Summary
miR-302/367 is the most abundant miRNA cluster in human embryonic stem cells (hESCs) and can promote somatic cell reprogramming. However, its role in hESCs remains poorly understood. Here, we studied functional roles of the endogenous miR-302/367 cluster in hESCs by employing specific TALE-based transcriptional repressors. We revealed that miR-302/367 cluster dually regulates hESC cell cycle and apoptosis in dose-dependent manner. Gene profiling and functional studies identified key targets of the miR-302/367 cluster in regulating hESC self-renewal and apoptosis. We demonstrate that in addition to its role in cell cycle regulation, miR-302/367 cluster conquers apoptosis by downregulating BNIP3L/Nix (a BH3-only proapoptotic factor) and upregulating BCL-xL expression. Furthermore, we show that butyrate, a natural compound, upregulates miR-302/367 cluster expression and alleviates hESCs from apoptosis induced by knockdown of miR-302/367 cluster. In summary, our findings provide new insights in molecular mechanisms of how miR-302/367 cluster regulates hESCs.
Graphical Abstract
Highlights
-
•
Knockdown of the endogenous miR-302/367 cluster attenuates hESC self-renewal
-
•
Endogenous miR-302/367 cluster dually regulates cell cycle and apoptosis in hESCs
-
•
miR-302/367 cluster regulates hESC self-renewal by inhibiting apoptosis pathway
-
•
Butyrate suppresses BNIP3L/Nix expression via miR-302/367 cluster
In this article, Wu and colleagues report that endogenous miR-302/367 cluster dually regulates cell cycle and apoptosis in hESCs. They demonstrate that miR-302/367 cluster regulates hESC self-renewal mainly by repressing expression of BNIP3L/Nix, a BH3-only proapoptotic protein. Furthermore, they provide evidence suggesting that Butyrate suppresses BNIP3L/Nix expression via miR-302/367 cluster.
Introduction
Human embryonic stem cells (hESCs) are valuable resources for regenerative medicine because of their unlimited and rapid self-renewal capacity and differentiation potential to generate all cell types in the body (Xu et al., 2009). However, culturing hESCs has been more technically challenging than culturing mouse ESCs because they have problematic properties such as slow growth and sensitivity to apoptosis upon cellular detachment and dissociation (Watanabe et al., 2007). hESCs usually undergo massive cell death particularly after complete dissociation, and cloning efficiency of dissociated hESCs is generally ≤1% (Amit et al., 2000; Pyle et al., 2006; Thomson et al., 1998). Although much recent efforts have been devoted to finding small molecules that can improve hESC survival after passage (Bajpai et al., 2008; Emre et al., 2010; Watanabe et al., 2007), the molecular mechanisms that govern hESC survival are not completely understood.
MicroRNAs (miRNAs) are 18–24 nucleotide-long non-coding RNAs that bind and cleave mRNAs or inhibit their translation (Ambros, 2004; Bartel, 2004). Recent studies demonstrate that miRNAs play important roles in modulating hESC self-renewal and differentiation and somatic cell reprogramming (Anokye-Danso et al., 2011; Lin et al., 2011; Miyoshi et al., 2011; Wang et al., 2008, 2014; Xu et al., 2009; Zhang et al., 2013). Among these miRNAs, miR-302/367 cluster is highly expressed in hESCs and human embryonic carcinoma cells, and overexpression of this miRNA cluster can maintain stemness of hESCs and promote somatic cell reprograming (Anokye-Danso et al., 2011; Suh et al., 2004). However, how the endogenous miR-302/367cluster regulates hESC self-renewal or growth remains largely unknown.
In the present study, we studied functional roles of the endogenous miR-302/367 cluster in hESCs using a new knockdown approach mediated by transcription activator-like effector (TALE)-based transcriptional repressor (TALE-KRAB). We demonstrated that miR-302/367 cluster dually regulates cell cycle and apoptosis pathways in hESCs in a gene dose-dependent manner. Consistent with this finding, we identified several key cell cycle regulators that are negatively regulated by miR-302/367 cluster. By performing a human apoptosis PCR array and 3′UTR luciferase reporter assay, we identified BNIP3L/Nix, a BH3-only proapoptotic gene, as a direct target gene of miR-302/367 cluster. We also revealed that miR-302/367 cluster modulates BCL-xL expression in hESCs and overexpression of BCL-xL rescues hESCs from apoptosis and their growth defect caused by knockdown of miR-302/367 cluster. Furthermore, we showed that butyrate, a natural compound and histone deacetylase inhibitor, can upregulate expression of miR-302/367 cluster in hESCs and thus alleviates their apoptosis induced by knockdown of miR-302/367 cluster. In summary, our data uncover previously unrecognized new functions of miR-302/367 cluster in dual regulation of both cell cycle and apoptosis pathways in hESCs.
Results
Knockdown of the Endogenous miR-302/367 Cluster Attenuates hESC Self-Renewal
We previously constructed TALE-based transcriptional repressors that specifically bind to the promoter region of human miR-302/367 cluster and could efficiently inhibit the elevated expression of primary miR-302/367 during reprogramming (Zhang et al., 2013). To understand functional roles of the endogenous miR-302/367 cluster in hESCs, we first determined whether TALE1-KRAB, an miR-302/367 cluster-specific TALE-based transcriptional repressor constructed previously (Zhang et al., 2013), could efficiently knock down the expression of five mature miR-302/367 members. We generated lentiviral particles expressing TALE1-KRAB or control-KRAB (with a GFP marker) and transduced them into hESCs, respectively. We sorted GFP+-transduced hESCs and measured the expression of five mature miR-302/367 members by qPCR. As shown in Figure 1A, TALE1-KRAB evenly inhibited expressions of five mature miR-302/367 members by 80% when compared with the control-KRAB group.
Figure 1.
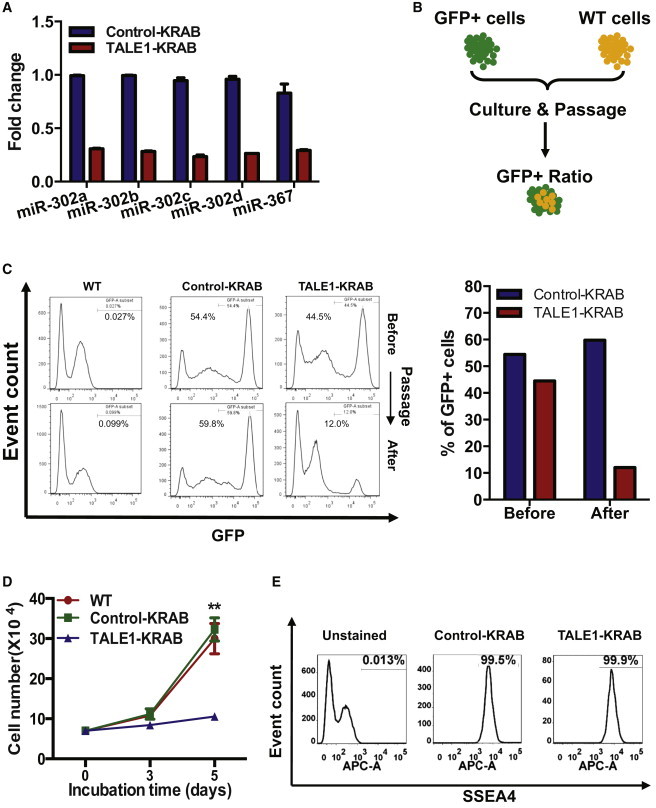
Role of the Endogenous miR-302/367 Cluster in Regulation of hESC Growth
(A) qPCR analysis of mature miR-302/367 members in hESCs stably expressing control-KRAB and TALE1-KRAB. hESCs were infected with control-KRAB or TALE1-KRAB. Transcripts of miR-302/367 members were analyzed by qPCR using specific primers. Data are represented as mean ± SD of technical replicates (n = 3).
(B) Scheme of a GFP fluorescence-based growth competition assay. GFP+ hESCs (control-KRAB or TALE1-KRAB) and GFP− hESCs (WT) were mixed at nearly 1:1 ratio and cultured together for two passages. The ratio of GFP+ and GFP− cells was determined before and after passaging.
(C) Percentage of GFP+ cell populations in hESCs stably expressing control-KRAB or TALE1-KRAB. (Left) A representation of flow cytometric analysis of GFP+ cells before and after passage. (Right) Percentage of GFP+ cells in hESCs stably expressing control-KRAB or TALE1-KRAB before and after passage. Data are a representative of two independent experiments.
(D) The effects of miR-302/367 cluster on the growth of hESCs. WT hESCs or stable expressing control-KRAB or TALE1-KRAB-hESCs were seeded alone in 12-well plate (7,000 cells per well). The cells were then counted at indicated time points. Data are represented as mean ± SD of three independent experiments (∗∗p < 0.01). See also Figure S1.
(E) Pluripotency analysis of hESCs stably expressing control-KRAB or TALE1-KRAB by flow cytometry. Pluripotency of GFP+ cell population was assessed by analysis of SSEA4 expression with anti-SSEA4 antibody. Data are a representative of two independent experiments.
It has been shown that overexpression of miR-302/367 cluster regulates G1-S transition in mouse ESCs and reduces the proliferation rate of cancer cells (Cai et al., 2013; Fareh et al., 2012; Lin et al., 2010; Wang et al., 2008). Thus, we first assessed effects of the endogenous miR-302/367 cluster on hESC growth. To do so, we performed a competitive growth assay by mixing hESCs stably expressing control-KRAB or TALE1-KRAB with a similar number of WT hESCs (Figure 1B). The percentage of GFP-positive cells before passaging was 54.4% and 44.5% of total cells for control-KRAB- and TAL1-KRAB-expressing hESCs, respectively. After two passages, the percentage of hESCs expressing control-KRAB remained almost the same (59.8%), but the percentage of hESCs expressing TALE-KRAB was decreased to 12% after passaging (Figure 1C). These data indicated that the endogenous miR-302/367 cluster is essential for hESCs growth during culture. To further verify this observation, we measured cell number of hESCs expressing control-KRAB and TALE1-KRAB. We found that proliferation rate of hESCs expressing control-KRAB and WT hESCs is nearly the same, but hESCs expressing TALE1-KRAB grow much slower than the control groups (Figure 1D). Furthermore, we measured the sizes and numbers of hESC colonies after seeding an equal number of hESCs expressing TALE1-KRAB or control-KRAB (control group). Our data showed that the TALE1-KRAB group had fewer total numbers of colonies, fewer large and medium colonies, and more small colonies when compared with control-KRAB (Figure S1).
In addition, we analyzed expression of hESC marker SSEA4 on the surfaces of hECSs expressing control-KRAB and TALE1-KRAB by flow cytometry. Our data showed that expression of SSEA4 on hESCs in the two groups is comparable, indicating that knockdown of the endogenous miR-302/367 cluster does not significantly affect hESC pluripotency under normal culturing conditions (Figure 1E). Therefore, we concluded that knockdown of the endogenous miR-302/367 cluster impairs hESC self-renewal capacity.
Knockdown of the Endogenous miR-302/367 Cluster Causes Cell Cycle Arrest and Apoptosis in hESCs
Self-renewing hESCs usually have a longer cell cycle S phase and lower apoptotic rate.
Our data showed that knockdown of the endogenous miR-302/367 cluster impairs hESC self-renewal capacity (Figure 1); we thus hypothesized that miR-302/367 cluster regulates cell cycle and/or apoptosis in hESCs. To test our hypothesis, we first assessed the effects of the endogenous miR-302/367 cluster on the hESC cell cycle. We dissociated stable hESC lines, incubated them with Vybrant DyeCycle Violet, and followed by flow cytometric analysis. Our data show that indeed hESCs expressing TALE1-KRAB accumulated in cell cycle G0/G1 phase, with a concomitant decrease in the fraction of cells in S or G2/M phrase (Figure 2A). Next, we measured proliferation of hESCs in the two groups by performing EdU incorporation assay. As shown in Figure 2B, EdU incorporation rate was significantly reduced in hESCs expressing TALE1-KRAB when compared with control-KRAB group, indicating that knockdown of the endogenous miR-306/367 cluster decreases hESC proliferation. Furthermore, we decided to determine whether knockdown of the endogenous miR-302/367 cluster causes apoptosis in hESCs during cell culture. To address this question, we dissociated hESCs expressing control-KRAB or TALE1-KRAB, stained them with Annexin V-APC, and performed analysis by flow cytometry. As shown in Figure 2C, hESCs expressing TALE1-KRAB exhibited a significantly higher apoptotic rate when compared with hESCs expressing control-KRAB. These data indicate that the endogenous miR-302/367 cluster is required to prevent hESCs from apoptosis during cell culture.
Figure 2.
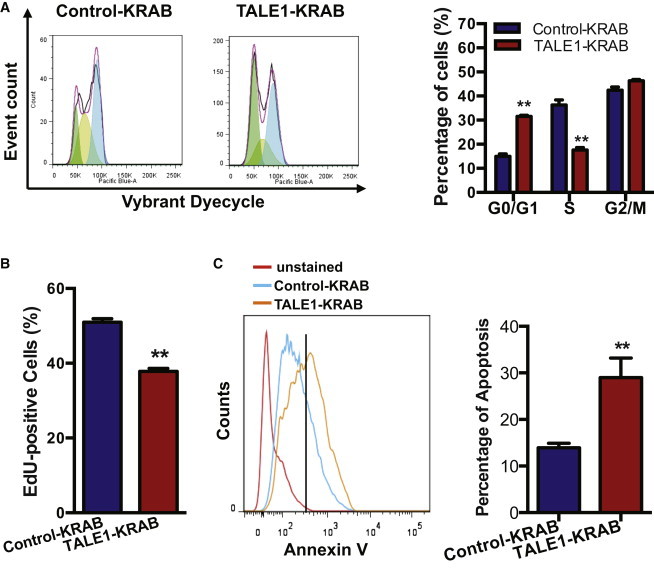
Role of miR-302/367 Cluster in Regulation of Cell Cycle and Apoptosis of hESCs
(A) Cell cycle analysis of hESCs-expressing control-KRAB or TALE1-KRAB by flow cytometry. Cells were dissected and stained by Vybrant Dyecycle according to manufacturer’s instructions. A representative graph of analyzing cell cycle processed with the FlowJo program was shown in the left, and analysis of cell cycle phase distribution was shown in the right. Data are represented as mean ± SD of three independent experiments (∗p < 0.05, ∗∗p < 0.01).
(B) Analysis of proliferating hESCs by EdU staining. hESCs expressing control-KRAB or TALE1-KRAB were cultured in 24-well plate overnight and then followed by the addition of EdU solution for 1 hr. Cells were dissected for EdU detection using the Click-iT detection kit. Data are represented as mean ± SD of three independent experiments (∗∗p < 0.01).
(C) Flow cytometric analysis of apoptotic hESCs. Control-KRAB- or TALE1-KRAB-expressing hESCs were stained with Annexin V-APC and then analyzed by flow cytometry (left). The percentage of Annexin V+ cells was determined (right). Data are represented as mean ± SD of three independent experiments (∗∗p < 0.01).
Endogenous miR-302/367 Cluster Dually Regulates Cell Cycle and Apoptosis in hESCs in a Dose-Dependent Manner
Collectively, our data show that the endogenous miR-302/367 cluster is essential for both hESC self-renewal and apoptosis (Figures 1 and 2). Now, a key question arises: how can this cluster regulate both self-renewal and apoptosis pathways? Based on miRNAs-specific modulation mechanism, one miRNA can regulate multiple target genes, and one gene can be targeted by multiple miRNAs. A previous study also showed that gene dose of each miRNA affects target selection and regulation (Shu et al., 2012). Therefore, we hypothesized that miR-302/367 cluster can dually regulate cell cycle and apoptosis pathways in hESCs in gene dose-dependent manner. To prove this hypothesis, we expanded hESCs expressing TALE1-KRAB and sorted four populations of hESCs based on GFP fluorescence: population I (P-I, GFPnegative), population II (P-II, GFPlow), population III (P-III, GFPmedium), and population IV (P-IV, GFPhigh). The three populations (P-II, P-III, P-IV) of hESCs express three different levels of GFP fluorescence: low, medium, and high. We extracted total RNAs from these four hESC populations and analyzed the transcripts of primary miR-302/367 by qPCR. Our data showed that the expression levels of pri-miR-302/367 in cell populations expressing low, medium, and high level of GFP was reduced to 70%, 40%, and 20% of the level in the control group (P-I), respectively (Figure 3B). These data indicated that the GFP fluorescence level is reversely correlated with the expression of the endogenous miR-302/367 cluster (Figures 3A and 3B). Next we stained these cells with both Vybrant DyeCycle Violet and Annexin V-APC and then analyzed cell cycle and apoptosis in each of the sorted populations by flow cytometry (Figures 3C–3E). Compared with the group with 70% of expression level (P-II), the group with 40% of expression level of miR-302/367 (P-III) accumulated in G0/G1-phase and had a decreased frequency of S-phase cells (Figure 3D). Interestingly, when the expression level of the miR-302/367 cluster was reduced to 20% (P-IV), we found a sharp decrease of hESCs in cell cycle G0/G1 and S phases and a dramatic increase of hESCs in G2/M phase (Figure 3D).
Figure 3.
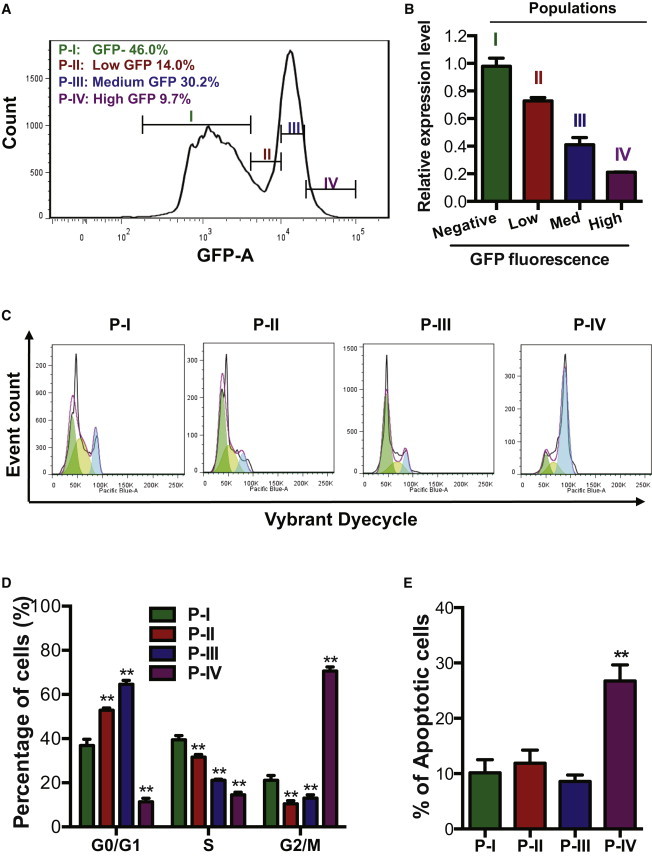
miR-302/367 Cluster Dually Regulates Cell Cycle and Apoptosis of hESC in a Dose-Dependent Manner
(A) Diagram of the gating strategy for hESC populations with different expression of TALE1-KRAB. hESCs were gated based GFP expression level (negative, low, medium, and high) and the following corresponding four populations were gated: P-I, GFPnegative; P-II, GFPlow; P-III, GFPmedium; P-IV, GFPhigh. Low, Med, and High were hESCs with low GFP fluorescence, medium GFP fluorescence, and high GFP fluorescence, respectively.
(B) qPCR analysis of pri-miR-302/367 in four populations of hESCs expressing different level of TALE1-KRAB indicated by GFP fluorescence. Data are represented as mean ± SD of technical replicates (n = 3).
(C) A representative flow cytometry analysis of cell cycle and apoptosis in the four populations of cells with different degree of GFP fluorescence (P-I, P-II, P-III, P-IV).
(D) Cell cycle phase distribution of hESCs expressing different level of TALE1-KRAB. The four cell populations (P-I, P-II, P-III, P-IV) were gated as shown in (A) and analyzed as shown in (C), and their cell cycle distribution was calculated and shown as a percentage. Data are represented as mean ± SD of three independent experiments (∗∗p < 0.01).
(E) Percentage of apoptotic cells in hESCs with a decreased expression level of miR-302/367 cluster. The apoptotic population of cells was represented by the sub-G0/G1 fraction of events in the four populations (P-I, P-II, P-III, P-IV). Data are represented as mean ± SD of three independent experiments (∗∗p < 0.01).
Furthermore, we measured apoptosis rate in each of the sorted populations by Annexin V-APC staining. Our data showed that the percentage of apoptotic cells was comparable in the groups II and III, which express approximately 70% and 40% of the expression level of the endogenous miR-302/367 cluster. Notably, we observed a sharp increase of apoptotic cells in the group IV that only express 20% of the expression level of the endogenous miR-302/367 (Figure 3E). These data clearly indicated that the endogenous miR-302/367 cluster dually regulates cell cycle and apoptosis in hESCs in a dose-dependent manner (Figures 3D and 3E).
miR-302/367 Cluster Regulates Molecular Targets Essential for hESC Cell Cycle and Apoptosis Pathways
Because transforming growth factor-β1 (TGF-β1) is involved in apoptotic pathways in several types of cells (Lee and Bae, 2002; Schuster and Krieglstein, 2002), we thus asked whether miR-302/367 cluster regulates apoptosis via TGF-β1 signaling. To address this question, we treated two groups of hESCs (control-KRAB versus TALE1-KRAB) with or without TGF-β1 or SB431542 (a chemical inhibitor of TGF-β1 receptor). Our data showed that TGF-β1 or SB431542 had little effect on apoptosis in hESCs expressing TALE1-KRAB or control-KRAB (Figure S2). To dissect the molecular mechanisms by which miR-302/367 cluster dually regulates cell cycle and apoptosis in hESCs, we examined the expression of 21 cell cycle regulators by qPCR and found that knockdown of the endogenous miR-302/367 cluster by TALE1-KRAB inhibits the expression of BMI-1, CDK2, CCND1, CCND2, CDK6 (Figure 4A; Table S1). It has been shown that these molecules play important roles in regulation of G0/G1- to S-phase transition (Abdelalim, 2013). Thus, it is likely that the endogenous miR-302/367 cluster controls hESC cell cycle progression through the regulation of these key cell cycle regulators.
Figure 4.
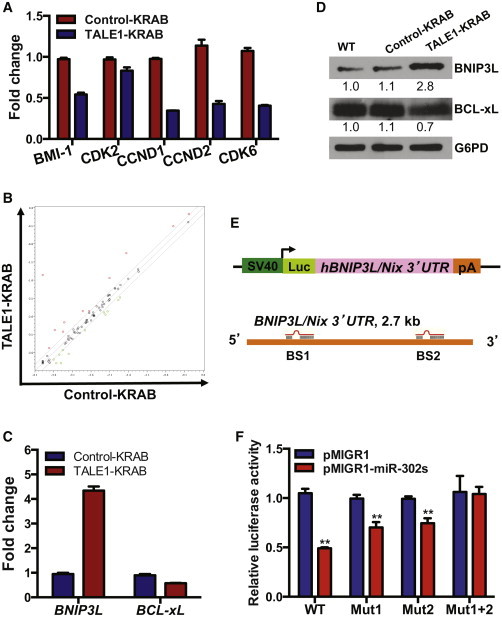
Target Genes of miR-302/367 Cluster Associated with Cell Cycle and Apoptosis in hESCs
(A) qPCR analysis of potential targets of miR-302/367 cluster regulating cell cycle in hESCs. Data are represented as mean ± SD of technical replicates (n = 3). See also Figure S2 and Table S1.
(B) Screening of apoptosis-related target genes of the miR-302/367 cluster. Patterns of gene expression were compared between the hESCs expressing control-KRAB or TALE1-KRAB using Apoptosis PCR Array. See also Table S2.
(C) qPCR analysis of BNIP3L and BCL-xL gene expression in control-KRAB- and TALE1-KRAB-expressing hESCs. Data are represented as mean ± SD of technical replicates (n = 3).
(D) Western blot analysis of BINP3L/Nix and BCL-xL in control-KRAB- and TALE1-KRAB-expressing hESCs. BINP3L/Nix and BCL-xL were detected by western blot analysis with each specific antibody. Relative expression of the BINP3L/Nix and BCL-xL was quantified by Image J (NIH) and normalized by G6PD.
(E) Schematic representation of the reporter construct containing the luciferase-coding sequence fused to the BNIP3L/Nix 3′UTR. Two predicted targeting sites for miR-302/367 cluster were designated as BS1 and BS2. See also Figure S3.
(F) BNIP3L/Nix 3′UTR luciferase reporter assay. 293T cells were transfected with the reporter plasmid pGL3-BNIP3L/Nix carrying mutations BS1 or BS2 or both, together with pMIGR1 (vector control) or pMIGR1_miR-302/367 and then cultured for 72 hr before luciferase activity assay. pCMV-LacZ was included in each transfection as an internal control to normalize luciferase activity. Data are represented as mean ± SD of three independent experiments (∗∗p < 0.01).
In contrast to cell cycle regulation, roles of miR-302/367 cluster in regulation of hESC apoptosis have not been explored. To identify potential targets of the endogenous miR-302/367 cluster in regulation of hESC apoptosis pathway, we compared expression profiles of human apoptosis-associated genes between the two groups of hESCs (control-KRAB versus TALE1-KRAB) by performing Human Apoptosis PCR array. We found that knockdown of miR-302/367 cluster results in upregulation of six genes and downregulation of five genes, respectively (Figure 4B; Table S2). Among these targets, we confirmed by qPCR that expression of BNIP3L/Nix was induced more than 4-fold and that BCL-xL was downregulated 2-fold in hESCs expressing TALE1-KRAB (Figure 4C). Our western blot analysis showed that protein expression of BNIP3L/Nix and BCL-xL was also affected in hESCs by TALE1-KRAB (Figure 4D).
miRNAs usually inhibit translation of their mRNA targets by binding with 3′UTR of their targets (Wang et al., 2008). We thus hypothesized that the endogenous miR-302/367 cluster inhibits apoptosis by suppressing the expression of proapoptotic genes. Among the six upregulated genes in hESCs expressing TALE1-KRAB, BNIP3L/Nix encodes a BH3-only proapoptotic factor and has been reported to induce apoptosis when its expression was induced or overexpressed in cells (Chen et al., 2010). Hence, we analyzed 3′UTR of BNIP3L/Nix by the miRNA target search software (http://www.microrna.org) and found that two sites in the 3′UTR of BNIP3L/Nix are potential recognition sites for miR-302/367 mature members (miR-302b, miR-302c, miR-302a, miR-302d) (Figure 4E). To determine whether BNIP3L/Nix is a true target of miR-302/367 cluster. We cloned the DNA fragment covering BNIP3L/Nix 3′UTR into pGL3-control luciferase vector and then muted its first (BS1) or second (BS2) or both potential recognition sites of miR-302/367 mature members (Figures 4E and S3). Next, we carried out a 3′UTR luciferase reporter assay to validate this bioinformatic prediction by transfecting each reporter together with an expression vector for miR-302/367 cluster. Our data revealed that the luciferase reporter containing WT BNIP3L/Nix 3′UTR was inhibited 50% by miR-302/367 cluster, whereas the mutations in either sites partially relieved the inhibition, and the double mutations fully rescued the inhibition, indicating that both sites contributed to the negative regulation by miR-302/367 cluster (Figure 4F). Interestingly, both BS1 and BS2 sites were only targeted by miR-302/367 cluster in human but not in mouse and rat, suggesting a unique regulatory role of the endogenous miR-302/367 cluster in hESC apoptosis.
miR-302/367 Cluster Regulates hESC Self-Renewal Mainly through the Inhibition of Apoptosis Pathway
So far, our data demonstrated that miR-302/367 cluster is required for hESC growth (Figure 1) and can dually regulate cell cycle and apoptosis in hESCs (Figures 3 and 4). To dissect molecular pathways by which the endogenous miR-302/367 cluster regulates hESC self-renewal, we overexpressed antiapoptotic gene BCL-xL (with a mCherry marker) in the TALE1-KRAB-expressing hESCs and examined the effects of BCL-xL on apoptosis in these cells. Significantly, our data showed that overexpression of BCL-xL not only rescued hESC from apoptosis caused by knockdown of the endogenous miR-302/367 cluster but also partially blocked the spontaneous apoptosis in WT hESCs (Figure 5A). Next, we assessed effects of BCL-xL on proliferation of hESCs expressing TALE1-KRAB using the competitive growth assay as described in Figure 1B. Our data showed that hESCs expressing TALE1-KRAB have growth disadvantage compared with WT hESCs, which is similar with the result shown in Figures 1C and 1D, but forced expression of BCL-xL enables TALE1-KRAB-expressing hESCs to regain their normal growth capacity. Because it was reported that BCL-xL is also involved in cell cycle regulation (Cheng et al., 2003; Janumyan et al., 2003), we analyzed cell cycle profile of the three groups of hESCs that express control-KRAB or TALE1-KRAB or both TALE1-KRAB and BCL-xL. As shown in Figure S4, BCL-xL only inhibits apoptosis but does not affect cell cycle progression in TALE1-KRAB hESCs. Collectively, our data undoubtedly demonstrated that the endogenous miR-302/367 cluster regulates hESC self-renewal predominantly through the inhibition of apoptosis in hESCs (Figure 5B).
Figure 5.
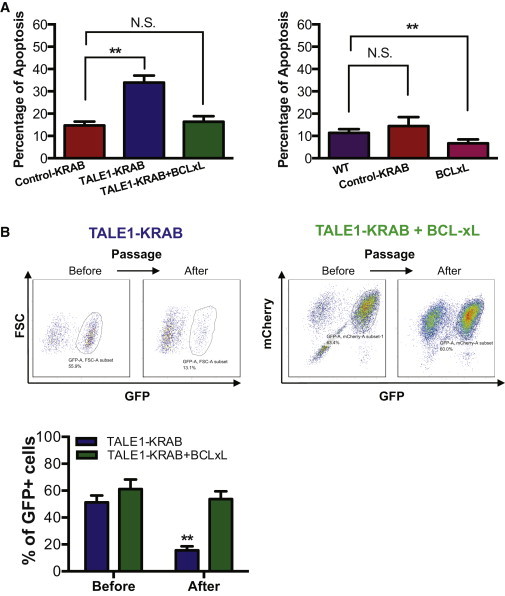
miR-302/367 Cluster Predominantly Regulates Apoptosis in hESCs
(A) Percentage of apoptotic cells in hESCs expressing control-KRAB, TALE1-KRAB, BCL-xL, or both TALE1-KRAB and BCL-xL. Cells were stained with Annexin V-APC and analyzed by flow cytometry. Data are represented as mean ± SD of three independent experiments (∗p < 0.05, ∗∗p < 0.01). See also Figure S4.
(B) Effect of apoptosis on cell growth of hESCs with knockdown of miR-302/367 cluster. hESCs expressing either TALE1-KRAB (left) or both TALE1-KRAB and BCL-xL (right) were cultured, passaged for several times, and then analyzed for GFP+ cells. The percentage of GFP+ cells was calculated for hESCs expressing TALE1-KRAB or both TALE1-KRAB and BCL-xL before and after passaging (lower). Data are represented as mean ± SD of three independent experiments (∗∗p < 0.01; N.S, no significance).
Butyrate Suppresses BNIP3L/Nix Expression through the Upregulation of miR-302/367 Cluster Expression
Our data established that knockdown of the endogenous miR-302/367 cluster impairs hESC self-renewal by triggering apoptosis. We thus predicted that increasing miR-302/367 cluster expression would restore normal growth of hESCs by alleviating them from apoptosis. Recently, we found that butyrate, a natural compound and histone deacetylase inhibitor, can enhance the expression of primary miR-302/367 during reprogramming process (Zhang and Wu, 2013). Thus, we hypothesized that butyrate might rescue, partially if not completely, hESC apoptosis induced by knockdown of endogenous miR-302/367 cluster. To test this hypothesis, we first tested the optimal concentrations of butyrate for modulating expression of miR-302/367 cluster because butyrate concentrations commonly used in cell culture (>1.0 mM) were toxic to hESCs. We found that lower concentrations of butyrate (0.25 to 0.5 mM) enhanced the expression of five mature miR-302/367 members (Figure S5). Interestingly, a low concentration of butyrate (0.5 mM) was previously shown to promote hESCs self-renewal in the absence of basic fibroblast growth factor (bFGF) (Ware et al., 2009). As expected, forced expression of TALE1-KRAB decreased the expression of miR-302/367 cluster by 2.5-fold, and butyrate induced expression of pri-miR-302/367 cluster in the control group of hESCs expressing control-KRAB by 2-fold (Figure 6A). Interestingly, butyrate treatment elevated expression of pri-miR-302/367 in hESCs expressing TALE1-KRAB to a level similar with that in the control group before butyrate treatment. Next, we measured by qPCR expression of BNIP3L/Nix, a target gene of miR-302/367 cluster in these cell groups. In agreement with our result (Figure 4), upregulation of miR-302/367 cluster by butyrate treatment downregulated BNIP3L/Nix expression to a level, which was comparable with the control groups (Figure 6B). Together, our data indicated that butyrate is able to suppress BNIP3L/Nix expression by upregulating the expression of endogenous miR-302/367 cluster.
Figure 6.
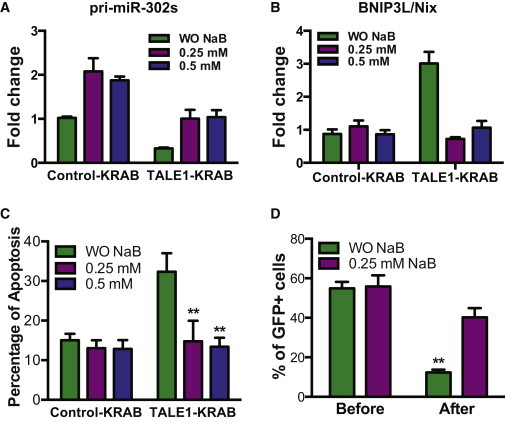
Butyrate Alleviates Apoptosis from hESC Induced by Knockdown of miR-302/367 Cluster
(A) qPCR analysis of pri-miR-302/367 transcripts in hESCs expressing control-KRAB- and TALE1-KRAB treated with or without butyrate (0.25, 0.5 mM) for 72 hr. Data are represented as mean ± SD of technical replicates (n = 3). See also Figure S5.
(B) qPCR analysis of BNIP3L/Nix transcripts in hESCs expressing control-KRAB- and TALE1-KRAB treated with or without butyrate (0.25, 0.5 mM) for 72 hr. Data are represented as mean ± SD of technical replicates (n = 3).
(C) Effect of butyrate on apoptosis induced by knockdown of miR-302/367 cluster. hESCs expressing control-KRAB or TALE1-KRAB-hESCs were treated with or without butyrate (0.25, 0.5 mM) for 72 hr and then assessed by flow cytometric analysis after staining with Annexin V-APC. Data are represented as mean ± SD of three independent experiments (∗∗p < 0.01).
(D) Effects of butyrate on growth of hESCs with knockdown of miR-302/367 cluster. hESCs stably expressing TALE1-KRAB were culture in medium with or without butyrate and percentage of GFP+ cells were analyzed by flow cytometry. Data are represented as mean ± SD of three independent experiments (∗∗p < 0.01).
Butyrate Inhibits Apoptosis and Restores Normal Growth in hESCs with Knockdown of miR-302/367 Cluster
Because our data suggested that BNIP3L/Nix is a primary mediator for apoptosis induced by knockdown of miR-302/367 cluster in hESCs, thus we asked whether restoring expression of the endogenous miR-302/367 cluster by butyrate would inhibit apoptosis in hESCs expressing TALE1-KRAB. To address this question, we measured apoptosis by Annexin V-APC staining in six groups of hESCs expressing either control-KRAB or TALE1-KRAB, which were treated with or without butyrate (Figure 6C). Significantly, our data showed that butyrate indeed suppressed apoptosis in hESCs expressing TALE1-KRAB, indicating that inhibiting BNIP3L/Nix expression is sufficient to suppress apoptosis pathway mediated by knockdown of miR-302/367 cluster. Since knockdown of miR-302/367 cluster induced apoptosis in hESCs and impaired their self-renewal (Figures 1 and 5), we thus applied a competitive growth assay as described in Figure 1B to compare relative growth rate of hESCs expressing TALE1-KRAB grown in medium with or without butyrate. As we expected, hESCs expressing TALE1-KRAB had growth disadvantage compared with WT cells when cultured in medium without butyrate (Figure 6D). In contrast, hESCs expressing TALE1-KRAB restored their normal growth rate when grown in medium with butyrate (Figure 6D). Collectively, these data demonstrate that butyrate inhibits apoptosis induced by a reduced expression of miR-302/367 cluster and therefore restores normal growth of these hESCs.
Discussion
Cell proliferation, differentiation, and death are fundamental processes in multicellular organisms (Alenzi, 2004) and are controlled by positive and negative regulators. miRNAs, as negative regulators, play critical roles in self-renewal and differentiation of hESCs (Xu et al., 2009). Here, using our TALE-based repressor approach to knock down the endogenous miR-302/367 cluster in hESCs, we reported that miR-302/367 cluster dually regulates cell cycle and apoptosis of hESCs, and such regulatory action is dependent on its expression level. Furthermore, we identified BNIP3L/Nix as a key direct target of miR-302/367 cluster and determined its predominant regulation on hESC apoptosis pathway.
The functions of miR-302/367 cluster have been investigated in various types of cells, including mouse ESCs, different cancer cells, and induced pluripotent stem cells (Cai et al., 2013; Fareh et al., 2012; Lin et al., 2010; Wang et al., 2008; Zhang et al., 2013). Wang et al. reported that miR-302 regulates cell cycle G1/S phase transition in hESCs (Lipchina et al., 2011). In cancer cells, forced expression of miR-302/367 cluster inhibited cell proliferation and tumor formation by blocking cell cycle G1/S transition (Cai et al., 2013; Fareh et al., 2012). Lin et al. (2010) recently showed that overexpression of miR302s (miR302a, miR302b, miR302c, miR302d) attenuates normal cell cycle rate without causing apoptosis in normal cells but causes massive apoptosis in multiple cancer cell lines. They also found that treatment of miR302s inhibits teratoma cell growth of Tera-2 pluripotent human embryonal carcinoma cells. In contrast, our data showed that knockdown of the endogenous miR-302/367 cluster induced G0/G1 phase arrest in hESCs, which is consistent with the previous findings in mouse and human ESCs (Wang et al., 2008; Lipchina et al., 2011). Thus, regulatory roles of the endogenous miR-302/367 cluster are different in cancer cells and mouse ESCs (mESCs) or hESCs. It was known that miR-302/367 was highly expressed in both hESCs and mESCs, but it is only slightly or not expressed in cancer cells. Therefore, the discrepancy from these studies may be partially due to different expression level of miR-302/367 cluster in different types of cells and the two different study approaches employed in these studies. Lin et al. (2010) overexpressed four members of miR302 (miR302a, miR302b, miR302c, miR302d) but not miR367 in a single vector in their study. In contrast, we applied a TALE-based repressor approach to knock down the whole miR-302/367 cluster in hESCs.
It has been reported that some genes maintain the balance between the rate of cell proliferation and apoptosis by regulating both cell cycle and apoptosis pathways (Alenzi, 2004). Although a recent study reported that both miR-17-92 and let-7a-7f clusters potentially select their targets in a dose-dependent and nonlinear fashion (Shu et al., 2012), it has not been reported previously that miRNAs can regulate both cell cycle and apoptosis pathways in dose-dependent manner. Our current findings clearly indicate that miR-302/367 dually regulates cell cycle and apoptosis in hESCs, and its action on both processes is dependent on its endogenous expression level.
Interestingly, Lin et al. (2010) showed that overexpression of miR302s (miR302a, miR302b, miR302c, miR302d) induces massive apoptosis in tumor/cancer cell lines. Scheel et al. (2009) reported that miR-302/367 cluster expression is reduced in testicular germ cell tumor and tumor suppressor p63 expression is elevated significantly. Based on these studies, it has been postulated that miR-302/367 cluster modulates apoptosis in both embryonic stem cells and cancer cells by suppressing p63 (Kuo et al., 2012). In the current study, we showed that knockdown of the endogenous miR-302/367 cluster upregulates BNIP3L/Nix expression in hESCs and verified BNIP3L/Nix as a direct target of this cluster. Recently, Pernaute et al. (2014) showed that three miRNA families (miR-20, miR-92, and miR-302) control apoptosis in mouse primed pluripotent stem cells through regulation of Bim. However, our apoptosis PCR array screening show that BIM is not a target of the endogenous miR-302/367 cluster in hESCs (data not shown). Thus, it is possible that miRNAs regulate apoptosis in mouse and human ESCs through distinctive molecular mechanisms.
We also revealed that p63 expression is not significantly affected in hESCs by knockdown of the endogenous miR-302/367 cluster (Figure S6). Thus, p63 is unlikely a target of miR-302/367 cluster in hESCs. BNIP3L/Nix belongs to the Bcl-2 family and is a BH3-only proapoptotic factor. Overexpression of BNIP3L/Nix is sufficient to induce apoptosis and necrosis-like cell death (Chen et al., 2010). Thus, BNIP3L/Nix should be a primary functional target of the endogenous miR-302/367 cluster in hESCs.
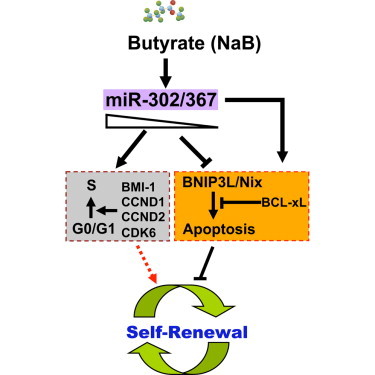
Butyrate is a histone deacetylase inhibitor and can promote ESC self-renewal across species, accumulate hESCs in S and G2/M phase, and delay the differentiation of hESCs (Ware et al., 2009). However, the molecular mechanisms underlying these actions of butyrate in hESCs remain elusive. We previously found that butyrate promotes cellular reprogramming by inducing the expression of miR-302/367 cluster (Zhang and Wu, 2013; Zhang et al., 2013). Here, we showed that butyrate could significantly induce expression of miR-302/367 cluster and downregulate its target gene BNIP3L/Nix in hESCs. Importantly, butyrate treatment rescues hESCs from apoptosis induced by knockdown of the endogenous miR-302/367 cluster. Thus, our data suggest that miR-302/367 cluster is a primary mediator for butyrate’s action in promoting hESC self-renewal and cell cycle progression (Figure 7).
Figure 7.
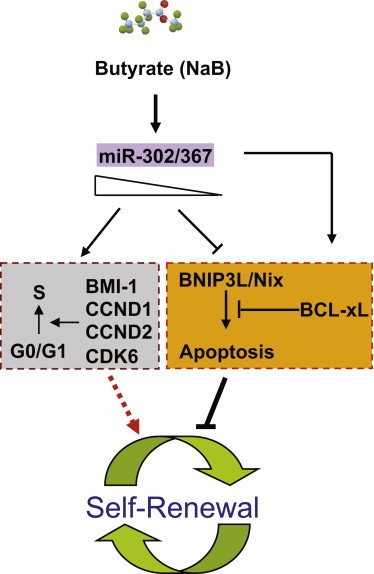
A Model for Action of miR-302/367 Cluster in Regulation of Cell Cycle and Apoptosis in hESCs
miR-302/367 cluster is inducible by sodium butyrate in hESCs, and it is required to sustain expression of key cell cycle regulators such as BMI-1, CCND1, CCND2, and CDK6. At the same time, a minimum level of miR-302/367 cluster is needed to inhibit spontaneous apoptosis in hESCs by modulates expression of BCL-xL and BH3-only proapoptotic factor BNIP3L/Nix.
In summary, our current findings highlight how miR-302/367 cluster functions as a critical positive regulator that fine tunes hESC self-renewal capacity by modulating cell cycle and apoptosis. Based on our findings, we proposed an action model for miR-302/367 cluster in hESCs (Figure 7). In this model, miR-302/367 cluster governs cell cycle and apoptosis in hESCs through regulation of distinct target genes, and it sequentially regulates cell cycle and apoptosis regulators in dose-dependent manner. Our findings provide substantial new insight into the molecular mechanisms for how miR-302/367 controls hESC self-renewal and apoptosis.
Experimental Procedures
Cell Culture
293T cells were cultured in DMEM containing 10% fetal bovine serum (FBS), and hESCs (H1 line) were cultured on mouse embryonic fibroblast (MEF) feeder cells in conventional human ESC culture medium (DMEM/F12, 20% knockout serum replacement, 1% Glutamax, 1% non-essential amino acids, 1% penicillin/streptomycin, 0.1-mM β-mercaptoethanol, and 20-ng/ml bFGF) or on BD gel in mTeSR1 medium (Stem Cell Technologies). All cell culture products were purchased from Invitrogen except where mentioned.
Construction of Luciferase Reporter and Luciferase Assay
To construct the luciferase reporter for BNIP3L/Nix, a DNA fragment containing the BNIP3L/Nix 3′UTR was amplified by PCR with specific primers (Table S3) and cloned into pGL3-Control vector (Promega), and the resultant plasmid was designed as pGL3-BNIP3L/Nix. To generate the mutant variants, point mutations in the two miR-302/367 binding sites were introduced by PCR. 293T were cultured in 24-well plate overnight and then co-transfected with 50 ng of the luciferase reporter, 925 ng of pMig or pMig-miR-302/367, and 25 ng of CMV-LacZ by using Fugene HD (Roche). After 72 hr of transfection, cells were lysed in 250 μl of the passive lysis buffer (Promega) and assayed with a luciferase assay kit (Promega), as directed by the manufacturer. The luciferase activities were expressed as relative luciferase/LacZ activities, normalized to those of control transfections in each experiments.
Lentivirus Production and Generation of Stable hESC Lines
For preparation of the lentiviruses, 293T cells were transfected with a mixture of DNA containing 2.5 μg of lentiviral vectors (control-KRAB or TALE1-KRAB) and 2.5 μg of the packaging mixture (Genecopoeia) by Fugene HD. Media containing lentiviruses was collected 24 hr after transfection and filtered through a 0.45-μm pore size filter. To generate stable hESC lines expressing control-KRAB or TLAE1-KRAB, hESCs were seeded in a 12-well plate at 5 × 104 cells per well 1 day before transduction and incubated with lentiviral particles (control-KRAB or TALE1-KRAB) containing supernatant supplemented with 5-μg/ml polybrene (Sigma), followed by centrifugation (1,000 g for 45 min). At 4 days after infection, infected cells were split using Accutase (Millipore) and plated on MEF feeders for expansion. Medium was changed every other day until 80% confluence. A pool of stable GFP+ hESCs was sorted by fluorescence-activated cell sorting (FACS) and seeded into 12-well plate precoated by BD Matrigel (BD Biosciences).
Western Blot Analysis
Cells were lysed directly with RIPA lysis buffer, supplemented with protease inhibitor cocktail (Sigma). Cell lysates were separated by electrophoresis on 12% SDS-PAGE and transferred to a nitrocellulose membrane (Pierce). The blot was blocked with TBST buffer (20-mM Tris-HCl [pH 7.6], 136-mM NaCl, and 0.05% Tween-20) containing 5% non-fat milk and then incubated with primary antibody solution at 4°C overnight. After washing with TBST buffer, the membrane was incubated with horseradish peroxidase (HRP)-conjugated secondary antibody for 1 hr at room temperature. Signals were detected with the Immobilon Western Chemiluminescent HRP substrate (Pierce). A list of primary antibodies is included in Table S4.
Flow Cytometry Analysis of Cell Cycle and Apoptosis
For analysis of cell cycle, hESCs were dissociated by Accutase, pelleted, and resuspended in Hank’s balanced salt solution (HBSS) at a cell concentration of 1 × 106 cells/ml. The cell suspensions were incubated with 5-μM Vybrant DyeCycle Violet at 37°C for 30 min and then subjected to flow cytometric analysis.
For EdU incorporation assay, hESCs stably expressing control-KRAB or TALE1-KRAB were sorted based on GFP fluorescence by FACS and cultured overnight in 12-well plate precoated BD matrigel and then incubated with 10-μM EdU (Invitrogen) in hES culture medium at 37°C for 1 hr. After incubation, cells were dissociated and labeled with AlexaFluor 647 using the Click-iT EdU Flow Cytometry Assay kit according to the manufacturer’s instructions (Invitrogen). EdU+ cells were analyzed by flow cytometry. For analysis of apoptotic hESCs, hESCs stably expressing control-KRAB or TALE1-KRAB were sorted by FACS and cultured in hESC medium for 2 days and dissociated. Cells were stained with Annexin V-APC antibody according to the manufacturer’s instruction (BD Biosciences). Annexin V+GFP+ cells were gated as apoptotic cells. The apoptotic percentage was determined by flow cytometric analysis.
Gene Expression Analysis by qPCR and PCR Array
Total RNA samples were extracted using the Quick-RNA MicroPrep Kit (Zymo Research). For qPCR analysis of individual mature miR-302/367 miRNAs, 200-ng RNA were reverse transcribed using the NCode VILO miRNA cDNA Synthesis Kit (Invitrogen). For qPCR analysis of target genes associated with cell cycle arrest and apoptosis, 500-ng RNA were reverse transcribed using Superscript III cDNA Synthesis Kit (Invitrogen). GADPH expression was used as internal control to normalize relative expression of each gene. For screening apoptotic gene candidates, human apoptosis PCR array was performed according to the manufacturer’s instructions (QIAGEN). The list of primers is included in Table S3.
Statistical Analysis
Results are presented as the mean ± SD. Data were analyzed by Student’s t test; p values ≤ 0.05 were considered significant.
Author Contributions
Z.Z. and W.-S.W. designed and interpreted all of experiments. Z.Z. planed and conducted most of the experiments. Y.H., D.X., P.Z., W.L, and E.W. were involved in some of the experiments. Z.Z., E.W., J.M, and W.-S.W wrote the manuscript.
Acknowledgments
This work was supported in part by an NICHD/NIH grant (R21 5R21HD061777) and the Jordan family’s endowment fund. Z.Z. was supported by a CIRM Berkeley scholarship (CIRM training grant TG2-01164).
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental Information
References
- Abdelalim E.M. Molecular mechanisms controlling the cell cycle in embryonic stem cells. Stem Cell Rev. 2013;9:764–773. doi: 10.1007/s12015-013-9469-9. [DOI] [PubMed] [Google Scholar]
- Alenzi F.Q. Links between apoptosis, proliferation and the cell cycle. Br. J. Biomed. Sci. 2004;61:99–102. doi: 10.1080/09674845.2004.11732652. [DOI] [PubMed] [Google Scholar]
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- Amit M., Carpenter M.K., Inokuma M.S., Chiu C.P., Harris C.P., Waknitz M.A., Itskovitz-Eldor J., Thomson J.A. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev. Biol. 2000;227:271–278. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- Anokye-Danso F., Trivedi C.M., Juhr D., Gupta M., Cui Z., Tian Y., Zhang Y., Yang W., Gruber P.J., Epstein J.A., Morrisey E.E. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell. 2011;8:376–388. doi: 10.1016/j.stem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajpai R., Lesperance J., Kim M., Terskikh A.V. Efficient propagation of single cells Accutase-dissociated human embryonic stem cells. Mol. Reprod. Dev. 2008;75:818–827. doi: 10.1002/mrd.20809. [DOI] [PubMed] [Google Scholar]
- Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Cai N., Wang Y.D., Zheng P.S. The microRNA-302-367 cluster suppresses the proliferation of cervical carcinoma cells through the novel target AKT1. RNA. 2013;19:85–95. doi: 10.1261/rna.035295.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Lewis W., Diwan A., Cheng E.H., Matkovich S.J., Dorn G.W., 2nd Dual autonomous mitochondrial cell death pathways are activated by Nix/BNip3L and induce cardiomyopathy. Proc. Natl. Acad. Sci. USA. 2010;107:9035–9042. doi: 10.1073/pnas.0914013107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S., Hsia C.Y., Leone G., Liou H.C. Cyclin E and Bcl-xL cooperatively induce cell cycle progression in c-Rel-/- B cells. Oncogene. 2003;22:8472–8486. doi: 10.1038/sj.onc.1206917. [DOI] [PubMed] [Google Scholar]
- Emre N., Vidal J.G., Elia J., O’Connor E.D., Paramban R.I., Hefferan M.P., Navarro R., Goldberg D.S., Varki N.M., Marsala M., Carson C.T. The ROCK inhibitor Y-27632 improves recovery of human embryonic stem cells after fluorescence-activated cell sorting with multiple cell surface markers. PLoS ONE. 2010;5:e12148. doi: 10.1371/journal.pone.0012148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareh M., Turchi L., Virolle V., Debruyne D., Almairac F., de-la-Forest Divonne S., Paquis P., Preynat-Seauve O., Krause K.H., Chneiweiss H., Virolle T. The miR 302-367 cluster drastically affects self-renewal and infiltration properties of glioma-initiating cells through CXCR4 repression and consequent disruption of the SHH-GLI-NANOG network. Cell Death Differ. 2012;19:232–244. doi: 10.1038/cdd.2011.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janumyan Y.M., Sansam C.G., Chattopadhyay A., Cheng N., Soucie E.L., Penn L.Z., Andrews D., Knudson C.M., Yang E. Bcl-xL/Bcl-2 coordinately regulates apoptosis, cell cycle arrest and cell cycle entry. EMBO J. 2003;22:5459–5470. doi: 10.1093/emboj/cdg533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C.H., Deng J.H., Deng Q., Ying S.Y. A novel role of miR-302/367 in reprogramming. Biochem. Biophys. Res. Commun. 2012;417:11–16. doi: 10.1016/j.bbrc.2011.11.058. [DOI] [PubMed] [Google Scholar]
- Lee K.Y., Bae S.C. TGF-beta-dependent cell growth arrest and apoptosis. J. Biochem. Mol. Biol. 2002;35:47–53. doi: 10.5483/bmbrep.2002.35.1.047. [DOI] [PubMed] [Google Scholar]
- Lin S.L., Chang D.C., Ying S.Y., Leu D., Wu D.T. MicroRNA miR-302 inhibits the tumorigenecity of human pluripotent stem cells by coordinate suppression of the CDK2 and CDK4/6 cell cycle pathways. Cancer Res. 2010;70:9473–9482. doi: 10.1158/0008-5472.CAN-10-2746. [DOI] [PubMed] [Google Scholar]
- Lin S.L., Chang D.C., Lin C.H., Ying S.Y., Leu D., Wu D.T. Regulation of somatic cell reprogramming through inducible mir-302 expression. Nucleic Acids Res. 2011;39:1054–1065. doi: 10.1093/nar/gkq850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipchina I., Elkabetz Y., Hafner M., Sheridan R., Mihailovic A., Tuschl T., Sander C., Studer L., Betel D. Genome-wide identification of microRNA targets in human ES cells reveals a role for miR-302 in modulating BMP response. Genes Dev. 2011;25:2173–2186. doi: 10.1101/gad.17221311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi N., Ishii H., Nagano H., Haraguchi N., Dewi D.L., Kano Y., Nishikawa S., Tanemura M., Mimori K., Tanaka F. Reprogramming of mouse and human cells to pluripotency using mature microRNAs. Cell Stem Cell. 2011;8:633–638. doi: 10.1016/j.stem.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Pernaute B., Spruce T., Smith K.M., Sánchez-Nieto J.M., Manzanares M., Cobb B., Rodríguez T.A. MicroRNAs control the apoptotic threshold in primed pluripotent stem cells through regulation of BIM. Genes Dev. 2014;28:1873–1878. doi: 10.1101/gad.245621.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyle A.D., Lock L.F., Donovan P.J. Neurotrophins mediate human embryonic stem cell survival. Nat. Biotechnol. 2006;24:344–350. doi: 10.1038/nbt1189. [DOI] [PubMed] [Google Scholar]
- Scheel A.H., Beyer U., Agami R., Dobbelstein M. Immunofluorescence-based screening identifies germ cell associated microRNA 302 as an antagonist to p63 expression. Cell Cycle. 2009;8:1426–1432. doi: 10.4161/cc.8.9.8324. [DOI] [PubMed] [Google Scholar]
- Schuster N., Krieglstein K. Mechanisms of TGF-beta-mediated apoptosis. Cell Tissue Res. 2002;307:1–14. doi: 10.1007/s00441-001-0479-6. [DOI] [PubMed] [Google Scholar]
- Shu J., Xia Z., Li L., Liang E.T., Slipek N., Shen D., Foo J., Subramanian S., Steer C.J. Dose-dependent differential mRNA target selection and regulation by let-7a-7f and miR-17-92 cluster microRNAs. RNA Biol. 2012;9:1275–1287. doi: 10.4161/rna.21998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh M.R., Lee Y., Kim J.Y., Kim S.K., Moon S.H., Lee J.Y., Cha K.Y., Chung H.M., Yoon H.S., Moon S.Y. Human embryonic stem cells express a unique set of microRNAs. Dev. Biol. 2004;270:488–498. doi: 10.1016/j.ydbio.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S., Jones J.M. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Wang Y., Baskerville S., Shenoy A., Babiarz J.E., Baehner L., Blelloch R. Embryonic stem cell-specific microRNAs regulate the G1-S transition and promote rapid proliferation. Nat. Genet. 2008;40:1478–1483. doi: 10.1038/ng.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Park J.W., Drissi H., Wang X., Xu R.H. Epigenetic regulation of miR-302 by JMJD1C inhibits neural differentiation of human embryonic stem cells. J. Biol. Chem. 2014;289:2384–2395. doi: 10.1074/jbc.M113.535799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware C.B., Wang L., Mecham B.H., Shen L., Nelson A.M., Bar M., Lamba D.A., Dauphin D.S., Buckingham B., Askari B. Histone deacetylase inhibition elicits an evolutionarily conserved self-renewal program in embryonic stem cells. Cell Stem Cell. 2009;4:359–369. doi: 10.1016/j.stem.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Ueno M., Kamiya D., Nishiyama A., Matsumura M., Wataya T., Takahashi J.B., Nishikawa S., Nishikawa S., Muguruma K., Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- Xu N., Papagiannakopoulos T., Pan G., Thomson J.A., Kosik K.S. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell. 2009;137:647–658. doi: 10.1016/j.cell.2009.02.038. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Wu W.S. Sodium butyrate promotes generation of human induced pluripotent stem cells through induction of the miR302/367 cluster. Stem Cells Dev. 2013;22:2268–2277. doi: 10.1089/scd.2012.0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Xiang D., Heriyanto F., Gao Y., Qian Z., Wu W.S. Dissecting the roles of miR-302/367 cluster in cellular reprogramming using TALE-based repressor and TALEN. Stem Cell Rep. 2013;1:218–225. doi: 10.1016/j.stemcr.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


