Abstract
Objective
To evaluate the effectiveness of physiotherapy intervention following lumbar spinal fusion.
Design
Systematic review and meta-analysis. 2 independent reviewers searched information sources, assessed studies for inclusion and evaluated risk of bias. Quantitative synthesis using standardised mean differences was conducted on comparable outcomes across trials with similar interventions.
Information sources
Predefined terms were employed to search electronic databases. Additional studies were identified from key journals, reference lists, authors and experts.
Eligibility criteria for included studies
Randomised control trials published in English prior to 30 September 2011 investigating physiotherapy outpatient management of patients (>16 years), following lumbar spinal fusion, with measurements reported on one or more outcome of disability, function and health were included.
Results
2 Randomised control trials (188 participants) from two countries were included. Both trials included a behavioural and an exercise intervention. 1 trial was evaluated as high risk of bias and one as unclear. 159 participants were incorporated in the meta-analysis. Although evidence from both trials suggested that intervention might reduce back pain short term (6 months) and long term (12 months and 2 years), and a behavioural intervention might be more beneficial than an exercise intervention, the pooled effects (0.72, 95% CI −0.25 to 1.69 at 6 months; 0.52, 95% CI −0.45 to 1.49 at 12 months and 0.75, 95% CI −0.46 to 1.96 at 2 years) did not demonstrate statistically significant effects. There was no evidence that intervention changes pain in the short (6 months) or long term (12 months and 2 years). The wide CI for pooled effects indicated that intervention could be potentially beneficial or harmful. Considerable heterogeneity was evident.
Conclusions
Inconclusive, very low-quality evidence exists for the effectiveness of physiotherapy management following lumbar spinal fusion. Best practice remains unclear. Limited comparability of outcomes and retrieval of only two trials reflect a lack of research in this area that requires urgent consideration.
Article summary
Article focus
Physiotherapy intervention is recommended following lumbar spinal fusion.
However, the most beneficial intervention and the effectiveness of physiotherapy management are unclear.
The objective was to evaluate effectiveness of physiotherapy intervention in patients following lumbar spinal fusion on clinically relevant outcomes, short and long term.
Key messages
Inconclusive, very low-quality evidence exists for the effectiveness of physiotherapy management following lumbar spinal fusion.
Best practice remains unclear.
Limited comparability of outcomes and retrieval of only two trials reflect a lack of research in this area that requires urgent consideration.
Strengths and limitations of this study
The strengths of this review are its focus to physiotherapy intervention and it being the first in this area; exploring the breadth of potential physiotherapy interventions.
The key limitation of this review is that differences were evident in the content and nature of interventions, selection of outcome measures and timing of assessment points, contributing to heterogeneity in treatment effects.
Introduction
Rationale
In the UK National Health Service, surgery is the greatest single component of expenditure for managing low back pain, with increasing numbers of lumbar fusions being performed.1 More than 4036 operations were performed in 2009/2010,2 reflecting a 14% increase from 2008/2009. The USA has also seen a well-documented increase in lumbar fusion surgery rates from 1990 to 2001 of 220%,3 4 with a corresponding 500% increase in spending for lumbar fusion from 1992 to 2003.5 The increased rates are partially attributable to advances in technology, including the Food and Drug Administration approval of intervertebral cage implants (1996) and pedicle screws (1998). Although overall lumbar surgical rates in the USA reduced from 2002 to 2007, fusion rates increased from 1.3 to 10.9 per 100 000 patients.6 In 1992, lumbar fusion accounted for 14% spending on back surgery in the USA, and by 2003, this had increased to 47%.5 Additional contributions to this increasing problem include more than 200 lumbar fusion revision operations performed annually in the UK,2 with a re-hospitalisation rate of 13% within 30 days of surgery documented in the USA.6
The primary indication for lumbar fusion is pain (back and/or leg pain) from joints with degenerative disease. Lumbar fusion is thought to stabilise the spine and reduce the need for further surgery.7 A Cochrane review of spinal surgery for lumbar spondylosis due to degenerative causes1 identified trials of variable quality, with an emphasis on surgical rather than patient outcomes, and little information available regarding occupational or long-term outcomes. The review concluded that there were conflicting results for surgery.1 These findings were confirmed by Sogaard et al 8 who summarised the literature and also concluded that there are minimal data on the reported success for patient outcome following lumbar fusion. Data from the Swedish National Spine Register reported that 25% patients reported no change or worsened pain following lumbar fusion (back and/or leg pain) and that at 12 months following surgery, 40% of patients reported dissatisfaction regarding the outcome of the surgery.9
Re-operation rates have recently become a focus of investigation. Martin et al 7 investigated the cumulative incidence of second operation for degenerative conditions in one USA state, finding that an increased proportion of fusion operations and the technical development of implants did not affect the rate of re-operation. Indeed, surgery in the late 1990s was more likely to be repeated than that in the early 1990s, contributing to a ‘substantial’ likelihood of re-operation.10
The existing variability of evidence to evaluate efficacy of lumbar fusion and some evidence of persisting symptoms and dissatisfaction following surgery highlights the necessity for evidence of the effectiveness of post-operative rehabilitation. Evidence for rehabilitation following surgical intervention in low back pain is an area of increasing interest, for example, post-discectomy.11 12 There is some debate in the literature regarding timing of intervention post-lumbar fusion owing to concern over the potential of early exercise to overload internal fixation. In view of this, Christensen et al 13 commenced rehabilitation after 3 months, although Rohlmann et al 14 found no evidence of compromise of internal fixation through exercises, except perhaps through walking as an exercise.
There is no systematic review investigating effectiveness of rehabilitation in a post-lumbar fusion population. Although physiotherapy intervention is recommended post-lumbar fusion, its effectiveness is unclear, with no evaluation of existing trials through a systematic review. Consequently, current practice and best physiotherapy practice are unclear.
Objective
To investigate the short- and long-term effectiveness of physiotherapy outpatient management following lumbar spinal fusion in terms of disability, function and health15 in patients aged >16 years.
Materials and methods
A protocol following method guidelines by the Back Review Group of the Cochrane Collaboration16 and Cochrane handbook17 informed the conduct of a systematic review, which is reported in line with the PRISMA statement.18
Eligibility criteria
Studies
Randomised control trials that evaluated the effectiveness of physiotherapy outpatient management of patients following lumbar spinal fusion.
Participants
Patients who had undergone lumbar spinal fusion, with no complications, aged >16 years.
Interventions
Any physiotherapy outpatient management intervention.
Outcome measures
Measurements reported on one or more outcome of disability, function and health15 in the short term (approximately 3–6 months post-surgery/intervention) and/or long term (12–24 months).
Information sources
The search employed sensitive topic-based strategies designed for each database (to 30 September 2011):
The Cochrane Library: Controlled Trials Register, Health Technology Assessment Database, NHS Economic Evaluation Database.
CINAHL, EMBASE, MEDLINE, PEDro, ZETOC Databases.
Selected Internet sites and Indexes: Turning Research into Practice, Health Services/Technology Assessment, PubMed.
National Research Register, Current Controlled Trials website (York).
Cochrane Back Review Group.
Hand searches key journals.
Science Citation Index and Social Science Citation Index.
Unpublished research:19 British National Bibliography for Report Literature, Dissertation Abstracts, Index to Scientific and Technical Proceedings, National Technical Information Service, System for Information on Grey Literature.
Search
Predefined search terms were used. Box 1 details an example of searches used: the Medline OvidSP search.
Box 1. Example of Medline OvidSP search strategy 1948—30 September 2011.
1. Lumbar spinal fusion or post lumbar spinal fusion.mp.
2. Spinal fusion or post spinal fusion.mp.
3. clinical trial or randomised controlled trial or RCT.mp.
4. Physical approach or physical intervention or physical management or physical therapy or physiotherapy.mp.
5. 2 and 4 and 3.
6. 2 and 4.
7. 1 and 4 and 3.
8. 1 and 4.
9. Conservative approach or conservative intervention or conservative management or conservative therapy.mp.
10. 2 and 9 and 3.
11. 2 and 9.
12. 1 and 9 and 3.
13. Exercise or active range of motion exercise$ or strengthening exercise$ or stretching exercise$ or therapeutic exercise$ or home exercise$ or proprioception exercise$ or balance exercise$ or postural exercise$.
14. Manual therapy or manipulation or massage.mp.
15. Pain management program$ or patient education or educational or self management program$.mp.
16. Transcutaneous electrical nerve stimulation or TENS or thermotherapy or electrical stimulation or heat or electrotherapy or ultrasound.mp.
17. Traction.mp.
18. 1 and 13 and 3.
19. 1 and 13.
20. 2 and 13 and 3.
21. 2 and 13.
22. 1 and 14 and 3.
23. 1 and 14.
24. 2 and 14 and 3.
25. 2 and 14.
26. Post spinal fusion and rehabilitation.
Study selection
Two subject experts (GE/E-JP to 15 March 2011 and GE/RB updated to 30 September 2011) searched information sources independently and assessed identified studies for inclusion, facilitated by grading each criterion (table 1) as eligible/not eligible/might be eligible.20 The full text of a study was reviewed and the study considered potentially relevant when it could not be clearly excluded on the basis of its Title and Abstract19 following discussion between the two independent reviewers. Full text was obtained for abstracts with insufficient information or in a situation of disagreement. A study was included when both reviewers independently assessed it as satisfying the inclusion criteria from the full text. A third reviewer (AR, methodological and subject expert) mediated in the event of disagreement following discussion.16
Table 1.
Criteria for inclusion and exclusion of studies in the review
| Criteria | |
| Inclusion criteria | |
| Study design | Randomised control trial (RCT) |
| Population | |
| Age | 16 years or older |
| Subjects | Human, male or female, outpatients |
| Condition | Post-lumbar spinal fusion |
| Intervention | Conservative physiotherapy outpatient management |
| Comparison group(s) | At least one comparison group, either placebo/other intervention/no intervention |
| Outcome | Measurement on at least one of the following outcomes: disability; functional status; physical impairment; impact on social and occupational levels of fitness; pain; quality of life |
| Measurement of short-term outcome (approximately 3–6 months post-surgery) and/or long-term outcomes (≥1 year post-surgery) | |
| Time frame | All studies conducted from 1979 onwards |
| Exclusion criteria | |
| Study design | Initial search: |
| ▶Studies stated as RCTs but do not have a comparison group or random allocation to groups | |
| Participant characteristics | ▶Multiple pathology |
| Intervention | None |
| Outcome | None |
| Language | Full article/report not written in English |
Risk of bias for each included trial was independently assessed by the same initial reviewers. Consistent with Cochrane,17 risk of bias and homogeneity of participants, interventions and outcome measures were important considerations informing potential inclusion of trials in meta-analyses, thereby ensuring meaningfulness of findings from a clinical perspective. The third reviewer mediated in situations of disagreement.16 Cohen's κ was used to assess agreement between reviewers.21 All tools and processes were piloted prior to use.
Data collection process
Using a standardised form, two reviewers (AR/CW) extracted the data independently.19 22 A third reviewer (NH) independently checked for consistency and clarity.
Data items
Data extracted for each trial included design, participants and indication, interventions and study setting, outcome measures, timing of assessments, and main results. Key outcome measures of interest were predefined as valid tools to measure pain, disability, function, physical impairment, social impact and patient satisfaction, as reflected in the domains from the WHO's International Classification of Functioning, Disability and Health.15
Risk of bias in individual studies
The Cochrane ‘risk of bias’ assessment tool23 was used to assess the internal validity of each included trial. This approach was developed through empirical research18 23 unlike most quality scales.24–26 Each ‘risk of bias’ component was reported independently, in relation to each outcome measure.23 27 Assessment by reviewers acknowledged that the component of ‘blinding’ the treating therapist is generally impossible23 and the Cochrane tool permitted evaluation of the likely influence of lack of blinding.
Summary measures
In accordance with the protocol, quantitative synthesis was conducted on comparable key outcomes across trials that had similar interventions (nature of intervention) and timing of assessments (at approximately 6 months, 12 months and 2 years post-surgery). Tools developed to measure the same underlying domain of the International Classification of Functioning15 were defined as comparable outcomes. Subject and methodological experts (AR/CW/GJ) identified the combinations of trials and outcomes on which to conduct meta-analyses. All results were reported in the context of overall risk of bias for a trial.
Meta-analyses, conducted through RevMan, compared standardised differences in means using DerSimonian–Laird random effects28 29 as the principal analyses to allow for systematic differences in effects estimated across the included trials.19 29 Ninety-five per cent confidence intervals were reported for summary statistics. For comparisons across trials that reported different measurement tools for the same outcome19 or a mixture of final value scores and change from baseline scores, standardised mean differences were selected.30
Planned methods of analysis
All authors were contacted to request either raw data or additional summary statistics to those reported. No raw data were supplied, and analyses were therefore conducted on the reported final summary statistics. Standard deviations were estimated from reported CIs or percentiles, as necessary.30 Change scores were used for studies when no other data were forthcoming, in-line with the use of random effects as primary analyses.29 Heterogeneity in treatment effects was considered by computation of I2. An analysis of the quality of the interventions was undertaken as the basis for interpretation of heterogeneity.19 31
Risk of bias across trials
Retrieval of too few trials reporting comparable outcome measures prohibited visual assessment of potential publication bias using Funnel plots.19 Consensus regarding the overall potential risk of bias was facilitated through tabulation of the summary assessment for risk of bias.
Additional analyses
With only two trials included in the review, there was a lack of information on which to conduct post-hoc supportive analyses beyond descriptive analysis.
Results
Study selection
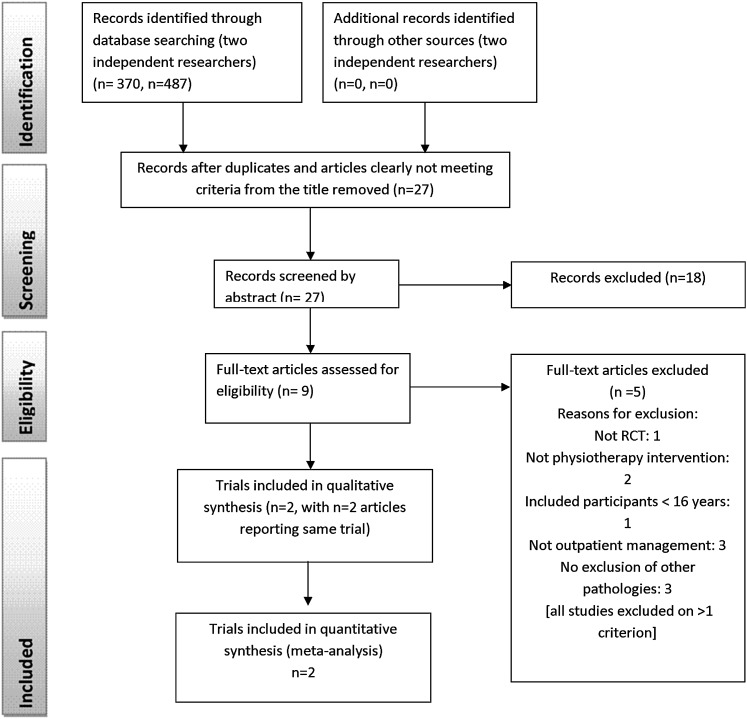
In total, four articles8 13 32 33 and two trials13 33 from two countries were included. For one trial,13 two further articles retrieved presented additional data to the original trial and were considered as part of the main trial.8 32 All but one of the retrieved trials were published in English. No relevant unpublished studies were found. Figure 1 presents the numbers of studies at each stage of selection. Complete inter-reviewer agreement was achieved on study inclusion following discussion.
Figure 1.
Study selection flow diagram (from Moher et al 18).
Study characteristics
Descriptive data for the two included trials are summarised in table 2.
Table 2.
Characteristics of eligible trials of patient management following lumbar spinal fusion surgery
| Trial | Design | Participants and indication | Intervention and setting | Outcome measures | Main results | Comments |
| Abbott et al (2010)33
Sweden |
RCT Two groups: A: Exercise therapy B: Psychomotor therapyRecruitment strategy: patients selected for lumbar spinal fusion by spinal surgeons at one University Hospital orthopaedic clinic. Recruitment over 2-year period 2005–2007. |
Patients aged 18–65 years, presenting with back
pain/sciatica >12 months without success for conservative
management; a primary diagnosis of spinal stenosis, spondylosis,
degenerative or isthmic spondylolisthesis or degenetaive disc disease;
patient selected for lumbar fusion with or without decompression;
competent in Swedish; with no previous lumbar fusion, rheumatoid
arthritis, ankylosing
spondylitis. Baseline: Pre-operative. A: n=54 Mean age (SD) 51 (11) 57% women B: n=53 Mean age (SD) 50 (10) 66% women |
Intervention: A: Exercise therapy. Inpatient respiratory and circulatory exercises, training in transfers, walking and other activities of daily living. Prior to discharge, 20 min instruction in home exercises including dynamic endurance exercises for back, abdominal and leg muscles, stretches, and cardiovascular exercises. Progression of intensity and quantity by patient's perceived level of pain. Home programme continued from 0 to 12 weeks. Activity restriction from contact sports, running, heavy lifting, outer-range lumbar movements for 6 months post-operation. B: Psychomotor therapy. Same inpatient physiotherapy and activity restriction as A. Prior to discharge, 20 min instruction in home exercises including therapeutic exercise for lumbo-pelvic stabilisation, based on cognitive–behavioural early intervention and motor re-learning principles. Home programme continued from 0–3 and then 9–12 weeks. Programme progressed during 90 min outpatient sessions at 3, 6 and 9 weeks post-operation. Setting: Outpatient physiotherapy clinic. Home exercise programme. |
Short term:
ODI V.2.0 (primary outcome) VAS back pain intensity latest week (0–100 mm)EQ-5D SF-36 mental health subscale (0–100) SES (8–64) BBQ (9–45) TSK (17–68) CSQ and subscales Long term: a/a Return to work Work status Compliance evaluated through self-reported diary. Assessments: Short term at 3 and 6 months post-operation. Long term at 12 months and 2–3 years post-operation. |
Statistically significant improvement for group B compared with group A
in: ODI, SES, BBQ and TSK at 3, 6 and 12 months, and 2–3 years; VAS at 3 and 6 months; EQ-5D at 12 months; CSQ-catastrophising subscale in group B at 6 months and 2–3 years; CSQ coping to control pain and ability to decrease pain subscales at 3, 6 and 12 months. More patients employed 2–3 years after surgery. And fewer patients had sickness leave duration >6 months after surgery. Authors did respond to request for data, but no additional data available. |
Primary outcome measure specified No primary end point specified A priori power calculation conducted on ODI (α=0.05; power =90%, MCID =10) Loss to follow-up: Dropouts: At 3 months (6%): A: n=3 withdrew B: n=3 treatment protocol violation No dropouts at 6-month follow-up. At 12 months (7%): A: n=2 (n=1 more pain, n=1 re-operation) At 2–3 years (19%): A: n=4 (n=1 not stated, n=3 pain free) B: n=8 (n=2 deceased, n=3 pain free, n=1 more pain, n=2 re-operation) No exclusions Management of losses: missing values imputed taking into account level of pain, when dropout was not associated with pain. Co-interventions not explored. ITT analyses performed. |
| Christensen et al (2003)13
Sogaard et al (2008)8 Sogaard et al (2006)32 Denmark |
RCT Three groups: A: VideoB: Back-cafe C: Training Recruitment strategy: Patients post-lumbar spondylodesis 3 months previously invited to participate. Recruitment period 1996–1999. Stratification for posterior lumbar access, posterior and anterior access combined.n=25 patients refused inclusion. Statistically significant differences between groups for climbing stairs without pain at baseline. |
Patients post-lumbar spondylodesis 3 months previously, with
severe chronic low back pain caused localised lumbar or lumbosacral
segmental instability caused by isthmic spondylolisthesis I/II, primary
degeneration (no previous surgery), secondary degeneration after
decompression surgery, accelerating degeneration after decompression
surgery; with no age <20 or >60 years, one or more
comorbidity (eg, metabolic bone disease, osteoporosis), clinical
indication of new lumbar nerve root compression, psychosocial
instability. Baseline: 1 week after 3 months post-operation follow-up clinical/radiograph. 60 women and 30 men Mean age 45 (range 24–60) A: 29 Median age (range) 45 (24–59) 83% women B: 26 Median age (range) 47 (24–60) 50% women C: 26 Median age (range) 48 (26–59) 67% women Conflicting reporting of n in each group. |
Intervention: A: Video. Video demonstration rehabilitation exercises, followed by one oral instruction by a physiotherapist. Exercises designed to provide dynamic muscular training to enhance endurance back, abdominal and leg muscles, stretching, warm up and restrictions to activity (no contact sports, training on machines at a fitness centre, running/jogging). Video and written instructions provided. B: Back-cafe. As A with additional back-cafe meetings with a physiotherapist and other patients to exchange experiences, doubts, reassurances, tips and psychological support. Involved tea/coffee, 10 min small talk, duration 1.5 h, three over period of 8 weeks. C: Training. Warm up of 15 min, condition training to improve heart–lung function, dynamic muscular endurance training of the back, abdominals and legs, and stretching exercises. Lasted 1.5 h, twice weekly over 8 weeks. Progressed repetitions individually. Unclear if a group intervention. Setting: Outpatient physiotherapy sections of hospital. Home exercise programme. |
Short term:
LBPRS: back and leg pain LBPRS: function and psychological capacity Long term: LBPRS: back and leg pain LBPRS: function and psychological capacity RTW Work status Back-related healthcare Assessments: Short term at 6 months post-operation. Long term at 12 and 24 months post-operation. |
Statistically significant improvement for: leg pain in groups A and B compared with group C at 24 months. Components of function for group B compared with groups A and C at 24 months. Sick leave for group B compared with groups A and C at 12 and 24 months. Working patients in groups B and C compared with group A at 12 months. Authors did not respond to request for data. |
No primary outcome measure specified No primary end point specified A priori power calculation reported but lacks clarity (α =0.05; power =90%). Reported that n=29 initially included in each group to ensure validity. n=9 lost to follow-up to give n=81. Loss to follow-up: Losses of n=9 (11%) overall (n=3 dissatisfied with allocation to group, n=2 re-operation, n=1 operation for malignancy, n=1 additional treatment, n=1 language issues, n=1 moved). Dropouts at each follow-up not reported. Lack of clarity over n in each group ateach assessment. No exclusions No management of losses described Co-interventions not explored No ITT analyses reported Data analysis and reporting results unclear Inconsistency in data between trial and subsequent reports |
BBQ, Back Beliefs Questionnaire; CSQ, Coping Strategies Questionnaire; EQ-5D, European Quality of Life Questionnaire; ITT, intention-to-treat; LBPRS, Low Back Pain Rating Scale; MCID, minimum clinically important difference; ODI, Oswestry Disability Index; RCT, randomised control trial; RTW, return to work; SES, Self-efficacy Scale; SF-36, Short Form 36-item health questionnaire; TSK, Tampa Scale for Kinesiophobia; VAS, Visual Analogue Scale.
Methods
Abbott et al 33 randomised participants across two groups and compared exercise therapy with psychomotor therapy. Christensen et al 13 randomised participants across three groups and compared a video of home exercises, a back-cafe and a training intervention. Both trials, therefore, included a behavioural and an exercise intervention. Duration of interventions ranged from 1 day to 9 weeks, starting between 1 day and 3 months post-surgery. Abbott et al,33 therefore, commenced interventions during the patient's inpatient stay. The number of assessments varied from 3 to 4, occurring 3 months to 2/3 years post-surgery.
Participants
Although the population for trial inclusion was broad and encompassed all patients following lumbar spinal fusion, both trials included patients of primarily a degenerative cause. The two trials randomised a total of 188 participants. Age varied from 18 to 65 years. One hundred and fifty-nine participants were included in the meta-analyses (table 2) that omitted one of the three groups from Christensen et al.13
Interventions
Both trials were conducted at single centres. Settings for interventions ranged from home to inpatient wards and to outpatient physiotherapy clinics/sections of the hospital. One trial13 included at least one intervention that was a group intervention (back-cafe), although it was unclear whether their exercise training intervention was also a group intervention. All other interventions were individual 1:1 interventions. Interventions could be grouped according to whether they were focused on exercise or a behavioural approach. Exercise interventions included exercise therapy commenced in an inpatient ward and progressed prior to discharge to provide a programme of home exercises33 and a 1.5 h training session twice a week.13 Both exercise interventions included components in the hospital environment (as an inpatient or outpatient) and one included home exercises.33 Behavioural interventions included psychomotor therapy using cognitive behavioural principles in addition to exercise33 and a back-cafe using physiotherapist and group support to continue exercises. Timing of interventions ranged from 1 day to 9 weeks, starting between 1 day and 3 months post-surgery.
Primary outcomes
One trial specified a primary outcome: the Oswestry Disability Index V.2.0.33
Secondary and additional outcomes
Both trials reported some assessment of back pain, psychological functioning and measures of occupational outcome. No other comparable outcome measures were used across the two trials. One aspect of the psychosocial outcomes, the influence of back pain on future life, was reported by both trials. However the quality of the outcome was unclear in one trial.13 Other outcomes included the following: Visual Analogue Scale back pain, subcomponents of the Low Back Pain Rating Scale (back and leg pain; function and psychological capacity), European Quality of Life Questionnaire EQ-5D, SF-36 mental health subscale, Self-efficacy Scale, Back Beliefs Questionnaire, Tampa Scale for Kinesiophobia, Coping Strategies Questionnaire, Return to Work, work status and back-related healthcare.
Risk of bias within studies
Good inter-reviewer agreement was achieved on risk of bias (Cohen's κ 0.613, 95% CI 0.359 to 0.868).21 Of the two included trials, one was evaluated overall as high risk of bias13 and one as unclear33 (table 3). Risk of bias was, therefore, considered in conjunction with other indicators of study differences (comparability of interventions, outcome measures and timings of assessments) to determine any appropriate quantitative synthesis of the trials.19 Interestingly, in the subsequent reporting of the Christensen trial,8 32 the risk of bias was improved, although, overall, it remained high (table 3). This suggests that poor reporting contributed to the rating of high risk of bias for multiple issues in the original trial report.
Table 3.
Summary assessment of the overall risk of bias for each trial
| Trial (authors, year, country) | Components of risk of bias/key risk criteria | Summary within trial | Comments on high-risk components | ||||||
| 1 | 2 | 3 | 4 | 5a | 5b | 6 | |||
| Abbott et al (2010)33 | L | L | L | L | U | U | U |
Unclear (3)
Low (4) |
|
| Christensen et al (2003)13 | U | U | U | U | U | U | H |
High (1)
Unclear (6) |
One high-risk component: 6 No primary outcome measure specified No primary end point specified No ITT reported Poor reporting, lacking detail across all components Data analysis and reporting results unclear |
| Sogaard et al (2006)32 | U | L | L | L | U | U | H |
High (1)
Unclear (3) Low (3) |
One high-risk component: 6 No primary outcome measure specified No primary end point specified No ITT reported Differences at baseline re gender |
| Sogaard et al (2008)8 | U | L | U | L | U | U | H |
High (1)
Unclear (4) Low (2) |
One high-risk component: 6 No primary outcome measure specified No primary end point specified |
Components of risk of bias/key risk criteria: 1, sequence generation; 2, allocation concealment; 3, blinding of participants, personnel and outcome assessors; 4, incomplete outcome data; 5a, short-term selective outcome reporting; 5b, long-term selective outcome reporting; 6, other potential threats to validity. Levels of risk of bias: H, high risk of bias; U, unclear risk of bias; L, low risk of bias. Summary WITHIN a study: L, low risk of bias for all key risk criteria; U, unclear risk of bias for one or more key risk criteria; H, high risk of bias for one or more key risk criteria.
ITT, intention-to-treat.
Risk of bias across studies
Christensen et al 13 had one high-risk component owing to poor reporting affording a lack of clarity across all components, no primary outcome being pre-specified, no primary end point being pre-specified and no intention-to-treat analysis reported. Both trials reported losses to follow-up. However, in both trials, losses were <20% and evaluated as acceptable.34 Interpretation of results could be affected by the high proportion of information from one trial identified as high risk of bias.23
Results of individual trials and synthesis of results
Only trials evaluated as high or unclear risk of bias were available for meta-analysis. Although the reasons for the high-risk components provided concern for potential bias, critical evaluation of results from meta-analyses enabled an overview of the current evidence and strength of effect to be presented, which permitted tentative conclusions to be proposed to advance research. Exploration of inter-trial compatibility of outcomes and assessment points identified back pain as the only possible comparison for exercise versus behavioural interventions, at 6 months, 12 months and 2 years. Reporting ‘mean change from baseline’ (SD)33 for back pain intensity during the previous week on a 0–100 mm Visual Analogue Scale was evaluated as a comparable outcome to reporting median (range)13 for ‘mean back pain intensity’ within the previous 14 days on a 0–10 scale as part of the Low Back Pain Rating Scale.
At 6 months, the evidence from one trial13 suggested that intervention might reduce back pain, with a behavioural intervention being beneficial compared with an exercise intervention (figure 2). The pooled random effects (0.72, 95% CI −0.25 to 1.69) did not support evidence of an effect at 6-month short term.
Figure 2.
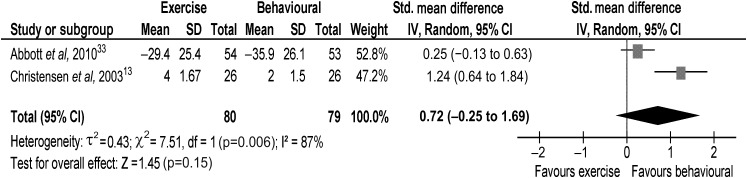
Back pain at 6-month short term.
At 12 months, the evidence from one trial13 suggested that intervention might reduce pain, with a behavioural intervention being beneficial compared with an exercise intervention (figure 3). The pooled random effects (0.52, 95% CI −0.45 to 1.49) did not support evidence of an effect at 12-month long term.
Figure 3.
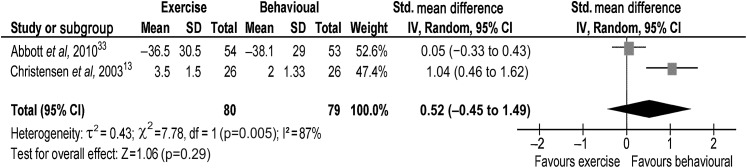
Back pain at 12-month long term.
At 2 years, the evidence from one trial13 suggested that intervention might reduce pain, with a behavioural intervention being beneficial compared with an exercise intervention (figure 4). The pooled random effects (0.75, 95% CI −0.46 to 1.96) did not support evidence of an effect at 2-year long term. Overall, there was no evidence that intervention changes pain in the shorter (6 months) or long term (12 months or 2 years).
Figure 4.
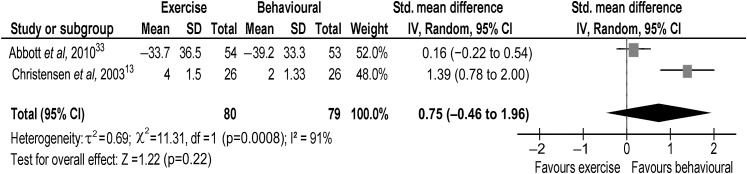
Back pain at 2-year long term.
Additional analyses
The wide CIs for pooled effects indicated intervention could be potentially beneficial or harmful. No evidence from supportive analyses conflicted with the primary analyses.
Discussion
Summary of evidence
Evidence was assessed from two randomised controlled trials (188 participants) conducted across two countries focused to lumbar fusion as a consequence of predominantly degenerative causes. Both trials included individualised 1:1 management and one trial13 included group management. Interventions were grouped into exercise versus behavioural comparisons. One trial was evaluated as high risk of bias and one as unclear. There were multiple issues contributing to the high risk of bias for Christensen et al 13 The number of issues did lessen in subsequent reporting of the Christensen trial,8 32 suggesting that trial reporting was problematic. One hundred and fifty-nine participants from the two trials were included in the meta-analyses. The only comparable outcome across the trials was back pain in the short (6 months) and long terms (12 months and 2 years). There was, consequently, limited comparability of outcomes to evaluate the potential benefits of physiotherapy intervention. No patients >65 years were included in either of the two trials. This is problematic with a documented increase in patients undergoing lumbar spinal fusion from 2000/2001 to 2009/2010 in patients in the UK aged 60–74 years of 14%–22% and aged 75+ years of 2%–6%.2
Results from the trial by Abbott et al,33 which was rated as unclear risk of bias, indicated no statistically significant difference across groups (n=54, n=53) with regard to pain, in the short or long term. Although findings from Christensen et al 13 indicated a statistically significant benefit of behavioural intervention reducing pain in the short and longer term, there were multiple issues contributing to the high risk of bias for this trial and low numbers of participants (n=29, n=26 and n=26 in the three groups). It is noted that for Christensen et al,13 the 95% CIs were positioned completely within the ‘favours’ behavioural intervention, illustrating some conflict within the pooled evidence from the two trials. Also of note were the narrower CIs for Abbott et al 33 reflecting the much larger sample size in that trial. The pooled random effects of results from the two trials provided no supporting evidence of an effect. Overall, there was no evidence that physiotherapy management changes back pain.
The strengths of this review are its focus to physiotherapy intervention and it being the first in this area, exploring the breadth of potential physiotherapy interventions. It is, therefore, not possible to compare the findings with previous reviews. The key findings of this review (only two trials, no evidence of the benefits of physiotherapy management) are a concern owing to the documented re-operation rate,2 7 10 re-hospitalisation rate6 and increasing numbers of patients undergoing lumbar spinal fusion surgery.2–4 6 When combined with existing literature reporting variability of conclusions and minimal data on the reported success for patient outcome following lumbar fusion, this concern contributes to a developing health problem, with available data supporting 25% patients reporting no change or worsened pain following surgery and 40% patients reporting dissatisfaction regarding their outcome 12 months following surgery.9 Consequently, there are major societal and economic implications from this ongoing disability, dissatisfaction and requirement for further intervention. Effective rehabilitation of patients following lumbar spinal fusion surgery is therefore an important issue.
Limitations
Differences were evident in the content and nature of interventions, selection of outcome measures and timing of assessment points, contributing to heterogeneity in treatment effects. Differences in components of the physiotherapy interventions might be explained by diversity in practice between countries. These differences limited the possible comparisons in the meta-analysis. Surprisingly, it was not possible to conduct analysis of occupational outcome measures, despite their importance being identified within the literature.1
Considerable heterogeneity was present in the evidence for behavioural intervention for pain at 6 months (I2 88%), 12 months (I2 88%) and 2 years (I2 92%),30 perhaps explaining no evidence of an effect for all evaluations. This anticipated heterogeneity was accounted for by using the random effects model.
Using GRADE35 (the Grading of Recommendations Assessment, Development and Evaluation system), the quality of the body of evidence for physiotherapy rehabilitation in the management of patients following lumbar fusion, based on the two trials included in the meta-analyses of behavioural versus exercise intervention, is very low for back pain in both the short and long term. These estimates are interpreted as ‘little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect’.35 Downgrading of quality was due to high risk of bias and issues of imprecision and inconsistency.35 The conclusions of cost-effectiveness8 in the subsequent reporting of Christensen et al 13 are uncertain considering the very low quality.
The few trials available of high and unclear risk of bias and considerable heterogeneity illustrate the necessity for a high-quality and properly powered trial to evaluate a post-lumbar spinal fusion population. Meta-analyses depend upon the availability of published trials, and in this topical area that is increasing in profile, only two trials were available. The very low quality of existing trials is consistent with earlier findings for physiotherapy management post-lumbar discectomy.12 36 Within physiotherapy, there is currently limited scope for good quality meta-analyses with well-reported and rigorous criteria for trial inclusion.36 Owing to the limited comparability of outcome measures possible, consensus for a minimum core set of outcome measures for specific populations is also required.
Conclusions
This systematic review has identified inconclusive very low-quality evidence35 for the effectiveness of physiotherapy management following lumbar spinal fusion. Best practice for physiotherapy management is, therefore, unclear. There is no identified potential benefit for improving pain. However, the limited comparability of outcome measures and retrieval of only two trials reflects a lack of published research in this area. This gap requires urgent consideration, in a properly powered clinical trial with attention to quality and, in particular, to ensuring a low risk of bias. Inclusion of other outcomes that are important to the WHO's classification15 is important, particularly occupational outcomes.1
Footnotes
To cite: Rushton A, Eveleigh G, Petherick E-J, et al. Physiotherapy rehabilitation following lumbar spinal fusion: a systematic review and meta-analysis of randomised controlled trials. BMJ Open 2012;2:e000829. doi:10.1136/bmjopen-2012-000829
Contributors: AR and GE are senior lecturers in physiotherapy and NH, RB and GJ are lecturers in physiotherapy. E-JP is a clinical physiotherapist. CW is a senior lecturer and statistician. AR and CW have long-standing professional interests in the quality and reporting of randomised controlled trials in physiotherapy. AR, GE, E-JP, NH and GJ have a professional focus to musculoskeletal physiotherapy. AR and CW were responsible for the conception of the study. All authors have contributed to the systematic review and have been involved in developing the content of the article. AR wrote the first draft of the paper and developed it initially with CW. AR has worked with all authors reworking content into subsequent drafts. All authors gave final approval of the version to be published. AR is the guarantor.
Funding: This research received no specific grant from any funding agency in public, commercial or not-for-profit sectors.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: There are no additional data.
References
- 1. Gibson JN, Waddell G. Surgery for degenerative lumbar spondylosis. Cochrane Database Syst Rev 2005;(4):CD001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. HES online. Primary Diagnosis: 3 Character Tables. The Health and Social Care Information Centre; http://www.hesonline.nhs.uk (accessed 20 Oct 2011). [Google Scholar]
- 3. Deyo RA, Gray DT, Kreuter W, et al. United States trends in lumbar fusion surgery for degenerative conditions. Spine (Phila Pa 1976) 2005;30:1441–5. [DOI] [PubMed] [Google Scholar]
- 4. Gray DT, Deyo RA, Kreuter W, et al. Population-based trends in volumes and rates of ambulatory lumbar spine surgery. Spine (Phila Pa 1976) 2006;31:1957–63. [DOI] [PubMed] [Google Scholar]
- 5. Weinstein JN, Lurie JD, Olson P, et al. United States trends and regional variations in lumbar spine surgery: 1992-2003. Spine (Phila Pa 1976) 2006;31:2707–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deyo RA, Mirza SK, Martin BI, et al. Trends, major medical complications and charges associated with surgery for lumbar stenosis in older adults. JAMA 2010;303:1259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martin BI, Mirza SK, Comstock BA, et al. Are lumbar spine reoperation rates falling with greater use of fusion surgery and new surgical technology. Spine (Phila Pa 1976) 2007;32:2119–26. [DOI] [PubMed] [Google Scholar]
- 8. Sogaard R, Bunger CE, Laurberg I, et al. Cost-effectiveness evaluation of an RCT in rehabilitation after lumber spinal fusion: a low-cost, behavioural approach is cost-effective over individual exercise therapy. Eur Spine J 2008;17:262–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stromqvist B, Fritzell P, Hagg O, et al. Follow-up of Lumbar Surgery in Sweden 2007, The Swedish National Spine Register. The Swedish Spinal Surgery Society, 2007. http://www.4s.nu/pdf/Ryggregisterrapport_2008_eng_version.pdf (accessed 26 Sep 2011). [Google Scholar]
- 10. Martin BI, Mirza SK, Comstock BA, et al. Reoperation rates following lumbar spine surgery and the influence of surgical procedures. Spine (Phila Pa 1976) 2007;32:382–7. [DOI] [PubMed] [Google Scholar]
- 11. Ostelo RW, Costa LO, Maher CG, et al. Rehabilitation after lumbar disc surgery. Cochrane Database Systematic Rev 2008;(4):CD003007. [DOI] [PubMed] [Google Scholar]
- 12. Rushton A, Wright C, Goodwin P, et al. Physiotherapy rehabilitation post first lumbar discectomy: a systematic review and meta-analysis of Randomised Controlled Trials. Spine (Phila Pa 1976) 2011;36:E961–72. [DOI] [PubMed] [Google Scholar]
- 13. Christensen FB, Laurberg I, Bunger CE. Importance of the back-cafe concept to rehabilitation after lumbar spinal fusion: a randomised clinical study with a 2-year follow-up. Spine (Phila Pa 1976) 2003;28:2561–9. [DOI] [PubMed] [Google Scholar]
- 14. Rohlmann A, Graichen F, Bergmann G. Loads on an internal spinal fusion device during physical therapy. Phys Ther 2002;82:44–52. [DOI] [PubMed] [Google Scholar]
- 15. World Health Organisation. International Classification of Functioning, Disability and Health: ICF. Geneva, Switzerland: World Health Organisation, 2001. [Google Scholar]
- 16. Furlan A, Pennick V, Bombardier C, et al. ; from the Editorial Board of the Cochrane Collaboration Back Review Group. Updated method guidelines for systematic reviews in the Cochrane Collaboration back review group. Spine (Phila Pa 1976) 2009;34:1929–41. [DOI] [PubMed] [Google Scholar]
- 17. Higgins JP, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. http://www.cochrane-handbook.org [Google Scholar]
- 18. Moher D, Liberati A, Tetzlaff J, et al. The PRISMA group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e100009. [PMC free article] [PubMed] [Google Scholar]
- 19. Centre for Reviews and Dissemination [CRD]. Systematic Reviews: CRD's Guidance for Undertaking Reviews in Healthcare. 3rd edn CRD University of York, York Publishing Services Ltd, York, 2009. [Google Scholar]
- 20. van Tulder M, Furlan A, Bombardier C. Bouter L and the Editorial Board of the Cochrane Collaboration back review group. Updated method guidelines for systematic reviews in the Cochrane Collaboration back review group. Spine (Phila Pa 1976) 2003;28:1290–9. [DOI] [PubMed] [Google Scholar]
- 21. Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas 1960;20:37–46. [Google Scholar]
- 22. Higgins JP, Deeks JJ, eds. Chapter 7: selecting studies and collecting data. In: Higgins JP, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. http://www.cochrane-handbook.org [Google Scholar]
- 23. Higgins JP, Altman DG, Sterne JA, eds. Chapter 8: assessing risk of bias in included studies. In: Higgins JP, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. http://www.cochrane-handbook.org [Google Scholar]
- 24. Moher D, Jadad AR, Nichol G, et al. Assessing the quality of randomised controlled trials: an annotated bibliography of scales and checklists. Controlled Clin Trials 1995;16:62–73. [DOI] [PubMed] [Google Scholar]
- 25. Katrak P, Bialocerkowski AE, Massy-Westropp N, et al. A systematic review of the content of critical appraisal tools. BMC Med Res Methodol 2004;4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Olivo SA, Macedo LG, Gadotti IC, et al. Scales to assess the quality of randomized controlled trials: a systematic review. Phys Ther 2008;88:156–75. [DOI] [PubMed] [Google Scholar]
- 27. Jüni P, Witschi A, Bloch R, et al. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA 1999;282:1054–60. [DOI] [PubMed] [Google Scholar]
- 28. DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 29. Green S, Higgins JP, eds. Chapter 2: Preparing a cochrane review. In: Higgins JP, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. http://www.cochrane-handbook.org [Google Scholar]
- 30. Deeks JJ, Higgins JP, Altman DG, eds. Chapter 9: Analyzing data and undertaking meta-analyses. In: Higgins JP, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. http://www.cochrane-handbook.org [Google Scholar]
- 31. Herbert RD, Bo K. Analysis of interventions in systematic reviews. BMJ 2005;331:507–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sogaard R, Christensen FB, Lauersen I, et al. Lumbar spinal fusion patients' demands to the primary health sector: evaluation of three rehabilitation protocols. A prospective randomized study. Eur Spine J 2006;15:648–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abbott AD, Tyni-Lenne R, Hedlund R. Early rehabilitation targeting cognition, behaviour and motor function after lumber fusion. Spine (Phila Pa 1976) 2010;35:848–57. [DOI] [PubMed] [Google Scholar]
- 34. Fewtrell MS, Kennedy K, Singhal A, et al. How much loss to follow-up is acceptable in long-term randomised trials and prospective studies? Arch Dis Child 2008;93:458–61. [DOI] [PubMed] [Google Scholar]
- 35. Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64:401–6. [DOI] [PubMed] [Google Scholar]
- 36. Rushton A, Calvert M, Wright C, et al. Physiotherapy trials for the 21st century – time to raise the bar? J R Soc Med 2011;104:437–41. [DOI] [PMC free article] [PubMed] [Google Scholar]