Abstract
Fibulin-1, a multi-functional extracellular matrix protein, has been demonstrated to be involved in many kinds of cancer, while its function in colorectal cancer (CRC) is unclear. So here we investigated the expression and function of fibulin-1 in CRC. The expression of fibulin-1 mRNA variants named A, B, C and D in human colorectal cancer cells and colorectal cancer specimens were determined by RT-PCR. Fibulin-1 protein expression in colorectal cancer and normal colorectal mucosa tissue was evaluated by western blot, and was further validated by immunohistochemistry and enzyme-linked immunosorbent assay at serum level. The correlations between fibulin-1 expression and the clinicopathological features of colorectal cancers were evaluated by Chi-square test and Fisher’s exact tests. The survival rates were calculated by the Kaplan-Meier method. Among fibulin-1 A-D variants, fibulin-1D is the predominant form expressed in colorectal cancer cell lines and colorectal cancer tissue, whereas only trace amounts of fibulin-1A-C were detectable. Fibulin-1 expressed higher in the CRC tissues and serum compared to normal control. So in the process of tumorigenesis of CRC, fibulin-1 is upregulated, however, high fibulin-1 expression showed longer survival time in colorectal cancer patients, especially in the patients with stage I/II. Low fibulin-1 expression was significantly associated with lymph node involvement, distant metastasis and Dukes’ C and D stage (P < 0.05 for each). Fibulin-1 protein expression may be useful as a diagnosis and prognosis marker for colorectal cancer.
Keywords: Fibulin-1, colorectal cancer, prognosis
Introduction
Colorectal cancer (CRC) is the third most common cancer and the fourth leading cause of cancer-related deaths globally [1]. CRC is a very heterogeneous disease that is caused by the interaction of genetic and environmental factors. Extensive research has established that cancer in humans arises from a cell that has acquired multiple genetic alterations in oncogenes and/or tumor suppressor genes. CRC also develops through a gradual accumulation of genetic and epigenetic changes, leading to the transformation of normal colonic mucosa into invasive cancer. In addition, tumors are not merely masses of neoplastic cells but complex tissues composed of cellular and noncellular elements such as the extracellular matrix (ECM). The dynamic and reciprocal interactions between tumor cells and cells of the tumor microenvironment as well as the ECM orchestrate events are critical to tumor progression and metastasis formation. That is, the development of migration and invasive abilities in cancers is associated with the modification of various matrix structures [2].
Fibulins are encoded by fibulin genes, a newly recognized family of extracellular matrix proteins. Fibulin-1 gene, a member of the fibulin family, is located on chromosome 22, band q13, it was discovered in affinity chromatography experiments that used the short cytoplasmic tail of β1 integrin receptors [3,4]. Fibulin-1 have been demonstrated to modulate the extracellular matrix (ECM) structure and mediate certain cell signaling transductions by binding to many ECM proteins such as fibronectin, laminin-1, fibrinogen, angiogenin, tropoelastin, proteoglycans aggrecan and versican. It plays a role in cell morphology, growth, adhesion, and mobility [5-7]. Furthermore, fibulin-1 may regulate ECM formation and stabilization which is implicated in processes such as cancer growth, cell migration and invasion [8].
Fibulin-1 was firstly considered as a tumour suppressor gene by the exploration of it’s expression and the association with estrogen in hormone dependent cancer, such as breast cancer and ovarian cancer [9-11]. Later, in gastric and prostate cancer, it was reported that fibulin-1 acted as a tumor suppressor gene and was regulated by promoter hypermethylation [12,13]. Recently, Kanda M et al, indicated that fibulin-1 was also a novel candidate tumor suppressor gene in hepatocellular carcinoma and promoter hypermethylation of fibulin-1 was associated with tumor progression [14]. Until now, expression and role of fibulin-1 in CRC is unclear. Here we investigated the expression and function of fibulin-1 in CRC.
Materials and methods
Patients and tissue samples
This study was approved by the Sir Run Shaw Hospital Ethics Committee and conducted with the consents of all patients. The specimens were collected by experienced surgeons from surgically removed colorectal cancer diagnosed at the Sir Run Shaw Hospital of Zhejiang University.
For the Western blot experiments, fresh CRC tissues and paired normal colorectal (NC) tissues from twenty patients were obtained in 2009. The CRC surgical specimens were collected from various locations in colon and rectum. Tumors were classified according to the 7th edition of the International Union against Cancer (UICC) TNM classification (Supplemental Table 1). The corresponding NC surgical specimens were taken from the margin of the resection where the tumors located at least over 10 cm apart from it. For the immunohistochemistry (IHC) experiments, A total of 133 colorectal cancer patients who underwent curative resection between February 2003 and June 2009 were enrolled. All the patients did not receive any treatment before operation. The subject population patients consisted of 78 men and 55 women, and the patients’ age ranged between 28 and 89 with a mean age of 62 years old. Two experienced pathologists reviewed the hematoxylin and eosin-stained slides of the different biopsies according to the World Health Organization (WHO) classification guidelines and chose one appropriate paraffin block. Differentiation status was divided into two types: (1) well- and moderately-differentiated type, including papillary adenocarcinoma, highly to moderately-differentiated tubular adenocarcinoma; (2) poorly-differentiated type, including poorly differentiated adenocarcinoma, signet-ring cell carcinoma, mucinous adenocarcinoma and undifferentiated carcinoma. Of the 133 cases, 114 were well/moderately differentiated and 19 were poorly differentiated cancers.
The conditional medium
The conditioned medium (CM) collection and concentration was completed according to published literature [15]. Briefly, each tissue sample produced an individual CM. The fresh tissues were washed three times with PBS and cut into 1-3 mm3 explants using a sterile blade. Then the tissues were incubated in serum-free Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Grand Island, NY) at 37°C for 24 h. After that, the CM of each tissue were centrifuged, collected, and added with protease inhibitor cocktail (Roche, Mannheim, Germany) before being stored at -80°C.
Western blot analysis
To validate the expression level of fibulin-1 in the CM, the 20 pairs of normal colorectal tissues (NCM) and CRC tissues (CCM) was pooled with an equal quantity of protein respectively. The NCM and CCM were individually concentrated by the Ultracel YM-10 centrifugal filter devices (Millipore, Bedford, MA), and protein concentration was determined using Bradford assay. Cell lysates (35 μg protein/line) were separated on a 10% SDS-PAGE gel for nitrocellulose membrane blotting. The blotted membranes were blocked with 5% skimmed milk for 1 h and incubated with mouse anti-human fibulin-1 antibody (sc-25281, Santa cruz, Dallas, USA) at 4°C overnight. The membrane was washed with TBST buffer four times and then incubated with horseradish peroxidase-conjugated goat antimouse antibody (MultiSciences Biotech, Hangzhou, China) for 1 h at room temperature. The membrane was washed with TBST buffer four times, and the detection was carried out using SuperSignal West Pico Chemiluminescent Substrate (Pierce Chemical, Rockford, IL).
Immunohistochemical staining
The ChemMate™ EnVision™ detection kit (Dako, Carpinteria, CA, USA) was used for immunohistochemistry (IHC) according to the company’s recommended procedure. Briefly, the sections were incubated with fibulin-1 antibody (sc-25281, Santa cruz, Dallas, USA) overnight at 4°C. Then Chem-Mate EnVision/HRP, Rabbit/Mouse (ENV) reagent was applied to the sections, followed by application of ChemMate DAB + chromogen included in the kit. The slides were lightly counterstained with hematoxylin. For the assessment, the fibulin-1 protein expression was assessed by the percentage of positive cells and the intensity of stained cells. The percentage of positive cells was evaluated and scored in the following categories: 0, less than 5%; 1, 5-25%; 2, 25-50%; 3, 50-75%; 4, greater than 75%. The intensity of staining cells was evaluated and scored in the following categories: 0: no staining; 1: weak staining; 2: strong staining. The two scores were summed to obtain an immunoreactivity score (IRS) value ranging from 0 to 6. To evaluate the association of fibulin-1 expression with clinical and pathological parameters, the patients were then grouped into two categories based on IRS values: low-expression (IRS 0-5) and high-expression (IRS 6).
Cell lines, primary tissues and RNA extraction
Several colorectal cancer cell lines (HCT116, HT29, DLD-1, LOVO) were studied, all primary colorectal cancer cell lines were obtained from the Sir Run Shaw Hospital of Zhejiang University. All CRC specimens were immediately snap-frozen in liquid nitrogen and stored at -80°C until further processing. Total RNA were extracted using Omega (Omega; Norcross, GA) according to the manufacturer’s instructions.
RT-PCR
The mRNA expression levels of the fibulin-1 were determined by RT-PCR with GoTaq polymerase (Promega, Madison, WI, USA) respectively. GAPDH sequence was also amplified as an internal control. Primers used for fibulin-1 variants RT-PCR was designed according to published literature [16]: fibulin-1A-AS: GATCGGGCTTGAGCAGGTCC, fibulin-1B-AS: GGGCCGTGGGGAAAAGGG, fibulin-1C-AS: CCTCCTCATTGCCGCCG, fibulin-1D-AS: CCGCAGGTTCCCTTCCG.
ELISA for fibulin-1
ELISA for fibulin-1 was performed as described previously [16], detection of fibulin-1 in serum was achieved by indirect ELISA using a Protein Detector ELISA Kit (KPL, Inc., Gaithersburg, MD). In short, The samples were diluted 100-fold with 1 × coating buffer, the 96-well microplates were coated with diluted serum (100 μL/well) and incubated overnight at 4°C. The plates were washed once with 1 × wash solution and incubated with 300 μL of 1 × bovine serum albumin (BSA) blocking solution per well for 1 h at room temperature. Then, they were incubated with fibulin-1 antibody (sc-25281, Santa Cruz, Dallas, USA) at dilution of 1:1000 and then incubated again with secondary antibody (goat antimouse IgG-HRP, MultiSciences Biotech, Hangzhou, China) for 2 h at room temperature after four washed, finally, they were incubated in mixed ABTS peroxidase substrate solution and peroxidase solution B with 1:1 for 30 min at room temperature to induce a color reaction. The absorbance at 405 nm was measured with an Emax automated microplate reader (Biotek Devices, Vermont, USA).
Statistical analysis
Statistical calculations were performed using SSPS version 18.0 for Windows (SPSS Inc., Chicago, IL, USA). All experiments were repeated at least three times. Results were summarized as means ± standard deviation (SD). Pearson’s Chi square tests and Fisher’s exact tests used to analyze the association of fibulin-1 protein expression with clinicopathological parameters. P < 0.05 was considered statistically significance.
Results
Fibulin-1 was mainly up-regulated in the CRC tissues and serum
We first validated the expression level of fibulin-1 in the CM of 20 paired CRC and NC tissues by Western blot experiments (Figure 1A). Fibulin-1 was found to be overexpressed in the CRC tissues of 17 paired samples. Fibulin-1 showed similar level in paired samples from patient 14 and only in two paired sample (patient 17, 18) fibulin-1 showed down-regulation in the CM of CRC tissue.
Figure 1.
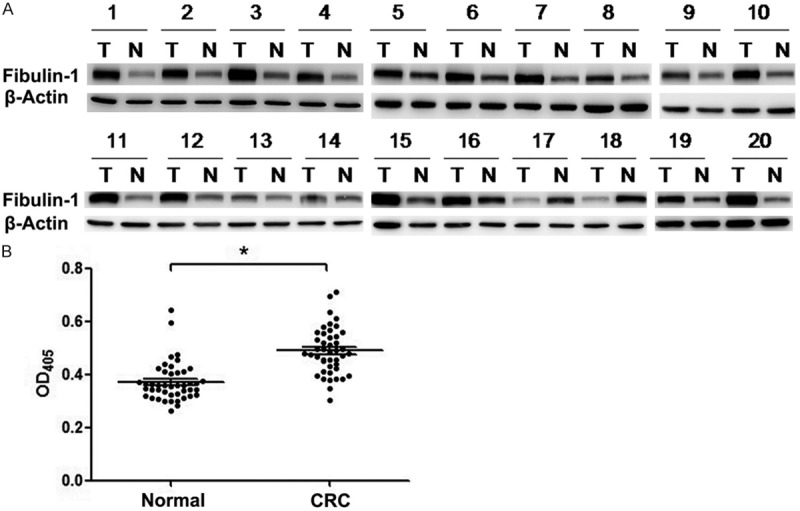
Fibulin-1 expression detected by Western blot and ELISA. A. Comparison of fibulin-1 expression level in the CM of 20 paired CRC tissue (T) and NC tissue (N) by Western blot. Fibulin-1 was up-regulated in the CRC tissues of 17 paired samples. B. ELISA experiments between 44 healthy controls and 44 CRC patients at the serum level of fibulin-1. The serum level of fibulin-1 was significantly elevated in colorectal cancer subjects when compared with healthy controls (OD: 0.37 ± 0.01 vs 0.49 ± 0.01, p < 0.001).
Then we stepped further to assess the serum level of fibulin-1 by ELISA experiments between 44 healthy controls and 44 CRC patients (Figure 1B). The serum level of fibulin-1 was significantly elevated in colorectal cancer subjects when compared with healthy controls (p < 0.001). Overall, fibulin-1 was mainly overexpressed in the CM of CRC tissues and serum.
Fibulin-1 expression in human colorectal cancer
To evaluate the fibulin-1 expression in colorectal cancer, fibulin-1 immunostaining in 133 primary colorectal cancer tissues and 9 normal colon mucosa were examined. In the normal colorectal tissues, cells showed no or weak staining for fibulin-1 protein. In the colorectal cancer tissues, the positively reactive substance of fibulin-1 protein was mainly localized in the stroma. For the comparison of fibulin-1 expression between colorectal cancer tissue and normal mucosa, the positive rate of fibulin-1 expression was significantly higher in colorectal cancer tissues (91.7%, 122/133) than in normal mucosa (0%, 0/9). Fibulin-1 expression was upregulated in human colorectal cancer stroma compared to normal colorectal tissue (Figure 2A: normal colorectal tissue; Figure 2B, 2C: colorectal cancer tissue).
Figure 2.
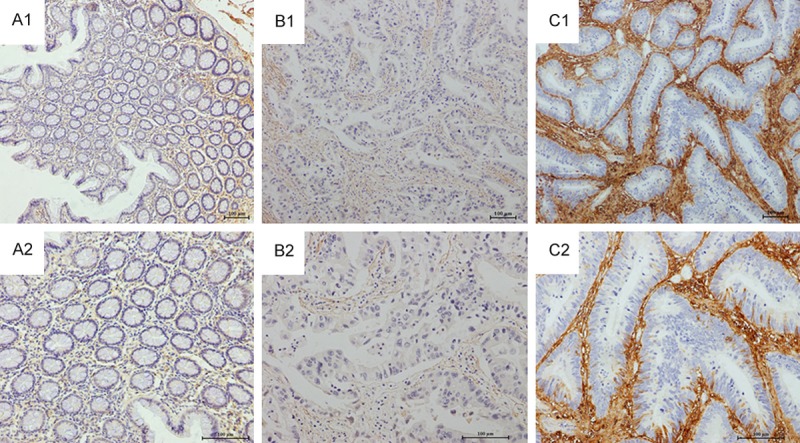
Representative immunohistochemical staining of fibulin-1 expression in normal colorectal mucosa and primary colorectal cancer. Weak fibulin-1 expression in normal colorectal mucosa (A). Typical examples of fibulin-1 staining in primary tumor samples: weak staining (B); intense staining (C). (Original magnification, A1, B1, C1: ×100; A2, B2, C2: ×200).
The relationship between fibulin-1 expression and clinicopathological parameters in patients with colorectal cancer
To evaluate the relationship between fibulin-1 protein and colorectal cancer progression, we analyzed the correlation between fibulin-1 protein expression and clinicopathological features of colorectal cancers (Table 1). Fibulin-1 expression in the stroma was not found to be associated with age, gender, tumor location, tumor histopathological grading, pT categories (P > 0.05).
Table 1.
Correlation between clinicopathological background and protein of FIBULIN-1 expression in 133 cases of colorectal cancer
| FIBULIN-1 expression | |||||
|---|---|---|---|---|---|
|
|
|||||
| N | Low expression (%) | High expression (%) | P-value | ||
| Total | 133 | ||||
| Gender | Male | 78 | 28 (35.9) | 50 (64.1) | 0.375 |
| Female | 55 | 24 (43.6) | 31 (56.4) | ||
| Age | |||||
| ≥ 62 | 67 | 29 (43.3) | 38 (56.7) | 0.376 | |
| < 62 | 66 | 23 (34.8) | 43 (56.2) | ||
| Histopathological grading | |||||
| Well/moderately | 114 | 43 (37.7) | 71 (62.3) | 0.075 | |
| Poorly | 19 | 9 (47.4) | 10 (52.6) | ||
| pT categories | |||||
| pT1 | 6 | 1 (16.7) | 5 (83.3) | 0.702 | |
| pT2 | 24 | 9 (37.5) | 15 (62.5) | ||
| pT3 | 98 | 40 (40.8) | 58 (59.2) | ||
| pT4 | 5 | 2 (40) | 3 (60) | ||
| pN categories | |||||
| pN0 | 66 | 16 (24.2) | 50 (75.8) | 0.000 | |
| pN1/2/3 | 67 | 36 (53.7) | 31 (46.3) | ||
| pM categories | |||||
| pM0 | 118 | 41 (34.7) | 77 (65.3) | 0.004 | |
| pM1 | 15 | 11 (73.3) | 4 (26.7) | ||
| Dukes stage | |||||
| A+B | 64 | 14 (21.9) | 50 (78.1) | 0.000 | |
| C+D | 69 | 38 (55.1) | 31 (44.9) | ||
The strongly positive rate of fibulin-1 expression was significantly lower in colorectal cancers with lymph node metastasis (46.3%, 31/67) than in cases without metastasis (75.8%, 50/66) (P=0.000). The strongly positive rate of fibulin-1 expression was also lower in colorectal cancers with distant metastasis (26.7%, 4/15) than in cases without distant metastasis (65.3%, 77/118) (P=0.004). Fibulin-1 expression also tended to correlate with Dukes stage (p=0.000), the strongly positive rate of fibulin-1 was significantly lower in colorectal cancers patients with Dukes C and D stage (44.9%, 31/69) than in cases with Dukes A and B stage (78.1%, 50/64).
These results suggested that thought the fibulin-1 expression in colorectal cancer tissues was much higher in colorectal cancer than that in paired normal tissues, and low-expression of fibulin-1 was significantly associated with lymph node involvement, distant metastasis and Dukes’ C and D stage (P < 0.05 for each).
Down-regulation of fibulin-1 was associated with poor survival of colorectal cancer patients
To further substantiate the importance of high fibulin-1 expression in colorectal cancer progression, the prognosis between the patients with high fibulin-1 expression and low fibulin-1 expression was compared (Figure 3C). The three-year survival rate in patients with high fibulin-1 expression and low fibulin-1 expression were 80.2% and 71.2% (P=0.199).
Figure 3.
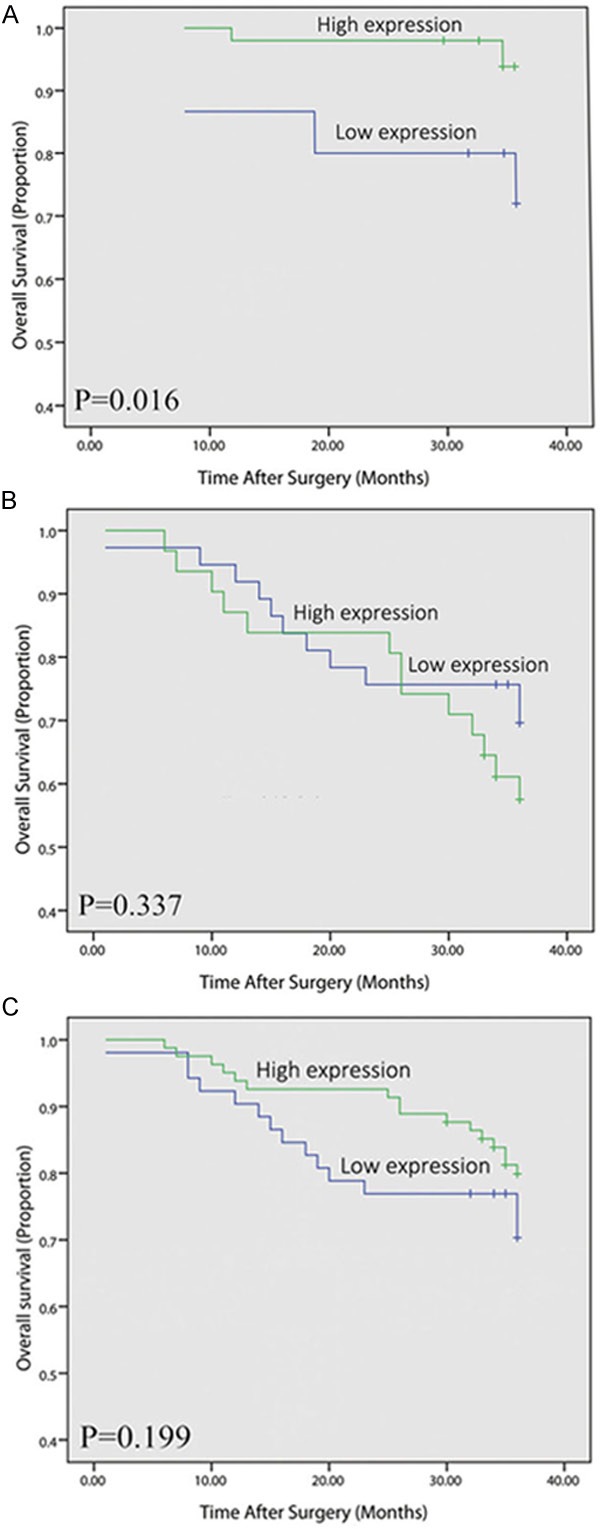
The association of fibulin-1 expression status with overall survival of colorectal cancer patients. Kaplan-Meier survival curves in regard to fibulin-1 expression in patients with early stage colorectal cancer (stage I/II, A) and advanced stage colorectal cancer (stage III/IV, B) and all colorectal cancer patients (C).
As staging is the most important prognostic factor to determine the clinical outcome of cancer patients, we stratified colorectal cancer patients with stage I/II and stage III/IV, respectively. Kaplan-Meier survival curve revealed that stage I/II patients with high-expression of fibulin-1 had remarkably longer overall survival time than those with low fibulin-1 expression. For the stage I/II, the three-year survival rate in patients with high expression and low fibulin-1 expression were 94.0% and 73.3% (P=0.016, Figure 3A). However, for the stage III/IV, the statistical difference was not significant (Figure 3B).
In conclusion, high fibulin-1 expression showed longer survival time in colorectal cancer patients, especially for the patients with stage I/II.
Fibulin-1 mRNA variant analysis in colorectal cancer cell lines and tissue
The expression of fibulin-1 A-D mRNA variants in colorectal cancer cell lines was analyzed by RT-PCR. Fibulin-1D is the predominant forms in all checked colorectal cancer cell lines (Figure 4A), whereas only trace amounts of fibulin-1A-C were detectable. The expression of fibulin-1A-D mRNA variants in 8 colorectal cancer tissues was also analyzed by RT-PCR (Figure 4B), Fibulin-1D is still the predominant forms in colorectal cancer tissue and trace amounts of fibulin-1A-C were detectable.
Figure 4.
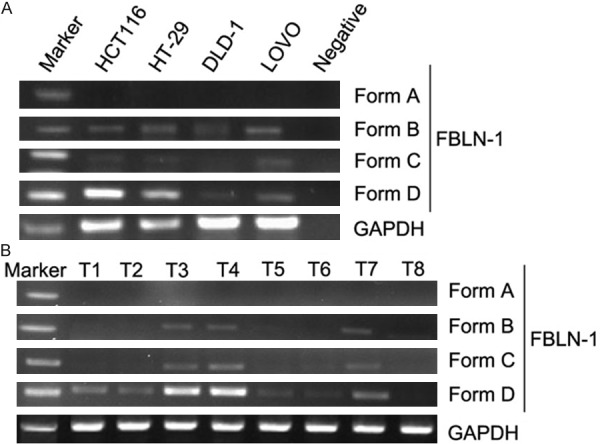
Expression of fibulin-1 mRNA variants in colorectal cancer cell lines (A) and colorectal cancer tissue (B) by RT-PCR. Fibulin-1D is the predominant form in colorectal cancer cell lines, whereas only trace amounts of fibulin-1A-C were detectable.
Discussion
The mutual and interdependent interaction between tumor and its microenvironment is a crucial topic in cancer research. Tumor progression is partly a result of evolving crosstalk between different cell types within the tumor and its surrounding supportive tissue or tumor stroma [17]. Tumor stroma interactions (cancer cell with non-neoplastic cells and cancer cell with ECM) determine not only cancer growth and metastasis but may also develop protective effects with respect to the tumor cells’ drug sensitivity/resistance. Recently, it was reported that targeting tumor stroma could improve efficacies of current therapeutics and prevent metastatic spreading. A deeper understanding of tumor microenvironment and these interactions will facilitate the design and development of novel mechanistically-acting or even individually designed drugs [2]. Our group once applied a lectin affinity based approach to enrich and increase the detection number of secreted proteins in the conditioned media of cultured tissues. They analyzed these captured proteins by the proteomic strategy of one-dimensional gel electrophoresis coupled to liquid chromatography-tandem mass spectrometry and found that 123 differentially expressed secreted proteins (DESPs) with 68 DESPs up-regulated in CRC tissues using quantification with label-free spectral counting. We found that fibulin-1 was one of the top 10 up-regulated DESPs. As one of the ECM components, fibulin-1 regulate cell morphology, growth, adhesion, and mobility and also may regulate ECM formation and stabilization [8,15]. In this study, we showed that fibulin-1 was mainly overexpressed in the CRC tissues and serum, and fibulin-1 protein expression was higher in human colorectal cancer stroma compared to normal colorectal mucosa. These results demonstrated that elevated fibulin-1 protein expression was associated with colorectal cancer and in consistent with our previous result [15].
The occurrence and development of tumor involves a dynamic process of cell proliferation and death, this process induce inflammatory responses in the stroma and proliferation of fibrous tissue constantly. On the one hand, the proliferation and division of tumor cells, activation and proliferation of stromal cells and disorder of extracellular matrix all lead to structural abnormalities of tumor tissue, On the other hand, the apoptosis and autophagic death of normal cells and partly tumor cells induce inflammatory responses which gather a large number of inflammatory cells and increase small vessels, this process produces fibroblast proliferation, epithelial-myofibroblast transition (EMT) and increased secretion of cellular matrix such as the ECM proteins. With the increased secretion of cellular matrix, fibulin-1, one of the ECM components, which modulate the ECM structure and mediate certain cell signaling transductions, was also increased expressed. Our investigation proved this supposition -- fibulin-1 was mainly up-regulated in the CRC.
There are four different splice variants of fibulin-1 named A, B, C and D, which vary in the COOH-terminal fibulin-type molecule. Fibulin-1C and fibulin-1D are expressed in a broad range of tissues and distributed mainly in vessels walls, basement membranes, microfibrils and elastic fibers [18-20]. It is widely believed that fibulin-1C acts as an oncogene and fibulin-1D acts as a tumor suppressor. Qing J, et al [21] have demonstrated that increased expression of fibulin-1D from a transfected gene in human fibrosarcoma-derived cell lines reduced anchorage-independent growth, delayed tumor growth in athymic mice and the invasive ability of these cells was greatly suppressed. These findings indicate that the loss of fibulin-1D expression contributes to the transformation and progression of human fibrosarcoma. In this study, The RT-PCR results demonstrated that fibulin-1D was the predominant form expressed in colorectal cancer cells, and fibulin-1 was considered as a tumour suppressor gene in many different types of tumor. So we supposed fibulin-1 may also act as a tumor suppressor in colorectal cancer. The immunohistochemical study showed that low-regulation of fibulin-1 expression was significantly associated with lymph node involvement, distant metastasis and Dukes’ C and D stage, and down-regulation of fibulin-1 was associated with poor survival of colorectal cancer patients, these results confirmed our speculation. Tumor studies demonstrated that fibulin-1 suppress HT1080 tumor growth, fibulin-1 suppression of HT1080 tumor growth is associated with diminished angiogenesis and also enhanced apoptosis of endothelial cells and tumor cells. It function as angiogenesis inhibitors and suppress tumor growth [22]. Moreover, fibulin-1 inhibit cellular processes such as cell adhesion and spreading. Research about the relationship between fibulin-1 and fibronectin showed that cells transfected to overproduce fibulin-1 displayed reduced velocity, distance of movement and persistence time on fibronectin substrata. The incorporation of fibulin-1 into fibronectin-containing type I collagen gels inhibited the invasion of endocardial cushion mesenchymal cells migrating from cultured embryonic heart explants. The findings indicate that fibulin-1 is an inhibitor of cell adhesion and motility [23,24]. In this study, we also observed significant overexpression of fibulin-1 protein in the colorectal cancer stroma, remodeling of the ECM is characteristic of the stromal response to cancer, contributes to the tumor microenvironment and tumor angiogenesis and invasion [25-27]. Fibulin-1 may involved in ECM remodeling and inhibit tumor angiogenesis and invasion.
In conclusion, in the process of tumorigenesis, with the increased secretion of cellular matrix, fibulin-1, as the ECM components, was also upregulated in colorectal cancer. However, the upregulation of fibulin-1 protein expression was associated with improved survival in patients, similarly, downregulation of fibulin-1 protein expression was more commonly seen in cases presenting with poor prognostic factors of colorectal cancer, leading to lymph node metastasis, late-stage and reduced survival time. Further studies needed to explore the mechanism.
Acknowledgements
This study was supported by National Natural Science Foundation of China, No. 81071651 and 81372622; the Program for Zhejiang Leading Team of ST innovation, No. 2010R10046-03; Major State Basic Research Development Program, No. 2010CB834303; National High Technology Research and Development Program of China, No. 2012AA02A601; Major Projects in Zhejiang Province, No. 2012C13014-1; and the Fundamental Research Funds for the Central Universities, No. 2012FZA7020
Disclosure of conflict of interest
None to disclose.
Supporting Information
References
- 1.Karsa LV, Lignini TA, Patnick J, Lambert R, Sauvaget C. The dimensions of the CRC problem. Best Pract Res Clin Gastroenterol. 2010;24:381–396. doi: 10.1016/j.bpg.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Ungefroren H, Sebens S, Seidl D, Lehnert H, Hass R. Interaction of tumor cells with the microenvironment. Cell Commun Signal. 2011;9:18. doi: 10.1186/1478-811X-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Timpl R, Sasaki T, Kostka G, Chu ML. Fibulins: a versatile family of extracellular matrix proteins. Nat Rev Mol Cell Biol. 2003;4:479–489. doi: 10.1038/nrm1130. [DOI] [PubMed] [Google Scholar]
- 4.Argraves WS, Dickerson K, Burgess WH, Ruoslahti E. Fibulin, a novel protein that interacts with the fibronectin receptor beta subunit cytoplasmic domain. Cell. 1989;58:623–629. doi: 10.1016/0092-8674(89)90097-4. [DOI] [PubMed] [Google Scholar]
- 5.Cooley MA, Kern CB, Fresco VM, Wessels A, Thompson RP, McQuinn TC, Twal WO, Mjaatvedt CH, Drake CJ, Argraves WS. Fibulin-1 is required for morphogenesis of neural crest-derived structures. Dev Biol. 2008;319:336–345. doi: 10.1016/j.ydbio.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barth JL, Argraves KM, Roark EF, Little CD, Argraves WS. Identification of chicken fibulin-1 homologs and characterization of the C. elegans fibulin-1 gene. Matrix Biol. 1998;17:635–646. doi: 10.1016/s0945-053x(98)90114-7. [DOI] [PubMed] [Google Scholar]
- 7.Roark EF, Keene DR, Haudenschild CC, Godyna S, Little CD, Argraves WS. The association of human fibulin-1 with elastic fibers: an immunohistological, ultrastructural, and RNA study. J Histochem Cytochem. 1995;43:401–411. doi: 10.1177/43.4.7534784. [DOI] [PubMed] [Google Scholar]
- 8.Williams SA, Schwarzbauer JE. A shared mechanism of adhesion modulation for tenascin-C and fibulin-1. Mol Biol Cell. 2009;20:1141–1149. doi: 10.1091/mbc.E08-06-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayashido Y, Lucas A, Rougeot C, Godyna S, Argraves WS, Rochefort H. Estradiol and fibulin-1 inhibit motility of human ovarian- and breast-cancer cells induced by fibronectin. Int J Cancer. 1998;75:654–658. doi: 10.1002/(sici)1097-0215(19980209)75:4<654::aid-ijc26>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 10.Greene LM, Twal WO, Duffy MJ, McDermott EW, Hill AD, O'Higgins NJ, McCann AH, Dervan PA, Argraves WS, Gallagher WM. Elevated expression and altered processing of fibulin-1 protein in human breast cancer. Br J Cancer. 2003;88:871–878. doi: 10.1038/sj.bjc.6600802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pupa SM, Argraves WS, Forti S, Casalini P, Berno V, Agresti R, Aiello P, Invernizzi A, Baldassari P, Twal WO, Mortarini R, Anichini A, Ménard S. Immunological and pathobiological roles of fibulin-1 in breast cancer. Oncogene. 2004;23:2153–2160. doi: 10.1038/sj.onc.1207323. [DOI] [PubMed] [Google Scholar]
- 12.Cheng YY, Jin H, Liu X, Siu JM, Wong YP, Ng EK, Yu J, Leung WK, Sung JJ, Chan FK. Fibulin 1 is downregulated through promoter hypermethylation in gastric cancer. Br J Cancer. 2008;99:2083–2087. doi: 10.1038/sj.bjc.6604760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wlazlinski A, Engers R, Hoffmann MJ, Hader C, Jung V, Müller M, Schulz WA. Downregulation of several fibulin genes in prostate cancer. Prostate. 2007;67:1770–1780. doi: 10.1002/pros.20667. [DOI] [PubMed] [Google Scholar]
- 14.Kanda M, Nomoto S, Okamura Y, Hayashi M, Hishida M, Fujii T, Nishikawa Y, Sugimoto H, Takeda S, Nakao A. Promoter hypermethylation of fibulin 1 gene is associated with tumor progression in hepatocellular carcinoma. Mol Carcinog. 2011;50:571–579. doi: 10.1002/mc.20735. [DOI] [PubMed] [Google Scholar]
- 15.Yao L, Lao W, Zhang Y, Tang X, Hu X, He C, Hu X, Xu LX. Identification of EFEMP2 as a serum biomarker for the early detection of colorectal cancer with lectin affinity capture assisted secretome analysis of cultured fresh tissues. J Proteome Res. 2012;11:3281–3294. doi: 10.1021/pr300020p. [DOI] [PubMed] [Google Scholar]
- 16.Moll F, Katsaros D, Lazennec G, Hellio N, Roger P, Giacalone PL, Chalbos D, Maudelonde T, Rochefort H, Pujol P. Estrogen induction and overexpression of fibulin-1C mRNA in ovarian cancer cells. Oncogene. 2002;21:1097–1107. doi: 10.1038/sj.onc.1205171. [DOI] [PubMed] [Google Scholar]
- 17.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 18.Catalano V, Turdo A, Di Franco S, Dieli F, Todaro M, Stassi G. Tumor and its microenvironment: a synergistic interplay. Semin Cancer Biol. 2013;23:522–532. doi: 10.1016/j.semcancer.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Muriel JM, Xu X, Kramer JM, Vogel BE. Selective assembly of fibulin-1 splice variants reveals distinct extracellular matrix networks and novel functions for perlecan/UNC-52 splice variants. Dev Dyn. 2006;235:2632–2640. doi: 10.1002/dvdy.20888. [DOI] [PubMed] [Google Scholar]
- 20.Muriel JM, Dong C, Hutter H, Vogel BE. Fibulin-1C and Fibulin-1D splice variants have distinct functions and assemble in a hemicentin-dependent manner. Development. 2005;132:4223–4234. doi: 10.1242/dev.02007. [DOI] [PubMed] [Google Scholar]
- 21.Qing J, Maher VM, Tran H, Argraves WS, Dunstan RW, McCormick JJ. Suppression of anchorage-independent growth and matrigel invasion and delayed tumor formation by elevated expression of fibulin-1D in human fibrosarcoma-derived cell lines. Oncogene. 1997;15:2159–2168. doi: 10.1038/sj.onc.1201385. [DOI] [PubMed] [Google Scholar]
- 22.Xie L, Palmsten K, MacDonald B, Kieran MW, Potenta S, Vong S, Kalluri R. Basement membrane derived fibulin-1 and fibulin-5 function as angiogenesis inhibitors and suppress tumor growth. Exp Biol Med (Maywood) 2008;233:155–162. doi: 10.3181/0706-RM-167. [DOI] [PubMed] [Google Scholar]
- 23.Twal WO, Czirok A, Hegedus B, Knaak C, Chintalapudi MR, Okagawa H, Sugi Y, Argraves WS. Fibulin-1 suppression of fibronectin-regulated cell adhesion and motility. J Cell Sci. 2001;114:4587–4598. doi: 10.1242/jcs.114.24.4587. [DOI] [PubMed] [Google Scholar]
- 24.Hayashido Y, Czirok A, Hegedus B, Knaak C, Chintalapudi MR, Okagawa H, Sugi Y, Argraves WS. Estradiol and fibulin-1 inhibit motility of human ovarian- and breast-cancer cells induced by fibronectin. Int J Cancer. 1998;75:654–658. doi: 10.1002/(sici)1097-0215(19980209)75:4<654::aid-ijc26>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 25.Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 26.Kunz-Schughart LA, Knuechel R. Tumor-associated fibroblasts (part I): Active stromal participants in tumor development and progression? Histol Histopathol. 2002;17:599–621. doi: 10.14670/HH-17.599. [DOI] [PubMed] [Google Scholar]
- 27.Kunz-Schughart LA, Knuechel R. Tumor-associated fibroblasts (part II): Functional impact on tumor tissue. Histol Histopathol. 2002;17:623–637. doi: 10.14670/HH-17.623. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


