Abstract
The podocyte is a key cell in the selective filtering action of the glomerular capillary wall. Podocyte injury is of pathogenetic and prognostic significance in human glomerular disease; podocyte repair and regeneration are important therapeutic targets. In particular, podocyte function is dependent on the cells' actin cytoskeleton: this maintains their complex structure. Alterations in the actin cytoskeleton arise from a variety of genetic and acquired causes. Therapeutic agents that are beneficial in proteinuric disease may act at least partly by restoring the cell shape via effects on the actin cytoskeleton. Recent studies of podocytes in vivo and in vitro are described, highlighting clinically relevant observations and those that help us understand the ways in which we may harness nature's own mechanisms to repair and/or renew these specialized glomerular cells, with a particular focus on their actin cytoskeleton. Drugs that have beneficial effects on podocytes can improve our ability to treat important renal diseases including diabetic nephropathy. Currently available agents can be applied in this way and the rapid progress in the study of podocytes is highlighting new therapeutic targets that can bring even more specificity.
Keywords: actin, cytoskeleton, podocyte, proteinuria
Introduction
A clinical nephrology readership needs no convincing of the importance of proteinuria: it has prognostic significance in patients with a wide variety of forms of renal disease and is a potent independent cardiovascular risk factor [1]. In health, proteinuria is prevented by the selective filtering action of the glomerular capillary wall. This has a tripartite structure comprising podocytes (visceral glomerular epithelial cells) on the outer (urinary) aspect, fenestrated glomerular endothelial cells on the inner (luminal) aspect and the highly negatively charged glomerular basement membrane lying between the two cell types. It should be stated at the outset that each of these three components exerts an important influence on the selective permeability of the glomerular capillary wall and it is a mistake to think of any of the components in isolation. Nevertheless, much of the focus in recent years has been on the podocyte and its actin cytoskeleton: these will be the focus of this article and I will attempt to highlight the clinical and therapeutic relevance of recent advances in the understanding of their cellular and molecular biology.
Podocyte injury and repair are relevant to the diverse forms of renal disease, including major clinical problems such as diabetic nephropathy [2], but many of the key principles are illustrated by focal segmental glomerulosclerosis (FSGS), a common and important cause of nephrotic syndrome. It is now generally accepted that the predominant glomerular lesion in FSGS is an injury to podocytes [3]. FSGS is one of the most difficult renal diseases to manage, with a real possibility that the cumulative toxicity of the various agents used in treatment has the potential to make the therapy as bad as or worse than the disease. Although our drugs are often effective in controlling the nephrotic state, we have all seen patients whose physical appearance, bones, skin, glucose tolerance, etc. have been wrecked by long-term treatment with corticosteroids or whose kidney function has been damaged by calcineurin inhibitor toxicity. We urgently need more specific agents: there is accumulating evidence that the podocyte is the appropriate cellular target [4], and in particular, agents that stabilize the actin cytoskeleton are likely to be beneficial.
Podocytes: cells of great beauty
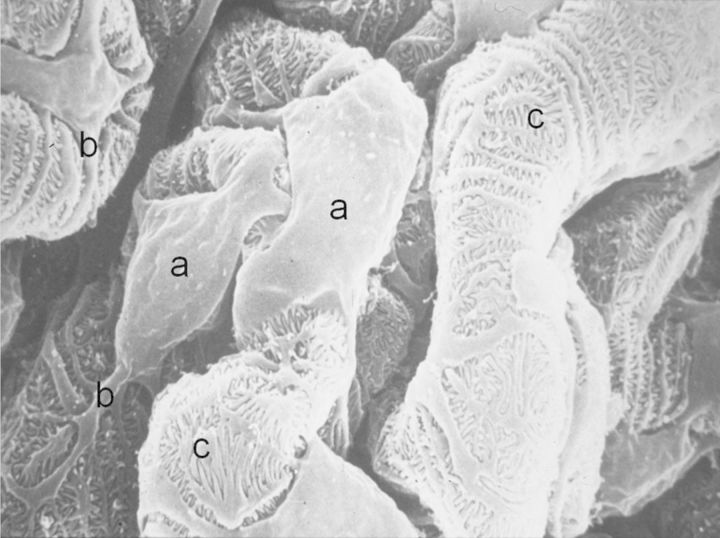
Podocytes imaged by scanning electron microscopy are, in the opinion of this author, aesthetically very pleasing (Figure 1). They have a cell body, long primary processes and a complex network of interdigitating secondary processes. This complex cellular architecture is maintained by a precise organization of microtubules and actin filaments in the cellular cytoplasm.
Fig. 1.
Scanning electron micrograph of podocytes on urinary aspect of normal glomerular capillary showing: (a) cell bodies, (b) primary processes and (c) interdigitating secondary processes that form filtration slits resembling the teeth of a zipper.
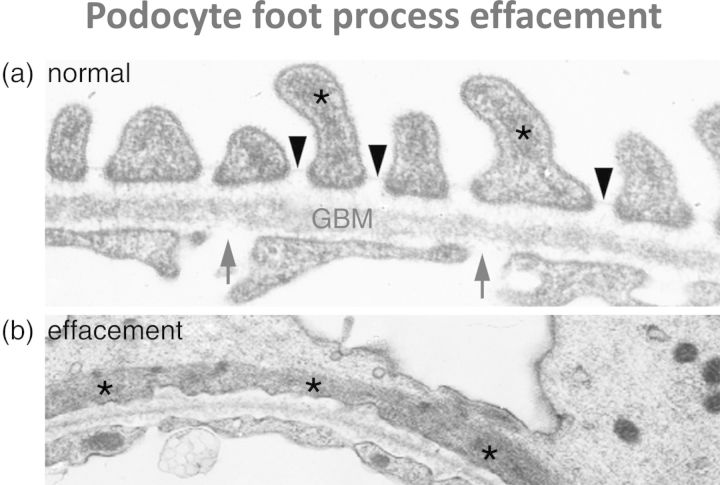
Transmission electron microscopic imaging will be more familiar to nephrologists from looking at real biopsies and shows that the normal glomerular capillary wall, on the urinary side, has a series of filtration slits between the foot processes of the podocytes (Figure 2a). It has been known for decades that a cardinal feature of the glomerular capillary wall in proteinuric states is flattening or effacement of the foot processes due to disruption of this precise organization. The key to understanding the importance of the actin cytoskeleton is the demonstration (Figure 2b) that this effacement of foot processes is associated with flattening of the actin filaments.
Fig. 2.
Transmission electron micrographs of the human glomerular capillary wall. (a) shows the normal appearance: arrows indicate endothelial fenestrations, arrowheads indicate filtration slits between podocyte foot processes and asterisks indicate actin filaments in podocyte cytoplasm. Note the actin filaments focused in podocyte foot processes. (b) shows podocyte foot process effacement as seen in proteinuric states: note flattening of the actin filaments (asterisked) longitudinally associated with the loss of normal foot process architecture.
In recent years, it has become ever clearer that the precise organization and regulation of the actin cytoskeleton in the podocyte are essential for the maintenance of its normal structure and function, that disruption thereof is a feature of podocyte diseases and is associated with proteinuria, and perhaps most importantly of all that therapeutic agents which have beneficial effects in nephrotic syndrome are capable of restoring the podocyte actin cytoskeleton.
Podocytes' actin cytoskeleton: insights from genetic conditions
Many of the recent advances in the understanding of podocyte biology have come from studies of rare inherited forms of nephrotic syndrome which have been found to be due to mutations in podocyte genes (reviewed in [5]). One example with particular relevance to the importance of the actin cytoskeleton concerns the gene encoding alpha-actinin IV, which plays an important role in actin polymerization. Elegant work from Martin Pollak's group showed that mutations in alpha-actinin IV were associated with autosomal dominant late-onset familial FSGS [6] and that the mutant form of the protein binds more avidly to actin and affects the mechanical properties of actin gels, providing an explanation for its effect on the podocyte structure [7]. Another group then showed that podocyte-specific transgenic expression of the mutant alpha-actinin IV gene in mice leads to FSGS [8], demonstrating that it is the effects of the gene in the podocyte rather than any other cell that is responsible for the disease. The final proof of the causative role of the mutation came in experiments in which Pollak's group showed that, when the mutant gene was ‘knocked-in’ in mice, the animals developed FSGS, showing that this gene defect alone is capable of causing the disease [9]. The mechanisms underlying the effects of the mutant protein have now been demonstrated in detail [10]. Pollak's group have provided further evidence that the physicochemical characteristics of actin fibres formed with the mutant alpha-actinin IV show altered flexibility that can explain the effects on the podocyte [11]. Most recently, the alpha-actinin IV protein mutants have been shown to mislocalize to the cell cytoplasm and lose their ability to associate with nuclear receptors and activate gene transcription [12]. Other groups have reported different gene mutations which affect the actin cytoskeleton and also cause FSGS: for instance, in the gene encoding CD2-associated protein which encodes a protein that is important in linking to actin fibres [13].
What about acquired forms of proteinuric disease?
Clinical nephrologists will inevitably ask whether these rare genetic forms of FSGS are analogous to the much more common sporadic forms of the disease. Shared mechanisms are likely to exist: for example, in relation to the glomerular disease associated with human immunodeficiency virus (HIV) in which podocytes are specifically targeted by the virus, a key HIV protein called Nef interacts with actin and alters the shape of podocytes [14]. Does the actin cytoskeleton play a similar role in idiopathic FSGS? We do not yet know for sure, but recent observations on the mechanisms of action of drugs which are effective in FSGS have caused real excitement and shown the way to more specific forms of treatment. Calcineurin inhibitors, especially cyclosporin, are widely used in the treatment of proteinuric diseases including FSGS; their use originally being based on the assumption that the diseases are immune-mediated and that, therefore, the immunosuppressive effects of such drugs are likely to be helpful. Faul et al. [15] made the novel observation that the anti-proteinuric effects of cyclosporin can be explained by direct effects on the podocyte actin cytoskeleton (and therefore the cell's shape) and are independent of its effects on T lymphocytes. The mechanism involves synaptopodin, a key stabilizer of the actin cytoskeleton in podocytes. When synaptopodin is phosphorylated, it is protected from degradation. Calcineurin (which is blocked by cyclosporin) dephosphorylates synaptopodin and allows its degradation. Thus, cyclosporin prevents the degradation of synaptopodin, stabilizes the actin cytoskeleton and protects against proteinuria. This suggests that nephrologists have been using the right drugs for the wrong reason [16]. This suggestion is further supported by analysis of the effects of corticosteroids, widely used in nephrotic syndrome, without us having much idea how they work [16]. Dexamethasone is the corticosteroid usually studied in vitro because it is not a pro-drug: it has potent effects on human podocytes including on their size and shape [17] and has recently been shown to interact with alpha-actinin IV in stabilizing the podocyte actin cytoskeleton and protecting it from the injurious effects of adriamycin [18]. Many of the effects of dexamethasone on podocytes can be mimicked by another currently available form of therapy, thiazolidinediones (‘glitazones’) [19] and this may help us explain the anti-proteinuric effects of this group of drugs.
What about novel, more specific forms of therapy?
The fact that many of the treatments that we currently use have potent effects on podocytes is of great interest, but clearly these agents are non-specific and have many unwanted effects. If we are to bring benefits to our patients from the recent scientific advances, we need to develop more specific therapeutic approaches. One pathway that can be selectively targeted by drugs is the system of small GTPase proteins of the Rho family. Activation of RhoA in podocytes induces FSGS [20] with disruption of the actin cytoskeleton [21]. Rho-kinase inhibition protected podocytes and reduced proteinuria in one mouse model [22]. There is still some controversy about which protein forms the most logical target, with another recent paper suggesting that RhoA is not the key member of the family in podocytes, instead providing evidence that another Rho family GTPase, cdc42, is the key one for maintenance of the podocyte actin cytoskeleton [23]. A protein which is activated by RhoA, Arhgap24, has also been suggested as a downstream target since this molecule is important in the maintenance of normal podocyte shape and a gene mutation in the ARHGAP24 gene was found in a family with FSGS [24]. Rho kinase inhibitors are already available for therapeutic use and should be tested in humans for their effectiveness in proteinuric disease. Other promising targets with protective effects on the podocyte actin cytoskeleton, and for which therapeutic agents are already available, include blockade of the tumour necrosis factor alpha pathway [25], and stimulation of the calcium-sensing receptor [26].
Finally, just to reiterate that podocytes are not the whole story: glomerular endothelial cells also depend for their integrity on an actin cytoskeleton, and this too is regulated by Rho-kinases [27]. Disease situations such as diabetes mellitus are likely to affect both glomerular cell types, and in thinking about novel therapeutic approaches, as we strive for greater specificity we need to remember that we may need to target more than one cell type.
Conclusions
The shape and structural complexity of podocytes underlie their function, and when disrupted lead to proteinuria. The actin cytoskeleton is a dynamic, highly regulated intracellular scaffold that maintains this shape and is disrupted in genetic (and probably also in acquired) forms of the nephrotic syndrome. Currently used therapies undoubtedly have effects on podocytes and this may explain some or all of their usefulness in some forms of glomerular disease. Other currently available therapies, as yet untested in proteinuric diseases, may have great potential. New pathways are being identified in glomerular cells that may provide even better therapeutic targets in future. Watch this space!
Points of interest
Podocytes are structurally and functionally complex cells which play a key role in the normal prevention of proteinuria
In many renal diseases, including FSGS and diabetic nephropathy, podocyte injury/loss is a major pathogenetic and prognostic factor
The actin cytoskeleton in podocytes is responsible for the maintenance of their shape and their function
Podocyte foot process effacement is associated with major alterations in the actin cytoskeleton
Currently used therapies including steroids and cyclosporin have direct effects on the podocyte actin cytoskeleton
Our ever-improving knowledge of glomerular cell biology in health and disease will allow new more specific forms of therapy to be developed
Conflict of interest statement. None declared.
References
- 1.Chronic Kidney Disease Prognosis Consortium. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jim B, Santos J, Spath F, et al. Biomarkers of diabetic nephropathy, the present and the future. Curr Diabetes Rev. 2012;8:317–328. doi: 10.2174/157339912802083478. [DOI] [PubMed] [Google Scholar]
- 3.D'Agati VD. Pathobiology of focal segmental glomerulosclerosis: new developments. Curr Opin Nephrol Hypertens. 2012;21:243–250. doi: 10.1097/MNH.0b013e32835200df. [DOI] [PubMed] [Google Scholar]
- 4.Mathieson PW. The podocyte as a target for therapies—new and old. Nat Rev Nephrol. 2011;8:52–56. doi: 10.1038/nrneph.2011.171. [DOI] [PubMed] [Google Scholar]
- 5.Benoit G, Machuca E, Antignac C. Hereditary nephrotic syndrome: a systematic approach for genetic testing and a review of associated podocyte gene mutations. Pediatr Nephrol. 2010;25:1621–1632. doi: 10.1007/s00467-010-1495-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaplan JM, Kim SH, North KN, et al. Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet. 2000;24:251–256. doi: 10.1038/73456. [DOI] [PubMed] [Google Scholar]
- 7.Pollak MR. Inherited podocytopathies: FSGS and nephrotic syndrome from a genetic viewpoint. J Am Soc Nephrol. 2002;13:3016–3023. doi: 10.1097/01.asn.0000039569.34360.5e. [DOI] [PubMed] [Google Scholar]
- 8.Michaud JL, Lemieux LI, Dubé M, et al. Focal and segmental glomerulosclerosis in mice with podocyte-specific expression of mutant alpha-actinin-4. J Am Soc Nephrol. 2003;14:1200–1211. doi: 10.1097/01.asn.0000059864.88610.5e. [DOI] [PubMed] [Google Scholar]
- 9.Yao J, Le TC, Kos CH, et al. Alpha-actinin-4-mediated FSGS: an inherited kidney disease caused by an aggregated and rapidly degraded cytoskeletal protein. PLoS Biol. 2004;2:e167. doi: 10.1371/journal.pbio.0020167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weins A, Schlondorff JS, Nakamura F, et al. Disease-associated mutant alpha-actinin-4 reveals a mechanism for regulating its F-actin-binding affinity. Proc Natl Acad Sci USA. 2007;104:16080–16085. doi: 10.1073/pnas.0702451104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ward SM, Weins A, Pollak MR, et al. Dynamic viscoelasticity of actin cross-linked with wild-type and disease-causing mutant alpha-actinin-4. Biophys J. 2008;95:4915–4923. doi: 10.1529/biophysj.108.131722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khurana S, Chakraborty S, Lam M, et al. Familial focal segmental glomerulosclerosis (FSGS)-linked α-actinin 4 (ACTN4) protein mutants lose ability to activate transcription by nuclear hormone receptors. J Biol Chem. 2012;287:12027–12035. doi: 10.1074/jbc.M112.345421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shih NY, Li J, Karpitskii V, et al. Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science. 1999;286:312–315. doi: 10.1126/science.286.5438.312. [DOI] [PubMed] [Google Scholar]
- 14.Tan R, Patni H, Tandon P, et al. Nef interaction with actin compromises human podocyte actin cytoskeletal integrity. Exp Mol Pathol. 2012 doi: 10.1016/j.yexmp.2012.06.001. doi:10.1016/j.yexmp.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faul C, Donnelly M, Merscher-Gomez S, et al. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med. 2008;14:931–938. doi: 10.1038/nm.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathieson PW. Clinical implications of basic research: proteinuria and immunity—an over-stated relationship? New Engl J Med. 2008;359:2492–2494. doi: 10.1056/NEJMcibr0806881. [DOI] [PubMed] [Google Scholar]
- 17.Xing CY, Saleem MA, Coward RJ, et al. Direct effects of dexamethasone on human podocytes. Kidney Int. 2006;70:1038–1045. doi: 10.1038/sj.ki.5001655. [DOI] [PubMed] [Google Scholar]
- 18.Liu H, Gao X, Xu H, et al. α-Actinin-4 is involved in the process by which dexamethasone protects actin cytoskeleton stabilization from adriamycin-induced podocyte injury. Nephrology (Carlton) 2012;17:669–675. doi: 10.1111/j.1440-1797.2012.01645.x. [DOI] [PubMed] [Google Scholar]
- 19.Agrawal S, Guess AJ, Benndorf R, et al. Comparison of direct action of thiazolidinediones and glucocorticoids on renal podocytes: protection from injury and molecular effects. Mol Pharmacol. 2011;80:389–399. doi: 10.1124/mol.111.071654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu L, Jiang R, Aoudjit L, et al. Activation of RhoA in podocytes induces focal segmental glomerulosclerosis. J Am Soc Nephrol. 2011;22:1621–1630. doi: 10.1681/ASN.2010111146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Ellis MJ, Gomez JA, et al. Mechanisms of the proteinuria induced by Rho GTPases. Kidney Int. 2012;81:1075–1085. doi: 10.1038/ki.2011.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer-Schwesinger C, Dehde S, Sachs M, et al. Rho-kinase inhibition prevents proteinuria in immune-complex mediated anti-podocyte nephritis. Am J Physiol Renal Physiol. 2012;303:F1015–F1025. doi: 10.1152/ajprenal.00380.2011. [DOI] [PubMed] [Google Scholar]
- 23.Scott RP, Hawley SP, Ruston J, et al. Podocyte-specific loss of cdc42 leads to congenital nephropathy. J Am Soc Nephrol. 2012;23:1149–1154. doi: 10.1681/ASN.2011121206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akilesh S, Suleiman H, Yu H, et al. Arhgap24 inactivates Rac1 in mouse podocytes, and a mutant form is associated with familial focal segmental glomerulosclerosis. J Clin Invest. 2011;121:4127–4137. doi: 10.1172/JCI46458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bitzan M, Babayeva S, Vasudevan A, et al. TNFα pathway blockade ameliorates toxic effects of FSGS plasma on podocyte cytoskeleton and β3 integrin activation. Pediatr Nephrol. 2012;27:2217–2226. doi: 10.1007/s00467-012-2163-3. [DOI] [PubMed] [Google Scholar]
- 26.Oh J, Beckmann J, Bloch J, et al. Stimulation of the calcium-sensing receptor stabilizes the podocyte cytoskeleton, improves cell survival, and reduces toxin-induced glomerulosclerosis. Kidney Int. 2011;80:483–492. doi: 10.1038/ki.2011.105. [DOI] [PubMed] [Google Scholar]
- 27.Swärd P, Rippe B. Acute and sustained actions of hyperglycaemia on endothelial and glomerular barrier permeability. Acta Physiol (Oxf) 2012;204:294–307. doi: 10.1111/j.1748-1716.2011.02343.x. [DOI] [PubMed] [Google Scholar]