Summary
Epiblast stem cells (EpiSCs) are pluripotent stem cells derived from epiblasts of postimplantation mouse embryos, and thus provide a useful model for studying “primed” pluripotent states. Here, we devised a simple and robust technique to derive high-quality EpiSCs using an inhibitor of WNT secretion. Using this method, we readily established EpiSC lines with high efficiency and were able to use whole embryonic portions without having to separate the epiblast from the visceral endoderm (VE). Expression analyses revealed that these EpiSCs maintained a homogeneous, undifferentiated status, yet showed high potential for differentiation both in vitro and in teratomas. Unlike EpiSCs derived by the original protocol, new EpiSC lines required continuous treatment with the Wnt inhibitor, suggesting some intrinsic differences from the existing EpiSCs. The homogeneous properties of this new version of EpiSCs should facilitate studies on the establishment and maintenance of a “primed” pluripotent state, and directed differentiation from the primed state.
Graphical Abstract
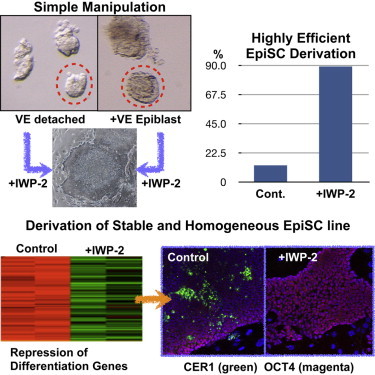
Highlights
-
•
Wnt inhibition (by IWP-2) dramatically increases the efficiency of EpiSC derivation
-
•
IWP-2 helps to maintain homogeneous EpiSC cultures by suppressing differentiation
-
•
Using IWP-2 to derive EpiSCs does not require the separation of epiblast from VE
-
•
New version of EpiSCs should facilitate studies on “primed” stem cells in mammals
Using an inhibitor of WNT secretion, Abe and colleagues established a simple and robust method that allows highly efficient derivation of mouse EpiSC lines. These EpiSCs maintain a homogeneous differentiation status but show a high differentiation potential. The homogeneous properties of these new EpiSCs should facilitate studies on the “primed” pluripotent state and directed differentiation from the primed state.
Introduction
Epiblast stem cells (EpiSCs) are pluripotent stem cells (PSCs) derived from the epiblasts of early postimplantation mouse embryos (Brons et al., 2007; Tesar et al., 2007). EpiSCs can proliferate indefinitely in culture and differentiate into derivatives of all three germ layers in vitro and in teratomas. However, EpiSCs possess several different characteristics compared with other PSCs such as mouse embryonic stem cells (mESCs). Whereas mESCs show dome-shaped colonies, EpiSCs show a flatter colony morphology. Other characteristics that differ between EpiSCs and mESCs are the status of X chromosome inactivation in female cells, the culture conditions needed, the expression of several genes/markers (e.g., mESC-specific Pecam1 and EpiSC-specific Fgf5), and their clonogenicity and contribution to chimeras when injected into blastocysts (Brons et al., 2007; Nichols and Smith, 2009). Most of these cellular characteristics of EpiSCs are shared by human PSCs such as human ESCs (hESCs) and human induced PSCs (hiPSCs). EpiSCs and human PSCs are considered “primed” PSCs, in contrast to “naïve”-type stem cells such as mESCs (Nichols and Smith, 2009). Therefore, comparisons between EpiSCs and hESCs/hiPSCs should contribute to our understanding of the nature of the primed state and provide insights into the processes underlying the naive-to-primed state transition.
One difficulty in deriving EpiSCs is the need for microdissection of small postimplantation embryos. The original protocols used only the epiblast layer separated from the surrounding visceral endoderm (VE) tissue as a source of the cell lines. The overall efficiency of EpiSC derivation from epiblast explants varies from <10% to 40% (Brons et al., 2007; Tesar et al., 2007; Kojima et al., 2014; this study). Han et al. (2010) reported that EpiSCs carrying the Oct4-GFP transgene contained both GFP-positive and GFP-negative populations in culture, and that the Oct4-GFP-positive minor population could contribute to the tissues of chimeras constructed by blastocyst injection, whereas the GFP-negative population could not. It is also known that EpiSCs express marker genes for the mesoderm, endoderm, or primitive streak (albeit at low levels and in conjunction with pluripotency markers), and that some of these lineage markers show heterogeneous expression among cells within the same culture (Bernemann et al., 2011; Sumi et al., 2013). It has been suggested that this heterogeneous expression of lineage markers might predispose or prime EpiSCs toward particular cell lineages even while the cells remain pluripotent (Bernemann et al., 2011; Kojima et al., 2014). Alternatively, the heterogeneous expression could reflect some degree of spontaneous differentiation in cultures of EpiSCs.
Wnt signaling has been described as a positive regulator of self-renewal in mESCs (Hao et al., 2006; Ogawa et al., 2006; Singla et al., 2006; ten Berge et al., 2011); however, the involvement of the canonical Wnt signaling pathway (i.e., β-catenin-dependent Wnt signaling, as opposed to non-canonical Wnt signaling, which is β-catenin independent) in pluripotency remains controversial (reviewed by Sokol, 2011). In fact, β-catenin null mESCs have been generated successfully in naive conditions (Lyashenko et al., 2011; Wray et al., 2011). Interestingly, Ying et al. (2008) showed that mESCs can be maintained in the ground state when cultured in medium containing leukemia inhibitory factor (LIF) plus two inhibitors (2i) for ERK signaling and GSK3 activity. However, although the GSK3 inhibitor they used was an effective agonist of the Wnt/β-catenin signaling pathway, the maintenance of ground-state mESCs requires dual inhibition. Blocking the secretion of all WNT proteins in ground-state mESCs by deleting the Porcupine gene also proved to be compatible with pluripotency, demonstrating that the WNT protein secretion may not be necessary for pluripotency (Biechele et al., 2013).
Given that the retention of β-catenin in the cytoplasm has been implicated in the self-renewal of both EpiSC and hESCs (Kim et al., 2013), we reasoned that inhibition of Wnt signaling might favor the growth of primed-state PSCs, i.e., EpiSCs. Here, we devised a simple and robust technique to derive high-quality EpiSCs using the small-molecule Wnt-inhibitor IWP-2, which acts on the protein Porcupine, blocking the secretion of WNT proteins and consequently pharmacologically inhibiting downstream Wnt signaling. Here, we show that the use of IWP-2 allows one to derive EpiSCs from epiblast cells without having to remove the VE, and to stably maintain EpiSCs by blocking the endogenous WNT-mediated spontaneous differentiation that otherwise would arise in culture. The homogeneous properties of the resulting EpiSCs at the cellular level, regarding morphology and expression of lineage markers, should facilitate studies on establishing and maintaining stable culture of cells in the primed pluripotent state, and directing differentiation from that state.
Results
Critical Effect of Inhibiting WNT Secretion on EpiSC Derivation
To test our hypothesis that Wnt inhibition would enhance the efficiency of EpiSC derivation, we separated epiblasts of embryonic day 5.5 (E5.5) mouse embryos obtained from C57BL/6 (B6) × 129S2/Sv (129) strain crosses from the VE and cultured them in EpiSC medium with or without the Wnt inhibitor IWP-2, as described in the Experimental Procedures (Figure 1A). Epiblast explants attached to the substratum on day 2 of culture, and epiblast cells formed flat colonies on day 3 irrespective of the presence or absence of IWP-2 (Figure S1). By day 5, only the epiblast explants cultured with IWP-2 still showed flat colonies of compact cells characteristic of EpiSCs (Figure 1B). In contrast, explants cultured without IWP-2 gave rise to colonies with heterogeneous morphologies, and differentiated cells emerged in most cases (Figure 1C). At 1 or 2 days after passage 3 or 4, we examined the morphologies of ten colonies, and if more than eight of the colonies showed “good” undifferentiated morphologies (see Figure S1), we judged that EpiSC lines had been established. Expression of alkaline phosphatase was also examined (Figure S1). EpiSC lines were derived from E5.5 epiblasts in the presence of IWP-2 with high efficiency (5/5), whereas EpiSC lines were established at a much lower rate in the absence of IWP-2 under our culture conditions (3/16 hybrid embryo explants; 19%) (Table 1). Using E6.5 embryos, we obtained essentially similar results (two lines of 24 E6.5 epiblasts (8%) in the absence of IWP-2, versus 6/6 in the presence of IWP-2; Table S1). These results suggest that inhibition of WNT secretion greatly enhanced the derivation of EpiSC lines.
Figure 1.
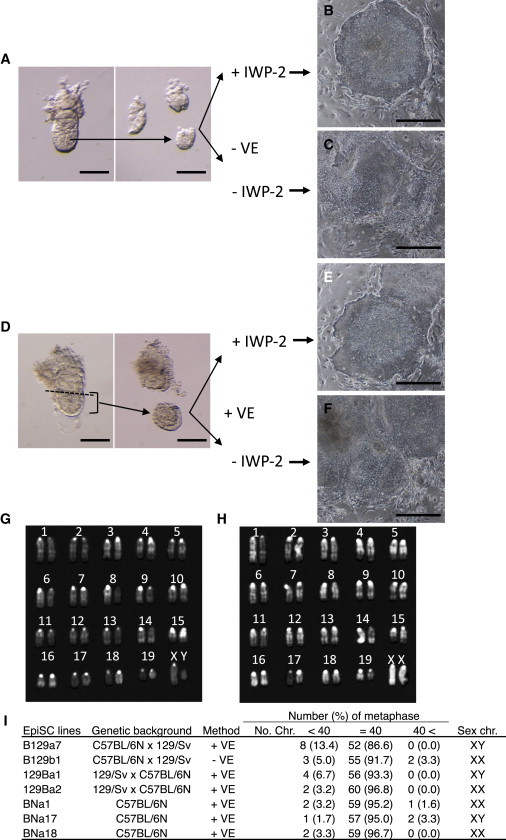
Establishment of EpiSC Lines by the IWP-2 Method
(A) E5.5 epiblasts without VE (−VE) were cultured with or without IWP-2.
(B and C) Epiblast outgrowth 5 days after plating of epiblasts without VE and with IWP-2 (B), and without VE and IWP-2 (C).
(D) Epiblasts with VE (+VE) of E5.5 embryos were cultured with or without IWP-2.
(E and F) Epiblast outgrowth 5 days after plating of epiblasts with VE and IWP-2 (E), and with VE and without IWP-2 (F).
(G and H) Representative karyotypes are shown for male (G) and female (H) EpiSC lines.
(I) List of the EpiSC lines used in this study, including their genetic background, the derivation method used, chromosome numbers, and compositions of sex chromosomes.
Scale bars, 100 μm (A and B) and 0.5 mm (C–F). See also Figure S1.
Table 1.
EpiSC Lines Established from E5.5 Epiblasts with or without Wnt Inhibitor
| Genetic Background | Visceral Endoderm | IWP2 | XAV939 | No. of Embryos | No. of Cell Lines (%) | Sex Ratio (Male/Female) | Total No. of Cell Lines/Embryos (%) |
|---|---|---|---|---|---|---|---|
| C57BL/6N × 129S2/Sv | − | − | − | 16 | 3 (19) | 2:1 | IWP2(−) 4/31 (13) |
| C57BL/6N × C57BL/6N | − | − | − | 9 | 1 (11) | 1:0 | |
| C57BL/6N × 129S2/Sv | + | − | − | 6 | 0 (0) | ND | |
| C57BL/6N × 129S2/Sv | − | + | − | 5 | 5 (100) | 2:3 | IWP2(+) 91/102 (89) |
| C57BL/6N × 129S2/Sv | + | + | − | 36 | 34 (94) | 18:16 | |
| 129S2/Sv × C57BL/6N | + | + | − | 19 | 18 (95) | 8:10 | |
| 129S2/Sv × 129S2/Sv | + | + | − | 12 | 11 (92) | 6:5 | |
| C57BL/6N × C57BL/6N | + | + | − | 30 | 23 (77) | 10:13 | |
| C57BL/6N × 129S2/Sv | + | − | + | 6 | 6 (100) | 2:4 | XAV939(+) 12/13 (92) |
| 129S2/Sv × C57BL/6N | + | − | + | 7 | 6 (86) | 4:2 | |
| Total | 146 | 107 |
ND, not determined. See also Table S1.
To simplify the procedure for EpiSC derivation even further, we next tested whether IWP-2 could facilitate EpiSC isolation from embryos when the VE was left intact. For this purpose, we used parts of E5.5 embryos without removing the VE (VE+ condition) and cultured them in EpiSC medium with or without IWP-2 (Figure 1D). On day 3 of culture, epiblast outgrowths were visible, whereas the VE tissues had shrunk in both conditions (Figure S1). Also, 86 EpiSC lines were eventually obtained from 97 VE+ embryo parts, but only when cultured with IWP-2. However, no EpiSC lines were isolated from VE+ conditions in the absence of IWP-2 (0/6 embryos; Figures 1E and 1F; Table 1). This result indicates that IWP-2 allowed EpiSC isolation even when the VE was present. To confirm the genome integrity of our EpiSCs, we selected two EpiSC lines obtained from a B6 × 129 strain cross and two lines from a 129 × B6 cross, and analyzed their karyotypes (Figures 1G–1I). In all cases, the cells possessed normal karyotypes: more than 85% of chromosome spreads had 40 chromosomes. No bias in the sex ratio was observed in these newly derived EpiSCs (Table 1).
We also tested another Wnt inhibitor, XAV939, which blocks the canonical pathway through inhibition of tankyrases (Huang et al., 2009). This inhibitor also promotes EpiSC derivation (Table 1; Sumi et al., 2013) and was found to be effective for stable maintenance of undifferentiated EpiSCs. Since both Wnt inhibitors with different modes of action showed similar promoting effects on EpiSC derivation, we conclude that Wnt inhibition is critical for establishing EpiSCs.
C57BL6 and its substrains are the most widely used mouse strains. They provide various biological resources and information, such as large numbers of genetically modified ESC lines and detailed available genomic data (e.g., Waterston et al., 2002). It is desirable to have EpiSC lines from a pure B6 strain, although to our knowledge, no EpiSC lines have been established from this strain to date. Therefore, we attempted to derive EpiSCs lines from C57BL/6N embryos. By culturing VE+ epiblasts from E5.5 B6 embryos, we successfully obtained 23 EpiSC lines in 30 trials with IWP-2, as opposed to one EpiSC line out of nine attempts in the absence of IWP-2 (Table 1). We obtained both male and female lines with no apparent sex bias, and we confirmed that three randomly selected EpiSC lines had normal karyotypes (Figure 1I).
Characterizations of EpiSCs Generated by Wnt Inhibition
To characterize our newly established EpiSCs, we examined the expression of pluripotency cell markers. Immunofluorescence analysis allowed us to detect the expression of SSEA1 and OCT4 in EpiSCs as well as in mESCs, whereas PECAM1 was detected only in mESCs, as reported previously (Figures 2A–2D; Rugg-Gunn et al., 2012). The NANOG protein was detected in the nuclei of mESCs and EpiSCs, albeit at much lower levels in the EpiSCs (Figures 2E and 2F). It is known that one of two X chromosomes in female EpiSCs is inactivated (Bao et al., 2009). As shown in Figures 2G and 2H, a single, strong immunofluorescence signal of histone H3K27 trimethylation (H3K27me3) was observed in the nuclei of female EpiSCs, whereas no such signals for H3K27me3 were observed in male EpiSCs, indicating that X inactivation occurred in our female EpiSC lines. Flow-cytometry analysis also confirmed that our EpiSCs were SSEA1-positive and PECAM1-negative (Figures 2I and 2J). Furthermore, we carried out qRT-PCR analyses to measure the expression levels of mRNAs for the mESC and EpiSC lines. As shown in Figure 2K, the EpiSC lines expressed Oct4 and Sox2 at levels similar to those observed in the mESCs. Nanog expression was much lower in our EpiSCs than in the mESCs, as expected from the immunofluorescence data described above. Three genes—Rex1, Pecam1, and Dppa3 (which are known to be highly expressed in naive mESCs)—were not detected in our EpiSC lines, consistent with previous reports (Brons et al., 2007; Tesar et al., 2007). Marker genes for epiblast (Fgf5, Cldn6, and Otx2) were expressed at much higher levels in the EpiSCs than in mESCs. We also examined marker gene expression in one of the EpiSC lines established by the original protocol, using the 129C1 line (Brons et al., 2007) (a gift from Dr. S. Pauklin) as a control. The expression profile of the marker genes in this cell line was essentially the same as those of our EpiSCs, except for Dppa3. These results indicate that the EpiSCs produced by our method using the Wnt inhibitor IWP-2 possess a transcription profile characteristic of “bona fide” EpiSCs.
Figure 2.
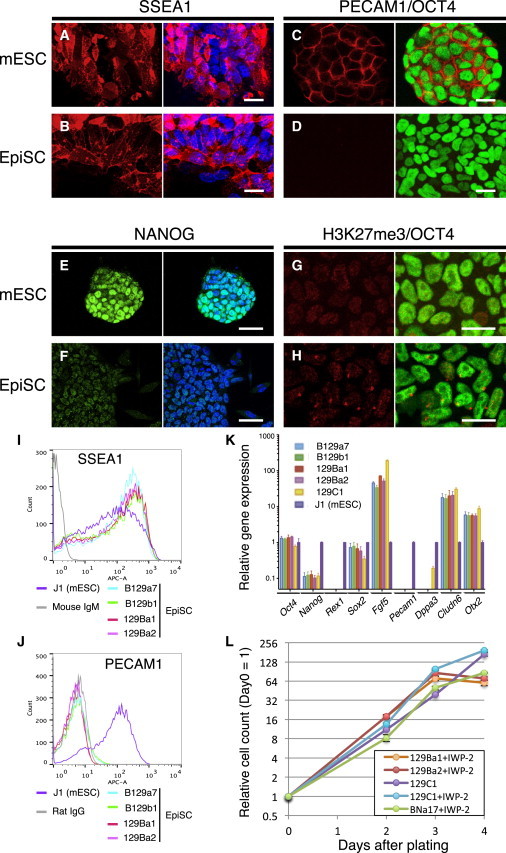
Cellular Pluripotency Marker Expression Levels in EpiSCs Derived by the IWP-2 Method
(A–J) Immunofluorescence images for SSEA1 (A and B), PECAM1 (red) and OCT4 (green, C and D), NANOG (E and F), and histone H3K27 trimethylation (G and H) in mESCs (A, C, E, and G) and EpiSCs (B, D, F, and H). Nuclear staining is shown using TO-PRO3 (blue). Cytograms of EpiSCs and mESCs are shown using anti-SSEA1 (I) and anti-PECAM1 (J) antibodies.
(K) Relative expression levels of marker genes in EpiSCs detected by qRT-PCR compared with J1 mESCs. For each gene, technical triplicate assays and two independent experiments were performed. Error bars represent the standard SEM.
(L) Growth curves of EpiSCs. At the indicated time points, 2.0 × 104 cells were plated and counted. Averaged data from three independent experiments were plotted.
Scale bars, 20 μm (A, B, C, D, G, and H) and 50 μm (E and F).
The population doubling time of hESCs is ∼36 hr, whereas mESCs have much shorter doubling times of 12–16 hr (Ware et al., 2006; Tamm et al., 2013). Although the growth rate of mouse EpiSCs was not defined in previous studies, EpiSCs are routinely passaged every 2–3 days (Tesar et al., 2007) or every 5 days (Brons et al., 2007), whereas mESCs are usually passaged every 2 days. Chang and Li (2013) reported that mESC-derived EpiSCs showed a population doubling time of ∼36 hr. Thus, one would expect murine EpiSCs to grow more slowly than mESCs. However, we noted that our EpiSCs derived in the presence of IWP-2 proliferated rapidly, and to maintain these cell lines we had to passage them routinely every 2 days. We determined the growth rates of the EpiSC lines 129Ba1, 129Ba2, and BNa17 (listed in Figure 1I). The EpiSCs showed logarithmic growth for the first 3 days with doubling times of 11.7 hr for 129Ba1, 11.2 hr for 129Ba2, and 12.7 hr for BNa17 (Figure 2L). We also determined the growth rate of the 129C1 line cultured in the presence or absence of IWP-2. In regular culture conditions, 129C1 cells proliferated with a doubling time of 13.5 hr, whereas 129C1 cells in the IWP-2 medium proliferated with a 10.8 hr doubling time. We also determined the doubling time of the J1 mESC line for comparison and found it to be 16.4 hr.
We noted that the 129C1 EpiSCs cultured in the IWP-2 medium showed a more homogeneous morphology than the same line cultured without IWP-2. Thus, some cells within the colonies changed from exhibiting the typical morphology of EpiSCs to displaying differentiated cells in the absence of IWP-2 (data not shown). Such spontaneous differentiation was rarely observed when the EpiSCs were cultured in the IWP-2 medium and passaged using a CTK cell dissociation solution (see Experimental Procedures).
EpiSCs Generated by the Wnt Inhibition Method Maintain Pluripotency
When IWP-2 was withdrawn from the EpiSC culture medium, our EpiSCs changed their morphologies and started to differentiate into cells expressing the mesoderm marker T or the endoderm marker GATA4 (Figures 3A, 3B, and S2). By contrast, 129C1, the EpiSC line established by the original protocol, could be maintained as an undifferentiated form in the absence of IWP-2. Flow-cytometry analysis of SSEA1-positive cells indicated that our EpiSC line downregulated SSEA1 expression, whereas a higher percentage of 129C1 cells continued to express SSEA1 4 days after IWP-2 removal (Figure S2).
Figure 3.
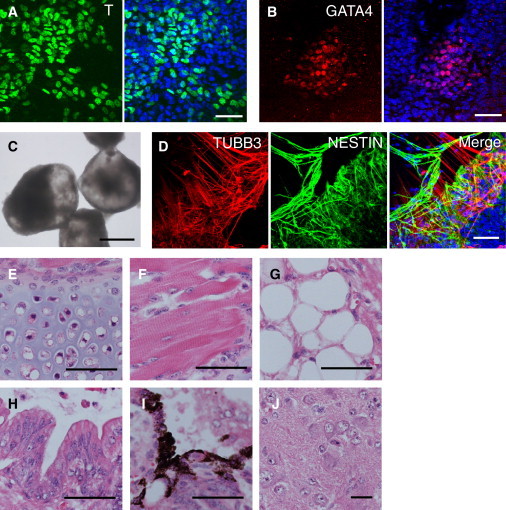
Differentiation Potential of EpiSCs Isolated by the IWP-2 Method
(A and B) Expression levels of Brachyury (T) (A) and GATA4 (B) were detected in B129a4 EpiSCs isolated by the IWP-2 method when they were cultured in medium without IWP-2 for 1 week.
(C) Bright-field image of EBs formed from 129Ba2 EpiSCs isolated with IWP-2 treatment.
(D) Immunofluorescence images for TUBB3 (red) and NESTIN (green), detecting neural differentiation in 129 Ba2 EBs.
(E–J) Hematoxylin and eosin-stained sections of teratomas from 129Ba1 EpiSCs: (E) cartilage, (F) skeletal muscle, (G) adipocytes, (H) gastrointestinal epithelium, (I) melanocytes, and (J) neural tissue.
Scale bars, 50 μm (A, B, and D–J) and 0.5 mm (C). See also Figures S2 and S3.
We found that differentiating EpiSCs developed efficiently into cystic embryoid bodies (EBs) that often contained pulsating areas, most probably containing cardiomyocytes (Figure 3C). After 7 days of EB formation in medium containing 10% fetal bovine serum (FBS), EBs were transferred to adhesive culture plates. After 14 days of adhesive culture, neuroectodermal cells expressing NESTIN, and more mature neural cells expressing β-III tubulin (TUBB3) were detected by immunofluorescence analysis (Figure 3D). In addition, we readily observed cells expressing the neural cell markers in EpiSC-derived EBs cultured in serum-free conditions (Figure S2) that are known to promote neural differentiation efficiently in mESC-derived EBs.
To assess the EpiSCs’ potency for developing into various tissues in vivo, we carried out teratoma-formation assays using two hybrid EpiSC lines and one B6 line. Clumps of EpiSCs were injected into the kidney capsule of immunodeficient SCID mice (Bosma et al., 1983). At 29 days and 72 days after transplantation of the hybrid EpiSC lines and the B6 line, respectively, teratomas were dissected out and subjected to histological analysis. The teratomas were found to contain extensively differentiated cell types from all three germ layers, including chondrocytes, skeletal myocytes, adipocytes, gastrointestinal epithelial cells, melanocytes, and neural cells (Figures 3E–3J). These results show unambiguously that the EpiSCs generated and maintained in the IWP-2 medium retained pluripotency and were able to differentiate efficiently into all three primary germ layers.
Differences in WNT Sensitivity between the EpiSCs Derived by the IWP-2 Method and Those Derived by the Original Protocol
As described above, EpiSC lines established in the presence of IWP-2 readily underwent differentiation after the withdrawal of IWP-2 (Figures 3A, 3B, S2A–S2D, and S2I–S2L), whereas the 129C1 line derived by the original method could be maintained even in the absence of IWP-2. Therefore, we investigated whether Wnt signals secreted from either feeder cells or the EpiSCs themselves caused EpiSC differentiation. We examined changes in the expression of OCT4 or SSEA1 in response to WNT stimulus by immunostaining and flow cytometry. As shown in Figures S3A and S3B, WNT3A administration induced loss of OCT4 expression in 129Ba1 cells, the EpiSC line established by the IWP-2 method. In the presence of IWP-2, 129Ba1 cells showed uniform OCT4 expression in their nuclei, whereas most of the cells lost OCT4 protein by WNT3A addition and only a small subset of cells retained OCT4 expression. Flow-cytometry analysis confirmed the immunostaining data (Figures S3E and S3F). Because IWP-2 is an inhibitor of WNT secretion, the direct supplementation of WNT protein to the culture medium overcomes the suppressive effect of IWP-2 on the Wnt pathway. We obtained essentially the same results from an SSEA1 expression analysis: WNT3A addition caused a reduction of SSEA1 expression in 129Ba1 culture (Figures S3I and S3J).
We performed similar experiments using 129C1 cells, the EpiSC line established by the original protocol (without using IWP-2). Unlike our 129Ba1 EpiSCs, the 129C1 cells could be maintained in an OCT4-positive pluripotent state without IWP-2, although we observed some spontaneous differentiation (Figure S3C). IWP-2 treatment reduced such spontaneous differentiation of 129C1, leading to a more homogeneous culture (not shown). In sharp contrast to 129Ba1, however, WNT3A addition to the 129C1 culture did not cause a reduction of OCT4-positive cells, as determined by both immunostaining and flow cytometry (Figures S3D, S3G, S3H, and S3M). Analysis of SSEA1 expression again confirmed that WNT3A addition caused only a small reduction in SSEA1 expression in the 129C1 line (Figures S3K, S3L, and S3N). These results suggest that the 129C1 cells cultured in the absence of IWP-2 may be less responsive to WNT ligands than EpiSC lines such as 129Ba1 (derived using IWP-2). This result might help explain the difference between 129Ba1 and 129C1 cells: Wnt pathway-dependent differentiation is promoted by WNT3A in the former cell line, but not efficiently in the latter one.
Molecular Characterization of EpiSC Lines Established in the Presence of IWP-2
To characterize the molecular features of our EpiSC lines, we determined global gene-expression profiles using microarrays and compared them with those of EpiSC lines established by other groups using conventional methods. The EpiSC lines 129Ba1 (XY) and 129Ba2 (XX) from E5.5 129 × B6 hybrid embryos, as well as BNa17 (XY) from E5.5 B6 embryos, were cultured in the IWP-2-containing medium and analyzed. The 129C1 EpiSC line (Brons et al., 2007) and Wt1 EpiSCs (derived from B6 × ICR embryos, a gift from Dr. M. Ema) were cultured in the absence of IWP-2 and analyzed. A 129C1 line cultured in IWP-2-containing medium was also analyzed. In addition to the EpiSC lines, two mESC lines and an mESC line cultured in 2i medium (Ying et al., 2008), and epiblast/embryonic ectoderm from E5.5, E6.5, and E7.5 mouse embryos were included in the analysis.
Principal-component analysis (PCA) of the expression profile data revealed that mESC lines, EpiSC lines, and epiblast/embryonic ectoderm were clustered at distinct positions on the PC1-PC2 plane (Figure 4A). Moreover, differences in the expression profiles of each group were revealed along the PC3 axis. For example, mESCs cultured in 2i medium were distantly located from mESCs cultured in KSR/FBS-containing medium, and EpiSC lines made by the IWP-2 method were located separately from the EpiSCs established by the original method. Hierarchical cluster analysis also revealed that three groups—mESCs, epiblast/ectoderm, and EpiSCs—displayed globally different expression profiles (Figure 4B; Table S2). Although the EpiSC lines generated by the IWP-2 method exhibited profiles similar to those of EpiSCs made by the original method, they were classified into different clusters. To examine differences among the EpiSC lines, we subjected 17,819 probes that showed differential expression among EpiSCs and epiblast/ectoderm to a k-means cluster analysis, which revealed nine clusters (Figure S4A). Clusters 2, 3, 6, and 8 represent probes that exhibited differences between EpiSCs and epiblast/ectoderm. Clusters 1 and 5 represent genes that were upregulated in 129C1 and Wt1 cells (the EpiSC lines established without IWP-2), and cluster 9 includes genes that were upregulated in the EpiSCs made by the IWP-2 method. Cluster 1 genes are particularly interesting because they were highly expressed in 129C1 cells but were repressed when 129C1 cells were cultured in the IWP-2 medium. These genes were also suppressed in 129Ba1, 129Ba2, and BNa17 cells cultured in the IWP-2 medium, suggesting that expression of the cluster 1 genes depends on WNT secretion, and therefore these genes function downstream of Wnt signaling.
Figure 4.
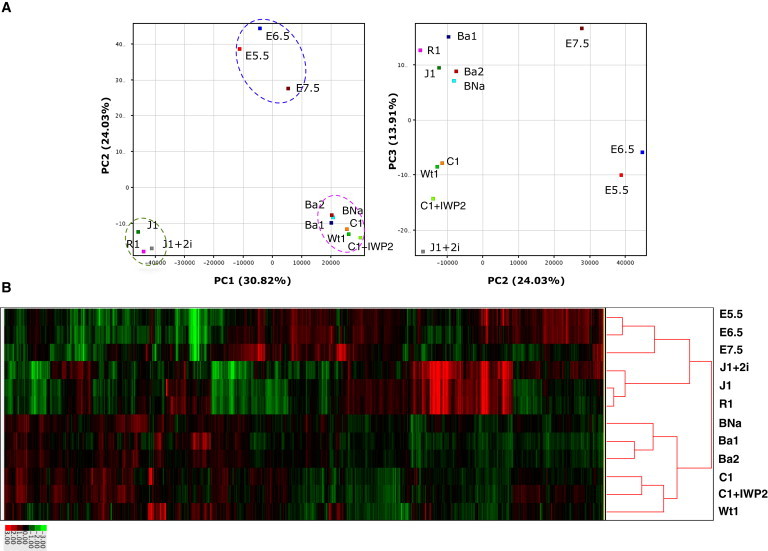
Global Gene-Expression Profiling of EpiSCs, mESCs, and Epiblasts
(A) PCA of global gene-expression profiles obtained from the indicated cell types. J1 and R1 are mESCs. J1+2i represents J1 cells cultured in 2i-containing medium. E5.5, E6.5, and E7.5 are epiblast cells isolated from embryos at the corresponding stages. Ba1, Ba2, BNa, Wt1, C1, and C1+IWP2 represent EpiSC lines 129Ba1, 129Ba2, 129BNa, Wt1, 129C1, and 129C1 cultured in the IWP-2-containing medium, respectively. Dotted circles indicate the mESC group (green), EpiSC group (pink), and epiblast group (blue).
(B) Hierarchical cluster analysis of expression profiles from EpiSCs, mESCs, and epiblast cells.
We then studied genes that were differentially expressed between 129C1 cells and those cultured in the IWP-2 medium (p < 0.05, fold change ≥ 2.0), and performed k-means clustering again (Figure S4B). We found that 229 gene probes were clearly upregulated in 129C1 and Wt1 cells but were repressed in the 129C1+IWP-2, 129Ba1, 129Ba2, and BNa17 EpiSCs (Figure S4B). Gene Ontology (GO) analysis suggested that GO terms such as “developmental process,” “anatomical structure development,” and “multicellular organismal development (biological process)” were enriched in these genes. Sixty-two of the 229 129C1-upregulated genes (34.4%) were associated with the GO term “developmental process” and included developmental regulatory genes such as Cer1, Cfc1, Foxa2, and Sox17 (data not shown). In the GO category of molecular function, “sequence-specific DNA binding” was enriched, and transcription factors such as Foxa2, Gata4, Gata6, Eomes, T, and Gsc were included. In the cellular-component GO category, “extracellular region” was overrepresented in the 129C1-upregulated genes. Genes such as Cer1, Dkk1, and Bmp2 are associated with this GO term. Collectively, many of the 129C1-upregulated genes that were suppressed by Wnt inhibition were developmental regulator genes acting in both extracellular and nuclear compartments.
Heterogeneous Expression of Developmental Regulators Was Suppressed in EpiSCs Cultured in the IWP-2 Medium
Next, we validated the suppression of several developmental regulators (GATA4, CER1, T, and SOX17) in 129C1 cells treated with IWP-2 by immunostaining (Figures 5 and S5). In 129C1 cells, GATA4- or SOX17-positive cells were clustered in OCT4-negative patches (Figures 5A–5D). However, such GATA4-positive cells or OCT4-negative regions were never observed in the EpiSCs cultured in the IWP-2 medium. Relatively large numbers of CER1-positive cells were found in the absence of IWP-2, whereas the IWP-2 treatment almost completely suppressed the expression of CER1. CER1-positive cells showed weaker OCT4 expression than the surrounding CER1-negative EpiSCs (Figure S5C). Small numbers of T-positive cells were found among the colonies in the absence of IWP-2 treatment, whereas T-positive cells were hardly seen in the IWP-2 cultures. T was expressed weakly in OCT4-positive cells, whereas cells weakly positive for OCT4 showed higher expression of this marker (Figure S5D). These results suggest that the EpiSC cultures were composed of subpopulations exhibiting different degrees of spontaneous differentiation: Cer1 was expressed in cells weakly positive for OCT4, while Gata4 and Sox17 were expressed in OCT4-negative cells. The IWP-2 treatment suppressed such developmental regulators by inhibiting WNT secretion, reducing the degree of spontaneous differentiation and thus producing a more homogeneous population of pluripotent EpiSCs. Whether or not “undifferentiated” EpiSCs cultured with IWP-2 differ significantly from EpiSCs cultured without IWP-2 remains to be clarified.
Figure 5.
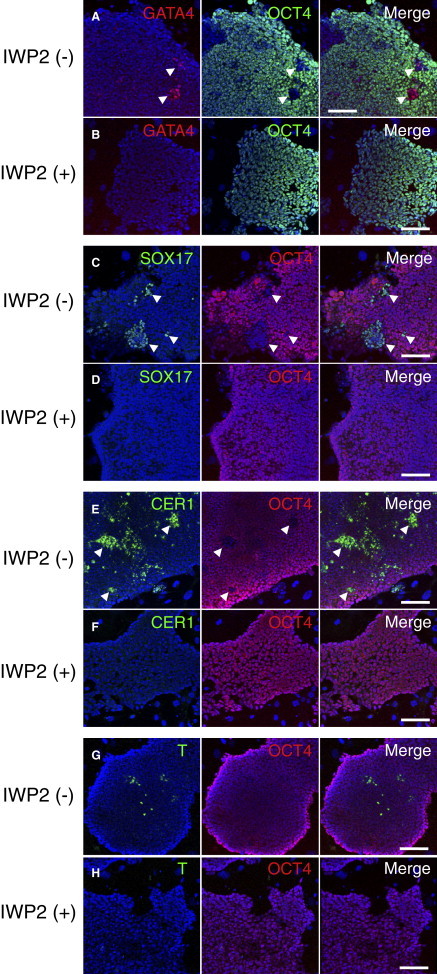
Effects of IWP-2 on Gene Expression in the 129C1 EpiSC Line
Immunofluorescence images for GATA4 (red) and OCT4 (green) (A and B), SOX17 (C and D), T (E and F), and CER1 (G and H) in EpiSCs cultured without IWP-2 (A, C, E, and G) or with IWP-2 (B, D, F, and H). Nuclei were stained with TO-PRO3 (blue). Scale bar, 100 μm. See also Figures S5 and S6.
To address this point, we sorted SSEA1-positive cells by fluorescence-activated cell sorting (FACS) from 129C1 cells cultured with or without IWP-2, and analyzed their global gene-expression profiles. The presence of SSEA1 likely represented “undifferentiated” EpiSCs, as SSEA1 was uniformly expressed in the IWP-2-treated (+) EpiSCs, but a small number of SSEA1-negative cells could be found in 129C1 cells cultured without IWP-2 (–). We collected both SSEA1 “high” and “low” fractions from cultures with or without IWP-2 (Figure S6) and performed microarray analysis. Although the overall profiles of the four kinds of samples were similar, significant differences could be detected. Out of 55,679 gene probes, 5,535 showed >2-fold differences between the SSEA1high;IWP-2(+) and SSEA1high;IWP-2(–) fractions. We found that 136 gene probes exhibited ≥10.0-fold differences between the two samples, and 101 of these were downregulated in the IWP-2(+) fraction. GO analysis of the up- or downregulated genes revealed that terms such as “pattern specification process,” “regionalization,” and “embryo development” were significantly enriched in the downregulated genes, whereas no GO terms were enriched in 35 upregulated genes. We noted that Gata4 or Sox17, which showed differential expression between bulk 129C1 and 129C1(+)IWP-2 cells, were not included in the data set. We next selected representative genes that exhibited significant differences between bulk 129C1 and 129C1 IWP-2(+) cells, and examined their expression in the SSEA1high cells treated with or without IWP-2, and SSEA1low cells treated with or without IWP-2 (Figure S6). We found that these genes could be classified into at least two classes. Gata4, Sox17, Cfc1, and Cer1 were barely detectable in both SSEA1high;IWP-2(+) and SSEA1high;IWP-2(–) fractions, but were highly expressed in the SSEA1low:IWP-2(–) cells, suggesting that these genes were more likely to be expressed in more differentiated cells than in the SSEA1high cells, and that they could be suppressed by the Wnt inhibitor. This result is consistent with the immunostaining data showing that GATA4 and SOX17 were expressed in the OCT4-negative population of the EpiSC culture (Figures 5A–5D). By contrast, Foxa2, Gsc, Evx1, and T were highly expressed in the SSEA1high;IWP-2(–) samples, suggesting that these genes were expressed in the SSEA1high cells in a Wnt signal-dependent manner.
Discussion
We have shown here that inhibiting WNT secretion by IWP-2 treatment was highly effective for the derivation and maintenance of EpiSCs. Expression analyses of the EpiSCs obtained by the IWP-2 method revealed that this Wnt inhibitor suppressed the heterogeneous expression of marker genes specific to the mesoderm, endoderm, or primitive streak. Thus, this inhibitor appears to play at least two roles: it favors the rapid propagation of epiblast cells and it inhibits Wnt-dependent spontaneous differentiation into particular germ-layer derivatives. As a result, we were able to obtain a homogeneous population of high-quality pluripotent EpiSCs under the culture conditions we used. Cell detachment using the CTK solution was also effective for maintaining a homogeneous population. Reductions in heterogeneous gene expression have been observed in EpiSCs treated with a different Wnt inhibitor (Sumi et al., 2013), hESCs treated with IWP-2 (Blauwkamp et al., 2012), and hiPSCs cultured in medium containing IWP-2 (M.K., M.S., and K.A., unpublished observation). The effect of IWP-2 must be specific to the Wnt signaling pathway, as administration of the WNT ligand WNT3A could reverse the effect of IWP-2. We also demonstrated that inhibition of the Wnt canonical pathway by the tankyrase inhibitor XAV939 (Huang et al., 2009) resulted in the derivation of homogeneous EpiSC lines. The EpiSC lines established by our method exhibited the typical morphologies of primed PSCs, with less spontaneous differentiation compared with EpiSCs made by the original protocol, but still showed robust differentiation into various cell lineages in vitro and in teratomas.
Note that our EpiSC lines required continuous treatment with IWP-2 because removal of this Wnt inhibitor caused spontaneous differentiation. Since the original EpiSCs can be propagated without IWP-2, our EpiSCs must be distinguished from them by some intrinsic difference. There may be heterogeneities in Wnt signaling activity among epiblast cell populations, as previously observed for hESCs (Blauwkamp et al., 2012). Given that the inhibition of WNT secretion (or inhibition of Wnt signaling) promotes rapid EpiSC expansion, cells with lower Wnt activity or responsiveness can be selected and established preferentially as EpiSCs under the original derivation conditions. On the other hand, IWP-2 blocks WNT secretion, leading to a reduced level of Wnt signaling. Since the WNT ligands available in culture would be greatly decreased, almost all of the epiblast cells, including cells with high Wnt responsiveness, would expand as EpiSCs. Therefore, EpiSCs derived by the IWP-2 method retain Wnt responsiveness and can differentiate in response to the Wnt stimulus. The fact that the 129C1 EpiSC line established by the original protocol (Brons et al., 2007) showed reduced responsiveness to WNT3A supports the notion described above.
Whether or not other existing EpiSC lines or primed PSC lines in general possess reduced Wnt responsiveness remains to be clarified. This is not a trivial question; because Wnt signaling is important for differentiation from pluripotent cells toward various cell lineages, reduced Wnt activity might hamper the subsequent differentiation processes. On the other hand, since stem cells established by the IWP-2 method retain robust Wnt responsiveness, they can be readily differentiated in various directions once IWP-2 is removed from the culture.
Global gene-expression analysis revealed that addition of IWP-2 to 129C1 EpiSC cultures suppressed the expression of a specific cluster of genes. This tight cluster of genes was highly expressed in EpiSC lines obtained by the original protocol (i.e., 129C1 and Wt1 cells), whereas it was barely detectable in the EpiSCs derived by the IWP-2 method, in 129C1 cells cultured with IWP-2, or in epiblast tissues from E5.5–E7.5 embryos. This cluster of genes is involved in the regulation of development into the mesoderm, endoderm, or primitive streak. Interestingly, some of these genes (e.g., Cer1, Dkk1, and Sox17) have been considered to be characteristic markers of EpiSCs (Brons et al., 2007; Tesar et al., 2007; Kojima et al., 2014). However, we show here that the expression of these marker genes is not essential for the derivation and self-renewal of EpiSC lines. Our EpiSCs made by the IWP-2 method appear to represent authentic pluripotent EpiSCs, but with low levels of Wnt-induced spontaneous differentiation. In a study using XAV939 together with the Rho kinase inhibitor Y27632, Sumi et al. (2013) suggested that Wnt signaling inhibition plays a role in promoting less spontaneous differentiation. In the present study, we further demonstrated that genes suppressed by Wnt inhibition could be classified into at least two groups: one expressed in a partially differentiated subpopulation in EpiSC cultures (e.g., Gata4 or Sox17), and one expressed in SSEA1high undifferentiated EpiSCs, including Foxa2, Gsc, and Evx1. Expression of genes in both classes was dependent on Wnt signaling. Therefore, the Wnt inhibitor suppressed spontaneous, Wnt-induced differentiation to endoderm or mesoderm lineages, and also suppressed the expression of Wnt-dependent genes in SSEA1high pluripotent cells.
As variations caused by Wnt signaling could be extinguished by the IWP-2 treatment of EpiSCs made by the original protocol, comparisons of IWP-2-treated EpiSCs should now reveal molecular differences caused by genetic differences or epigenetic features that arise during the establishment of these cell lines. Through further analyses of our new version of EpiSCs, the origins of inter- and intrastrain heterogeneities and their functional consequences, as well as common and distinct features of mouse EpiSCs and human PSCs, can now be addressed.
Experimental Procedures
Mice and Embryo Dissection
The mouse strains C57BL/6NJcl (B6) (CLEA Japan) and 129S2/Sv (129) (RBRC00123, provided by RIKEN BRC through the National Bio-Resource Project of MEXT, Japan) were used to establish EpiSC lines. SCID mice (CLEA Japan) were used for teratoma-formation assays.
E5.5 embryos were obtained from crosses of the mouse strains B6 × 129, 129 × B6, 129 inter se, and B6 inter se. Embryos were dissected out from decidua in DMEM/F-12 containing 10% FBS, and Reichert’s membrane was removed with finely sharpened tungsten needles. Epiblasts were separated from the other embryonic tissues as previously described (Sugimoto et al., 2012). In brief, embryos were incubated in pancreatin/trypsin enzyme solution (2.5% pancreatin, 0.5% trypsin, 0.5% polyvinylpyrrolidone in Ca2+/Mg2+-free Tyrode Ringer’s saline) for 15 min on ice, and the enzyme reaction was stopped in culture medium with 10% FBS. The embryonic region containing both epiblast and VE was isolated by cutting embryos at the border of the embryonic-extraembryonic portions using a pair of fine tungsten needles. The epiblast was separated from the VE by pipetting through a fine glass capillary tube.
All animal experiments were approved by the Institutional Animal Experiment Committee of RIKEN Tsukuba Institute.
Derivation and Maintenance of EpiSCs
The EpiSC culture medium was composed of DMEM/F-12 GlutaMAX medium (Life Technologies) supplemented with 15% KSR (Life Technologies), 1× nonessential amino acids (Life Technologies), 0.1 mM 2-mercaptoethanol (Life Technologies), 0.5× penicillin/streptomycin mix (Life Technologies), 12 ng/ml of human basic fibroblast growth factor (FGF; Peprotech), and 20 ng/ml of activin A (Peprotech). The Wnt inhibitor IWP-2 (Stemgent) was added to the EpiSC medium at 2 μM when necessary. The tankyrase inhibitor XAV939 (X3004; Sigma-Aldrich) was added to the medium at 10 μM when necessary. Single epiblast explants or embryonic parts of E5.5 embryos were placed in each well of four-well dishes (Thermo Scientific) and cultured in the EpiSC medium with or without IWP-2. The four-well dishes were precoated with FBS for 1 hr at 37°C, and mitomycin C (Sigma-Aldrich)-treated mouse embryonic fibroblast feeder cells were seeded at 1.25–2.50 × 104 cells/cm2. After 4–7 days of explant culture in the EpiSC medium, emerging colonies were cut into several pieces using a sterile 25 G (0.5 mm) needle, and detached from the dishes by gentle pipetting or scraping. The cell clumps were then plated on new dishes. EpiSCs cultured in the EpiSC medium with IWP-2 were passaged every other day and ∼1/8 of the cells were transferred regularly to new dishes in the same culture environment. EpiSCs were cryopreserved in FBS containing 10% DMSO (Sigma-Aldrich). Alternatively, EpiSCs were detached from the dishes by treating them with CTK solution containing 0.25% trypsin (215240; BD Diagnostic Systems), 1 mg/ml collagenase (17104-019; Life Technologies), 20% KSR, and 1 mM CaCl2 in PBS (Suemori et al., 2006) for 1–3 min at room temperature. After excess CTK solution was removed, EpiSC colonies were resuspended in culture medium and dissociated into small cell clumps by gentle pipetting using a 1 ml micropipette. The cell clumps were plated onto new dishes for passaging.
To calculate doubling times, EpiSCs were precultured under feeder-cell-free conditions overnight. Cells were treated with 10 μM of the Rho-associated protein kinase inhibitor Y-27632 (Wako Pure Chemical Industries) for 1 hr before they were dissociated with 0.25% trypsin-EDTA (Life Technologies). EpiSCs that dissociated into single cells were suspended in EpiSC medium supplemented with 2 μM of IWP-2 and 10 μM of Y-27632, and plated into 24-well dishes precoated with FBS at concentrations of 2.0 × 104 cells/well. The medium was changed every 12 hr. Cell numbers were counted using an automated TC10 cell counter (Bio-Rad) every day or every other day after dissociation into single cells. The EpiSC lines described in this work will be made available to the research community through the Cell Bank of the RIKEN BioResource Center (http://cell.brc.riken.jp).
Culture of mESCs
The J1 mESC line (Sado et al., 2001) was a gift from Dr. Takashi Sado (Kinki University). The mESCs were maintained on a feeder layer in Glasgow-minimal essential medium (G-MEM; Sigma-Aldrich) supplemented with 14% KSR, 1% ESC culture-grade FBS (Life Technologies), 1 mM sodium pyruvate (Nacalai Tesque), 1× nonessential amino acids, 0.1 mM 2-mercaptoethanol, 0.25× penicillin/streptomycin mix, and 1,000 U/ml of LIF (ESGRO; Millipore).
Karyotyping
EpiSCs were cultured in EpiSC medium containing 20 ng/ml of colcemid (KaryoMAX; Life Technologies) for 30 min and dissociated into single cells using 0.25% trypsin-EDTA (Life Technologies). After hypotonic treatment with 0.075 M KCl, EpiSCs were fixed in Carnoy’s solution (methanol/glacial acetic acid 3:1). Chromosome spreads were prepared by an air-drying method (Takagi et al., 1983) on clean glass slides and stained with Hoechst 33258 (Sigma-Aldrich).
EB Formation
EpiSCs were precultured in the EpiSC medium containing IWP-2 on FBS-coated dishes without feeder cells for 2 days. EpiSC colonies were cut into small fragments using a 25 G needle, and cell clumps were cultured on a 10 cm low-cell-binding dish (Lipidure Coat; NOF) in DMEM/F-12 GlutaMAX medium supplemented with 10% FBS, 1× nonessential amino acids, 0.1 mM 2-mercaptoethanol, and 0.5× penicillin/streptomycin mix.
For neural differentiation, EBs of day 7 cultures were plated on a gelatinized glass-bottomed dish and cultured for another 14 days in the FBS medium described above. Alternatively, 10% FBS was replaced with 15% KSR, and EBs were cultured to induce neural differentiation in this medium (Eiraku et al., 2008).
Immunofluorescence Analysis
EpiSCs and EBs were fixed with 4% paraformaldehyde in PBS at 4°C overnight. Samples were washed three times with a wash buffer (PBS containing 0.1% Tween-20), permeabilized with 0.5% Triton X-100, and incubated in 1% BSA (Sigma-Aldrich) or 5% goat serum (Jackson ImmunoResearch) to block any nonspecific binding of antibodies. Samples were incubated overnight at 4°C with a primary antibody diluted in blocking buffer (0.1% Triton X-100, 1% BSA in PBS). Primary antibodies were diluted as follows: anti-stage-specific mouse embryonic antigen (SSEA1; 1:100; Developmental Studies Hybridoma Bank); anti-OCT4 (1:300, sc-8628 [Santa Cruz Biotechnology] or ab19857 [Abcam]); anti-platelet/endothelial cell adhesion molecule 1 (PECAM1; 1:25, 550274; BD Biosciences); anti-NANOG (1:500, RCAB0001P; ReproCELL); anti-Brachyury (T, 1:300, sc-17743; Santa Cruz Biotechnology); anti-GATA4 (1:300, sc-9053; Santa Cruz Biotechnology); anti-neuron-specificβ-III tubulin (TUBB3; 1:100, MAB1195; R&D Systems); anti-NESTIN (1:1,000, ab81755; Abcam); anti-CER1 (1:200, AF1986; R&D Systems); and anti-histone H3K27 trimethylation (1:1,000, 07-449; Merck Millipore). The secondary antibodies were anti-mouse immunoglobulin M (IgM) tagged with Alexa Fluor 594 (1:500, A21044; Life Technologies); anti-rat IgG-Alexa Fluor 633 (1:500, A21094; Life Technologies); anti-goat IgG-Alexa Fluor 488 (1:500, A11055; Life Technologies); anti-rabbit IgG-Alexa Fluor 488 (1:500, A11008; Life Technologies); anti-rabbit IgG-Alexa Fluor 594 (1:500, A21207; Life Technologies); and anti-chicken IgY-DyLight 488 (1:500, 703-485-155; Jackson ImmunoResearch). Nuclei were stained with TO-PRO3 (Life Technologies). Immunofluorescence images were taken with an LSM510 meta confocal laser scanning microscope (Zeiss).
Flow Cytometry
EpiSCs or mESCs were dissociated with 0.25% trypsin-EDTA and plated for 30 min on a gelatinized dish to remove feeder cells. The cells were washed with ice-cold PBS containing 1% FBS (FBS/PBS) and then incubated at 4°C for 30 min with primary antibodies. Primary antibodies were diluted with FBS/PBS as follows: anti-SSEA1 (0.5 μg/ml, 560079; BD Biosciences) and anti-PECAM1 (1:25, 550274; BD Biosciences). As isotype controls, mouse IgMκ (0.5 μg/ml, 555581-81; BD Biosciences) and rat IgG2a (0.5 μg/ml, 14-4321-81; eBioscience) were used. The cells were then washed twice in ice-cold FBS/PBS and incubated at 4°C for 30 min with APC rat anti-mouse IgM (562032; BD Biosciences) or APC donkey anti-rat IgG F(ab′)2 (17-4822-82; eBioscience). The cells were washed twice in ice-cold FBS/PBS and then stained with 7-aminoactinomycin D (7-AAD, 559925; BD Biosciences) for 10 min on ice to discriminate between dead and live cells. Usually, 2 × 104 live cells were analyzed using an LSRFortessa cell analyzer (BD Biosciences). For sorting of SSEA1-positive EpiSCs, Alexa Fluor 488 anti-mouse/human CD15 (SSEA1, 125609; BioLegend) was used.
RNA Extraction and qRT-PCR
Total RNAs were extracted from mESCs and EpiSCs using TRizol Reagent (Life Technologies) according to the manufacturer’s procedure. Each sample was treated with RNase-free TURBO DNase (Life Technologies) to remove genomic DNA contamination. Then, 2 μg of total RNA was reverse-transcribed with SuperScript III Reverse Transcriptase (Life Technologies) and oligo dT18 primers. qRT-PCR was performed using a LightCycler 480 system (Roche Applied Science) with LightCycler 480 Probes Master mix (Roche Applied Science). Primer sequences were designed using the Roche Universal ProbeLibrary Assay Design Center web site.
Gene-Expression Profiling
A 60K mouse gene expression microarray (Agilent Technologies) was used for gene-expression profiling throughout this study. Hybridization was performed according to the protocol of the supplier. Hybridized slides were scanned using a microarray scanner (Agilent Technologies) and the signals were processed using Feature Extraction software (v. 10.5.1.1; Agilent Technologies). The processed signal data were normalized and analyzed using Gene Spring GX12.1 software (Agilent Technologies). The microarray experiments were conducted using duplicate samples.
Teratoma Formation
Teratoma formation assays were carried out as previously described (Honda et al., 2010). In brief, 1–2 × 106 EpiSCs were injected under the kidney capsule of SCID mice. At 29 days and 73 days after transplantation for two of the hybrid EpiSC lines and a B6 line, respectively, teratomas were dissected out and fixed with Bouin’s solution. Teratomas embedded in polyester wax were sectioned and stained with H&E.
Author Contributions
M.S., S.M.C.d.S.L., and K.A. conceived and designed the experiments. M.S., S.M.C.d.S.L., M.K., Y.K., H.S., M.H., R.I., and A.M. performed the experiments. M.S., S.M.C.d.S.L., A.O., A.Y., and K.A. analyzed the data. M.S., S.M.C.d.S.L., and K.A. wrote the paper.
Acknowledgments
We thank Drs. T. Sado, M. Ema, A. Smith, P. Tesar, and S. Pauklin for kind gifts of cell lines. We also thank Dr. J.-L. Guenet for the 129S2/Sv strain. This work was supported in part by a grant to M.S. and K.A. from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and in part by a grant to S.M.C.d.S.L. and K.A. from the Japan Society for the Promotion of Science (JSPS S12186).
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Accession Numbers
The GEO accession number for the gene-expression microarray data reported in this paper is GSE58735.
Supplemental Information
References
- Bao S., Tang F., Li X., Hayashi K., Gillich A., Lao K., Surani M.A. Epigenetic reversion of post-implantation epiblast to pluripotent embryonic stem cells. Nature. 2009;461:1292–1295. doi: 10.1038/nature08534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernemann C., Greber B., Ko K., Sterneckert J., Han D.W., Araúzo-Bravo M.J., Schöler H.R. Distinct developmental ground states of epiblast stem cell lines determine different pluripotency features. Stem Cells. 2011;29:1496–1503. doi: 10.1002/stem.709. [DOI] [PubMed] [Google Scholar]
- Biechele S., Cockburn K., Lanner F., Cox B.J., Rossant J. Porcn-dependent Wnt signaling is not required prior to mouse gastrulation. Development. 2013;140:2961–2971. doi: 10.1242/dev.094458. [DOI] [PubMed] [Google Scholar]
- Blauwkamp T.A., Nigam S., Ardehali R., Weissman I.L., Nusse R. Endogenous Wnt signalling in human embryonic stem cells generates an equilibrium of distinct lineage-specified progenitors. Nat. Commun. 2012;3:1070. doi: 10.1038/ncomms2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma G.C., Custer R.P., Bosma M.J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301:527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Brons I.G.M., Smithers L.E., Trotter M.W.B., Rugg-Gunn P., Sun B., Chuva de Sousa Lopes S.M., Howlett S.K., Clarkson A., Ahrlund-Richter L., Pedersen R.A., Vallier L. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- Chang K.-H., Li M. Clonal isolation of an intermediate pluripotent stem cell state. Stem Cells. 2013;31:918–927. doi: 10.1002/stem.1330. [DOI] [PubMed] [Google Scholar]
- Eiraku M., Watanabe K., Matsuo-Takasaki M., Kawada M., Yonemura S., Matsumura M., Wataya T., Nishiyama A., Muguruma K., Sasai Y. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell. 2008;3:519–532. doi: 10.1016/j.stem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Han D.W., Tapia N., Joo J.Y., Greber B., Araúzo-Bravo M.J., Bernemann C., Ko K., Wu G., Stehling M., Do J.T., Schöler H.R. Epiblast stem cell subpopulations represent mouse embryos of distinct pregastrulation stages. Cell. 2010;143:617–627. doi: 10.1016/j.cell.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Hao J., Li T.G., Qi X., Zhao D.F., Zhao G.Q. WNT/β-catenin pathway up-regulates Stat3 and converges on LIF to prevent differentiation of mouse embryonic stem cells. Dev. Biol. 2006;290:81–91. doi: 10.1016/j.ydbio.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Honda A., Hirose M., Hatori M., Matoba S., Miyoshi H., Inoue K., Ogura A. Generation of induced pluripotent stem cells in rabbits: potential experimental models for human regenerative medicine. J. Biol. Chem. 2010;285:31362–31369. doi: 10.1074/jbc.M110.150540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S.-M.A., Mishina Y.M., Liu S., Cheung A., Stegmeier F., Michaud G.A., Charlat O., Wiellette E., Zhang Y., Wiessner S. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- Kim H., Wu J., Ye S., Tai C.-I., Zhou X., Yan H., Li P., Pera M., Ying Q.-L. Modulation of β-catenin function maintains mouse epiblast stem cell and human embryonic stem cell self-renewal. Nat. Commun. 2013;4:2403. doi: 10.1038/ncomms3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima Y., Kaufman-Francis K., Studdert J.B., Steiner K.A., Power M.D., Loebel D.A.F., Jones V., Hor A., de Alencastro G., Logan G.J. The transcriptional and functional properties of mouse epiblast stem cells resemble the anterior primitive streak. Cell Stem Cell. 2014;14:107–120. doi: 10.1016/j.stem.2013.09.014. [DOI] [PubMed] [Google Scholar]
- Lyashenko N., Winter M., Migliorini D., Biechele T., Moon R.T., Hartmann C. Differential requirement for the dual functions of β-catenin in embryonic stem cell self-renewal and germ layer formation. Nat. Cell Biol. 2011;13:753–761. doi: 10.1038/ncb2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J., Smith A. Naive and primed pluripotent states. Cell Stem Cell. 2009;4:487–492. doi: 10.1016/j.stem.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Ogawa K., Nishinakamura R., Iwamatsu Y., Shimosato D., Niwa H. Synergistic action of Wnt and LIF in maintaining pluripotency of mouse ES cells. Biochem. Biophys. Res. Commun. 2006;343:159–166. doi: 10.1016/j.bbrc.2006.02.127. [DOI] [PubMed] [Google Scholar]
- Rugg-Gunn P.J., Cox B.J., Lanner F., Sharma P., Ignatchenko V., McDonald A.C., Garner J., Gramolini A.O., Rossant J., Kislinger T. Cell-surface proteomics identifies lineage-specific markers of embryo-derived stem cells. Dev. Cell. 2012;22:887–901. doi: 10.1016/j.devcel.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sado T., Wang Z., Sasaki H., Li E. Regulation of imprinted X-chromosome inactivation in mice by Tsix. Development. 2001;128:1275–1286. doi: 10.1242/dev.128.8.1275. [DOI] [PubMed] [Google Scholar]
- Singla D.K., Schneider D.J., LeWinter M.M., Sobel B.E. wnt3a but not wnt11 supports self-renewal of embryonic stem cells. Biochem. Biophys. Res. Commun. 2006;345:789–795. doi: 10.1016/j.bbrc.2006.04.125. [DOI] [PubMed] [Google Scholar]
- Sokol S.Y. Maintaining embryonic stem cell pluripotency with Wnt signaling. Development. 2011;138:4341–4350. doi: 10.1242/dev.066209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suemori H., Yasuchika K., Hasegawa K., Fujioka T., Tsuneyoshi N., Nakatsuji N. Efficient establishment of human embryonic stem cell lines and long-term maintenance with stable karyotype by enzymatic bulk passage. Biochem. Biophys. Res. Commun. 2006;345:926–932. doi: 10.1016/j.bbrc.2006.04.135. [DOI] [PubMed] [Google Scholar]
- Sugimoto M., Kondo M., Hirose M., Suzuki M., Mekada K., Abe T., Kiyonari H., Ogura A., Takagi N., Artzt K., Abe K. Molecular identification of t(w5): Vps52 promotes pluripotential cell differentiation through cell-cell interactions. Cell Rep. 2012;2:1363–1374. doi: 10.1016/j.celrep.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Sumi T., Oki S., Kitajima K., Meno C. Epiblast ground state is controlled by canonical Wnt/β-catenin signaling in the postimplantation mouse embryo and epiblast stem cells. PLoS ONE. 2013;8:e63378. doi: 10.1371/journal.pone.0063378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi N., Yoshida M.A., Sugawara O., Sasaki M. Reversal of X-inactivation in female mouse somatic cells hybridized with murine teratocarcinoma stem cells in vitro. Cell. 1983;34:1053–1062. doi: 10.1016/0092-8674(83)90563-9. [DOI] [PubMed] [Google Scholar]
- Tamm C., Pijuan Galitó S., Annerén C. A comparative study of protocols for mouse embryonic stem cell culturing. PLoS ONE. 2013;8:e81156. doi: 10.1371/journal.pone.0081156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Berge D., Kurek D., Blauwkamp T., Koole W., Maas A., Eroglu E., Siu R.K., Nusse R. Embryonic stem cells require Wnt proteins to prevent differentiation to epiblast stem cells. Nat. Cell Biol. 2011;13:1070–1075. doi: 10.1038/ncb2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesar P.J., Chenoweth J.G., Brook F.A., Davies T.J., Evans E.P., Mack D.L., Gardner R.L., McKay R.D.G. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- Ware C.B., Nelson A.M., Blau C.A. A comparison of NIH-approved human ESC lines. Stem Cells. 2006;24:2677–2684. doi: 10.1634/stemcells.2005-0452. [DOI] [PubMed] [Google Scholar]
- Waterston R.H., Lindblad-Toh K., Birney E., Rogers J., Abril J.F., Agarwal P., Agarwala R., Ainscough R., Alexandersson M., An P., Mouse Genome Sequencing Consortium Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420:520–562. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- Wray J., Kalkan T., Gomez-Lopez S., Eckardt D., Cook A., Kemler R., Smith A. Inhibition of glycogen synthase kinase-3 alleviates Tcf3 repression of the pluripotency network and increases embryonic stem cell resistance to differentiation. Nat. Cell Biol. 2011;13:838–845. doi: 10.1038/ncb2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Q.-L., Wray J., Nichols J., Batlle-Morera L., Doble B., Woodgett J., Cohen P., Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


