64%) patients, attendance at the clinic led directly to a change in therapy or management (Figure 2). Most commonly, this included cessation of tenofovir, addition of angiotensin-converting enzyme inhibitors, optimization of blood pressure or diabetes, change of anti-retroviral other than tenofovir, specific investigation such as magnetic resonance angiogram (MRA) of renal arteries or renal biopsy. Fifty (50%) of the patients reviewed had improved renal parameters as a result of the clinic visit. The mean improvement in eGFR was 4.5 mL/min/1.73 m2 (SD 116) at 3 months and 9.5 mL/min/1.73 m2 (SD 10) at 1 year. The mean improvement in the urine protein:creatinine ratio was 55 mg/mmol (SD) at 1 year. Thirty-three (33%) patients had stable and 10 (10%) had worse renal parameters. For this group, the mean deterioration at 1 year in eGFR was 6.15 mL/min/1.73 m2 (SD 10.76) and the mean urine protein:creatinine ratio was 45.6 mg/mmol (SD 120) higher. Forty (40%) continued to have joint specialist HIV–renal follow-up, while 60% were discharged back to their HIV physician with a clear renal management plan.
Fig. 2.
Interventions made at combined HIV–renal clinic. TDF: tenofovir; ACEi: angiotensin-converting enzyme inhibitor; ARV: anti-retroviral agent; MRA: magnetic resonance angiogram.
Discussion
This service review highlights the high prevalence of renal disease in an HIV-infected population and the benefits of providing combined care by both a nephrologist and an HIV specialist. We cannot estimate the overall prevalence of renal disease since some patients were seen in other renal settings (inpatient reviews and other renal outpatient clinics) and referrals came from a wide catchment area in West London. The renal diseases identified were from multiple causes including direct HIV-induced renal damage, HAART drug toxicity, but also commonly non-HIV, non-antiretroviral related comorbidities, notably diabetes and hypertension. Most diagnoses were clinical since the proportion of patients having a renal biopsy were small, and this will of course be a limitation in the study. It does, however, reflect real nephrological practice. Management of this cohort of patients has benefited enormously from the joint clinic with both a renal and an HIV consultant present. The combined approach allows decisions regarding new drug therapies, avoiding drug interactions, to be made within the clinic, and review or change of HAART therapy, strict optimization of medication for blood pressure and diabetes, and appropriate choice of patients requiring renal biopsy, specialized renal investigations or even renal transplant assessment. Without a combined clinic, patients would have required further attendance in an alternative outpatient setting or review off-line and further communication. In all cases, a diagnosis and management plan was made, although in 32% of patients this centred upon the fact that no significant renal disease was present, in itself an important contribution. Interestingly, a very significant proportion of patients had low-level proteinuria (urinary PCR < 100 mg/mmol) but with an eGFR between 60 and 90 mL/min/1.73 m2, or non-progressive mild CKD (eGFR 40–66 mL/min/1.73 m2 with urinary PCR <50 mg/mmol) for whom it was impossible to identify a precise cause, and in whom a renal biopsy was not felt warranted. Despite extensive imaging, blood and serological tests, no cause was identified. Whether HIV was directly or indirectly responsible is not clear, but this group of patients do require appropriate renal follow-up with repeated serum creatinine measurements and urine protein quantification, and may proceed to renal biopsy should either change in the future. Such patients may be at higher risk for renal damage should they have further renal insults. Other series of patients with HIV have also been reported to have a high prevalence of CKD, suggesting that this is caused by an HIV-related factor [8–11]. There were few patients with hepatitis B or C infection, and no association between the length of HIV infection and clinical diagnosis, but most patients had had HIV infection for over 10 years.
Drug interactions are a particular problem in patients with HIV since many of the antiretroviral agents have significant potential for interaction and are hepatic cytochrome p450 enzyme inducers or inhibitors. Even simple therapeutic decisions such as choice of statin or angiotensin-converting enzyme (ACE) inhibitors are complicated by the potential for drug interactions. Furthermore, patients often require careful advice on dosing and timing for their HAART medications. The presence of both HIV and renal consultants makes this considerably easier. For example, it would be completely inappropriate for a nephrologist to alter HAART medications without proper discussion with the HIV team, given the need for detailed knowledge of previous regimens, the HIV-resistance patterns, mutation risk and side-effect profile of HAART drugs. The diagnosis of possible tenofovir-associated nephrotoxicity (often not biopsy proven) in patients in this series subsequently led to a change of therapy in a number of patients. We cannot comment on the overall prevalence or incidence of tenofovir-associated renal damage since this was a highly selected cohort of patient referred specifically because of possible renal damage. However, we also note the high prevalence of hypertension, cardiovascular disease and diabetes in this population. This is important since there has been considerable debate currently over the choice between tenofovir- or abacavir-containing regimens given the potential risk of tenofovir-induced renal damage and the possible association of abacavir and cardiovascular disease [12–15]. Increasingly, such choices need to be made in the context of the patients overall renal and cardiovascular risks.
Many patients seen in this clinic were discharged back to their primary HIV team without further HIV–renal clinic follow-up since a clear diagnosis had been made and a management plan instituted. This varied from no intervention required, cessation of creatine supplements, ongoing monitoring of renal function or optimization of BP control and use of angiotensin system blockers. Since these patients come to HIV clinics regularly and have ongoing blood testing, many patients can be left under the care of their HIV clinic once an appropriate plan has been made, usually including a careful review of future serum creatinine and urine protein:creatinine ratios, minimizing clinic reviews for the patient. The combined approach can ensure that this happens as quickly as possible with both time savings for patients and cost savings for the health service.
Relatively few cases of directly HIV-related renal dysfunction were seen, although many patients where this was suspected were seen in the routine renal clinic as urgent referrals. This is clearly the case since eight patients had a renal biopsy performed over this period but were not seen in the combined HIV–renal clinic. The major directly HIV-related renal problem was in fact renal dysfunction related to anti-retroviral usage, particularly tenofovir, which was identified as a diagnosis in 10 patients, 2 patients on atazanavir/ritonavir and 1 patient on darunavir/ritonavir in the absence of a tenofovir backbone. In all cases, this was reflected in a reduced eGFR with or without low-level proteinuria, and usually was not biopsy proven but suspected clinically. Patients in whom anti-retroviral toxicity was suspected who were able to undergo a simple switch of therapy were usually offered the option of change in HAART using a non-tenofovir containing regimen with careful review of serum creatinine and eGFR. Those in whom the eGFR continued to decline were then offered a renal biopsy. The reduced eGFR seen in all of the cases of HAART nephrotoxicity reversed on cessation of the offending medication. Two confirmed cases of tenofovir-associated Fanconi's syndrome were also diagnosed with hypophosphataemia, phosphaturia, aminoaciduria, tubular glycosuria and tubular proteinuria. A wide variety of antiretroval regimens were in use for these patients and no other associations were identified between renal dysfunction, clinical diagnosis and treatment.
The complexity of renal disease in patients with HIV warrants joint specialist input. Our combined service has demonstrated a significant change in therapy which might otherwise not have taken place in a timely manner, focussed investigations and optimal management of both HIV and non-HIV related renal diseases and improved the renal outcome in most patients seen. We have also identified a large cohort of individuals living with HIV with low-level proteinuria and mildly reduced eGFR of undefined cause who require protection from future renal insults.
Conflict of interest statement
None declared.
Acknowledgement
The combined clinic takes place at the HIV/GUM Directorate, Chelsea and Westminster Hospital.
References
- 1.Szczech LA, Gange SJ, van der Horst C, et al. Predictors of proteinuria and renal failure among women with HIV infection. Kidney Int. 2002;61:195–202. doi: 10.1046/j.1523-1755.2002.00094.x. [DOI] [PubMed] [Google Scholar]
- 2.Gupta SK, Mamlin BW, Johnson CS, et al. Prevalence of proteinuria and the development of chronic kidney disease in HIV-infected patients. Clin Nephrol. 2004;61:1–6. doi: 10.5414/cnp61001. [DOI] [PubMed] [Google Scholar]
- 3.Gallant JE, Staszewski S, Pozniak AL, et al. 903 Study Group. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292:193–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 4.Jones R, Stebbing J, Nelson M, et al. Renal dysfunction with tenofovir disoproxil fumarate-containing highly active antiretroviral therapy regimens is not observed more frequently: a cohort and case–control study. J Acquir Immune Defic Syndr. 2004;37:1489–1495. doi: 10.1097/01.qai.0000138983.45235.02. [DOI] [PubMed] [Google Scholar]
- 5.Cheng JT, Anderson HL, Jr, Markowitz GS, et al. Hepatitis C virus-associated glomerular disease in patients with human immunodeficiency virus co-infection. J Am Soc Nephrol. 1999;10:1566–1574. doi: 10.1681/ASN.V1071566. [DOI] [PubMed] [Google Scholar]
- 6.Szczech LA, Gupta SK, Habash R, et al. The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int. 2004;66:1145–1152. doi: 10.1111/j.1523-1755.2004.00865.x. [DOI] [PubMed] [Google Scholar]
- 7.Kimmel PL, Phillips TM, Ferreira-Centeno A, et al. Brief report: idiotypic IgA nephropathy in patients with human immunodeficiency virus infection. N Engl J Med. 1992;327:702–706. doi: 10.1056/NEJM199209033271006. [DOI] [PubMed] [Google Scholar]
- 8.Veiga C, Rama A, Crespo P, et al. Prevalence of chronic kidney disease in older patients with HIV infection on antiretroviral treatment. Eur J Hosp Pharm. 2012;19:122. [Google Scholar]
- 9.Menezes AM, Torelly J, Jr, Real L, et al. Prevalence and risk factors associated to chronic kidney disease in HIV-infected patients on HAART and undetectable viral load in Brazil. PLos One. 2011;6:10. doi: 10.1371/journal.pone.0026042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cailhol J, Nkurunziza B, Izzedine H, et al. Prevalence of chronic kidney disease among people living with HIV/AIDS in Burundi: a cross-sectional study. BMC Nephrol. 2011;24:40. doi: 10.1186/1471-2369-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naftalin C, Nathan B, Hamzah L, et al. HIV-associated kidney disease in the context of an aging population. Sex Health. 2011;8:485–492. doi: 10.1071/SH10146. [DOI] [PubMed] [Google Scholar]
- 12.Scherzer R, Estrella M, Li Y, et al. Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS. 2012;26:867–875. doi: 10.1097/QAD.0b013e328351f68f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friis-Moller N, Thiebaut R, Reiss P, et al. Predicting the risk of cardiovascular disease in HIV-infected patients: the data collection on adverse effects of anti-HIV drugs study. Eur J Cardiovasc Prev Rehabil. 2010;17:491–501. doi: 10.1097/HJR.0b013e328336a150. [DOI] [PubMed] [Google Scholar]
- 14.Worm SW, Sabin C, Weber R, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J Infect Dis. 2010;201:318–330. doi: 10.1086/649897. [DOI] [PubMed] [Google Scholar]
- 15.Cruciani M, Znichelli V, Serpelloni G, et al. Abacavir use and cardiovascular disease events: a meta-analysis of published and unpublished data. AIDS. 2011;25:1993–2004. doi: 10.1097/QAD.0b013e328349c6ee. [DOI] [PubMed] [Google Scholar]