Abstract
Introduction
We previously reported a contemporaneous onset of cancer and scleroderma in patients with RNA polymerase III (pol) antibodies and identified a biological link between cancer and scleroderma. This investigation was designed to further evaluate whether autoantibody status and other characteristics associate with cancer and a clustering of cancer with scleroderma onset.
Methods
Logistic regression analysis was performed to assess the relationship between two outcomes, cancer (model-1) and a close (±2 years) cancer-scleroderma interval (model-2), as a function of autoantibody status and scleroderma covariates.
Results
Of 1044 scleroderma patients, 168 (16.1%) had cancer. In the adjusted model-1, only older age at scleroderma onset (OR 1.04, 95% CI 1.02,1.05) and white race (OR 2.71, 95% CI 1.22,6.04) were significantly associated with cancer risk overall. In the adjusted model-2, only pol positivity (OR 5.08; 95% CI 1.60,16.1) and older age at scleroderma onset (OR 1.04; 95% CI 1.00,1.08) were significantly associated with a close cancer-scleroderma interval. While pol was associated with a short cancer-scleroderma interval independent of age of scleroderma onset, the cancer-scleroderma interval shortened with older age at scleroderma onset in other antibody groups (Spearman’s p<0.05), particularly among patients with anti-topoisomerase-1 (topo) and patients negative for centromere, topoisomerase-1 and pol antibodies.
Conclusions
Increased age at scleroderma onset is strongly associated with cancer risk overall. While pol status is an independent marker of coincident cancer and scleroderma at any age, a clustering of cancer with scleroderma is also seen in patients developing scleroderma at older ages with topo and other autoantibody specificities.
Keywords: systemic sclerosis, malignancy, autoantibodies, paraneoplastic syndromes
Systemic sclerosis (scleroderma) is a heterogeneous, complex autoimmune disease characterized by vascular derangement, immune system abnormalities, and widespread tissue fibrosis. As in many other autoimmune rheumatic diseases, patients with scleroderma have an increased risk of malignancy compared to the general population.(1) In addition, a subset of patients have contemporaneous onset of both cancer and scleroderma that is reminiscent of the striking temporal relationship between cancer and autoimmunity seen in dermatomyositis.(2–4) While this clustering of cancer with scleroderma has largely been appreciated in scleroderma patients with breast cancer,(5, 6) our prior data suggest that coincident cancer and scleroderma may be seen across a variety of tumor types.(7) In our initial study examining the relationship between scleroderma and cancer, we demonstrated that patients with scleroderma and cancer who produce RNA polymerase III autoantibodies have a strong clustering of cancer diagnosis with the first clinical signs of scleroderma.(7) Subsequent studies have confirmed the close temporal relationship between cancer and scleroderma among anti-RNA polymerase III positive patients (8–10); 2 of these 3 studies also detected an increased prevalence of cancer in patients with RNA polymerase III antibodies compared to other autoantibody subgroups, whereas the third study did not detect a difference. We have recently demonstrated that some scleroderma patients with cancer and anti-RNA polymerase III antibodies have somatic mutations in POLR3A in their tumors, which appears to initiate the immune response against this antigen.(11) Cancers from anti-RNA polymerase III-positive patients (but not other scleroderma antibody subgroups) also demonstrated loss of heterozygosity at the POLR3A locus, strongly suggesting that this immune response applies negative selection against the cancer.(11) Since the immune response in scleroderma might effectively control emergence of cancer in RNA polymerase-3 positive patients (11), it is of importance to define whether similar mechanisms may underlie the other serological subgroups in scleroderma, where such temporal clustering is not readily apparent.
In this exploratory study, we sought to examine whether autoantibody status and clinical features (i) are risk factors for cancer in scleroderma and (ii) are associated with a clustering of cancer diagnosis with scleroderma onset. After demonstrating that older age at scleroderma onset and RNA polymerase III autoantibodies are strongly associated with a close cancer-scleroderma interval, we further examined the relationship between age, autoantibody status, and cancer. We demonstrate that an older age at the clinical onset of scleroderma is associated with a significant shortening of the cancer-scleroderma interval in patients with topoisomerase-1 antibodies and patients who are negative for RNA polymerase III, topoisomerase-1, and centromere antibodies.
PATIENTS AND METHODS
These cross-sectional analyses were performed with data from our IRB-approved Johns Hopkins Scleroderma Center cohort database, which was established in 1990. All consecutive, consenting patients who meet 1980 ACR criteria for scleroderma,(12) at least 3 out of 5 CREST (calcinosis, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly, telangiectasia) syndrome features, or have definite Raynaud’s phenomenon, abnormal nailfold capillaries, and a scleroderma specific autoantibody are included in this dynamic entry cohort at their first visit to our Scleroderma Center. All data, including patient reported cancer diagnosis site and date, have been collected prospectively.
Study population
Eligible participants consisted of patients with known anti-centromere, topoisomerase-1 (topo) and RNA polymerase III (pol) antibody status from clinically obtained test results (N=1070). Patients who were negative for these 3 autoantibodies (hereafter referred to as the “Other” group) were also included. Because autoantibodies in scleroderma are generally mutually exclusive (13) and the relationship between autoantibody type and cancer was our primary focus, our primary analyses were restricted to participants who were positive for only one scleroderma autoantibody. Twenty-five subjects were excluded from our primary analyses given multiple antibody positivity (12 positive for both pol and topo, 4 positive for topo and centromere, and 9 positive for centromere and pol). One subject with a suspected yet unconfirmed diagnosis of malignancy was excluded from the analysis to avoid misclassification of cancer status. Therefore our study population comprised 1044 subjects.
Exposure assessment
Demographic data, disease onset dates (Raynaud’s and first non-Raynaud’s symptoms), disease characteristics, smoking history, pulmonary function test data, echocardiography results, and clinically obtained autoantibody test results were abstracted from the database. Age at scleroderma onset was defined by the age at the first non-Raynaud’s scleroderma symptom. Scleroderma cutaneous phenotype was defined by established criteria.(14) A restrictive ventilatory defect suggestive of interstitial lung disease was defined by a forced vital capacity (FVC) ever < 70% of predicted. Measurements of FVC and diffusing capacity were standardized by age and gender.(15, 16) Echocardiography evidence of pulmonary hypertension (PH) was defined as a right ventricular systolic pressure ever ≥ 45 mmHg.(17) Severe Raynaud’s phenomenon was defined by whether a patient’s maximum Medsger Raynaud’s severity score was ≥ 2.(18) Myopathy was defined by a history of abnormal muscle enzymes, EMG, muscle biopsy or MRI.
Outcome assessment
Cancer diagnosis site, histology and date were reviewed in all subjects and confirmed by obtaining the original cancer biopsy pathology report, oncology records, and/or review of other physicians’ medical records. All cancer diagnoses, including non-melanoma skin cancers, that developed prior to 7/26/12 were analyzed. The interval between cancer and scleroderma was determined from the cancer diagnosis date and the date of the first non-Raynaud’s scleroderma symptom.
Statistical analysis
Demographic and clinical variables were compared between patients with and without cancer using a Student’s t test for continuous variables and a chi-square test for dichotomous or categorical variables.
Model 1: In our first analysis, we explored whether demographic and scleroderma characteristics, including autoantibody type, associated with the presence of cancer. Simple (Supplement 1) and multivariable logistic regression analyses were performed to assess the association between cancer and the following covariates: autoantibody status, age at scleroderma onset, gender (female vs. male), race (white vs. all others), smoking status (ever vs. never smoking), and scleroderma cutaneous subtype (diffuse vs. limited). These variables were fixed in the analyses because of their clinical relevance.
Model 2: In our second analysis, we explored whether demographic and scleroderma characteristics, including autoantibody type, associated with a short cancer-scleroderma interval (± 2 years) that would be suggestive of cancer-associated scleroderma. This definition of a close interval was chosen as a conservative estimate based on the cancer-associated dermatomyositis literature that defines this as a ± 3-year interval.(19) Simple (Supplement 1) and multivariable logistic regression was performed to examine the association between a close interval and autoantibody status along with other relevant scleroderma characteristics.
Because age at scleroderma onset and autoantibody status were both strong predictors of a close cancer-scleroderma interval, further analyses were performed to examine the relationship between age, cancer, and autoantibody status. The correlation between the cancer-scleroderma interval and age at scleroderma onset, both examined as continuous variables, was evaluated overall and in each autoantibody group with a Pearson’s or Spearman’s correlation test as appropriate. The mean age at scleroderma onset was examined as a continuous variable and compared (i) by cancer status and (ii) by cancer status stratified by autoantibody groups by Student’s t test. The relationships between age at scleroderma onset, age at cancer diagnosis, cancer-scleroderma interval, and autoantibody status were examined graphically.
Statistical analyses were performed using Stata version 10.1 (College Station, TX, USA). Statistical significance was defined by a 2-sided p-value ≤ 0.05.
Sensitivity and Other Analyses
We evaluated whether patients without cancer had less follow-up time from the onset of scleroderma than those with cancer, as this might suggest an insufficient observation period for the detection of cancer. The follow-up duration was compared by cancer status using the Student’s t-test. Because the association between increased cancer prevalence with older age at scleroderma onset could reflect a bias that we are more likely to be aware of a cancer diagnosis if a patient develops scleroderma at an older age, follow-up duration was also compared in patients with and without cancer stratified by the age of scleroderma onset.
We examined whether exclusion of patients who had less than 2 years of follow up from the onset of scleroderma changed our primary findings in the two multivariable logistic regression models (model 1-cancer; model 2-close cancer-scleroderma interval). We also examined whether exclusion of skin cancers, which were only present in white individuals, affected our model of cancer risk (model 1). All primary analyses were also performed defining scleroderma onset as the first scleroderma symptom, either Raynaud’s or non-Raynaud’s.
RESULTS
One thousand forty-four scleroderma patients met our inclusion criteria for study, 168 of whom (16.1%) had a known cancer diagnosis. Among these 1044 patients, 306 (29.3%), 191 (18.3%), and 199 (19.1%) patients were positive for centromere, RNA polymerase III (pol) and topoisomerase 1 (topo) antibodies, respectively. While 348 patients were negative for these 3 autoantibodies (the “Other” group), 329 of these patients were ANA positive, and 149 of this group had a nucleolar pattern. Cancer had been diagnosed in 20.9% of pol positive patients, 13.6% of topo positive patients, 16.0% of centromere positive patients, and 14.9% of “Other” patients (p=0.2). The proportion of patients with cancer in each autoantibody group is illustrated in Supplemental Figure 1. The 168 patients with cancer had a wide variety of tumor types (detailed in Supplement 1).
Clinical characteristics of the entire study population are detailed in Table 1. Scleroderma patients with cancer were older when they developed Raynaud’s phenomenon (mean age 48.1 vs. 42.0 years) and the first non-Raynaud’s scleroderma symptom (mean age 51.9 vs. 45.0 years) compared to patients without cancer (p<0.0001 for both). In addition, scleroderma patients with cancer were more likely to be white (p=0.035), more frequently had PH by echocardiography criteria (p=0.007), were less likely to have severe RP (p=0.040) and were more likely to be pol positive (p=0.044) than patients without cancer. After a Bonferroni adjustment for multiple comparisons, only age at scleroderma onset (examined as Raynaud’s onset, first non-Raynaud’s symptom onset, or first either symptom) was significantly different between those with and without cancer.
Table 1.
Comparison of clinical characteristics between scleroderma patients with and without cancer.
| Variable | Cancer (N=168) | Without Cancer (N=876) |
p-value |
|---|---|---|---|
| Age at RP onset (years), mean (SD) | 48.1 (15.2), N=165 | 42.0 (14.2), N=866 | <0.0001 |
| Age at 1st non-RP symptom onset, mean (SD) | 51.9 (13.6), N=166 | 45.0 (13.3), N=873 | <0.0001 |
| Age at 1st symptom, either RP or non-RP (years), mean (SD) | 47.7 (15.2) | 41.5 (14.1) | <0.0001 |
| Age at cancer diagnosis, mean (SD) | 56.1 (13.2), N=165 | NA | NA |
| Interval between RP onset and 1st non-RP symptom (years), mean (SD) | 3.7 (9.2), N=163 | 3.0 (8.6), N=863 | 0.3662 |
| Time to SSc diagnosis^ from first symptom (years), mean (SD) | 6.4 (9.3) | 5.3 (8.9) | 0.1688 |
| Female gender, no. (%) | 137 (81.6) | 726 (82.9) | 0.677 |
| Race, no. (%) | (N=166) | (N=860) | 0.035 |
| White | 159 (95.8) | 752 (87.4) | |
| Black | 5 (3.0) | 91 (10.6) | |
| Indian subcontinent | 0 (0) | 3 (0.4) | |
| Asian | 1 (0.6) | 8 (0.9) | |
| Mid-East/Arabian | 1 (0.6) | 6 (0.7) | |
| Cutaneous subtype, no. (%) | 0.291 | ||
| Diffuse | 68 (40.5) | 317 (36.2) | |
| Limited | 100 (59.5) | 559 (63.8) | |
| 1980 ACR criteria for SSc, no. (%) | 145 (86.3) | 774 (88.4) | 0.454 |
| Smoking: ever vs. never, no. (%) | 86 (51.2) | 378 (43.2) | 0.055 |
| mRSS at first visit to Center, mean (SD) | 11.1 (12.2), N=159 | 9.8 (10.8), N=818 | 0.1637 |
| Renal crisis, no. (%) | 10 (6.0) | 37 (4.2) | 0.322 |
| Myopathy | 21 (12.5) | 137 (15.7) | 0.296 |
| ILD* | 61 (36.3) | 331 (37.8) | 0.717 |
| Pulmonary hypertension** | 60 (35.7) | 224 (25.6) | 0.007 |
| Baseline FVC, % predicted | 82.9 (18.5), N=150 | 81.0 (18.4), N=795 | 0.2393 |
| Baseline DLCO, % predicted | 78.6 (22.5), N=135 | 78.2 (23.7), N=722 | 0.8300 |
| Baseline RVSP | 35.2 (11.1), N=108 | 34.4 (13.2), N=488 | 0.5584 |
| Severe RP, no. (%) | 84 (50) | 513 (58.6) | 0.040 |
| Immunosuppressive therapy, ever use, no. (%) | |||
| Prednisone | 47 (28.0) | 290 (33.1) | 0.190 |
| Methotrexate | 22 (13.1) | 122 (13.9) | 0.771 |
| Mycophenolate Mofetil | 45 (26.8) | 199 (22.7) | 0.257 |
| Azathioprine | 5 (3.0) | 41 (4.7) | 0.323 |
| Cyclophosphamide | 14 (8.3) | 56 (6.4) | 0.359 |
| D-penicillamine | 5 (3.0) | 30 (3.4) | 0.766 |
| IVIG | 6 (3.6) | 38 (4.3) | 0.649 |
| TNF inhibitors | 5 (3.0) | 34 (3.9) | 0.569 |
| Autoantibody status, ever positive, no. (%) | |||
| Centromere | 49 (29.2) | 257 (29.3) | 0.964 |
| RNA polymerase III | 40 (23.8) | 151 (17.2) | 0.044 |
| Topoisomerase 1 | 27 (16.1) | 172 (19.6) | 0.281 |
RP: Raynaud’s phenomenon, ACR: American College of Rheumatology, SSc: systemic sclerosis, mRSS: modified Rodnan skin score, ILD: interstitial lung disease, FVC: forced vital capacity, DLCO: diffusing capacity, RVSP: right ventricular systolic pressure;
SSc diagnosis determined by any physician,
ILD defined as FVC ever <70% of predicted,
pulmonary hypertension defined as RVSP ever ≥45 mmHg, severe RP defined by whether maximum Medsger Raynaud’s severity score ≥2 indicating digital pits, ulcers, or gangrene.
Older age of scleroderma onset and white race are associated with an increased risk of cancer overall (Model 1)
Existing published data on whether pol positive patients have an increased risk of cancer overall has been conflicting to date (8–10). Therefore, we sought to examine whether pol positivity associates with an increased risk of cancer in scleroderma and define any other factors that associate with an increased cancer risk. Multivariable logistic regression analysis was performed to assess the association between cancer and autoantibody status, age at scleroderma onset, race, gender, smoking status, and scleroderma cutaneous subtype. This analysis demonstrated that an older age at scleroderma onset (OR 1.04, 95% CI 1.02, 1.05) and white race (OR 2.71, 95% CI 1.22, 6.04) were significantly associated an increased risk of cancer overall, but smoking status, gender, cutaneous subtype, and autoantibody status were not (Table 2). While our unadjusted logistic regression models (detailed in Supplement 1) demonstrated an association between cancer risk and pol antibody status, the relative odds of cancer was not significantly increased in pol positive patients in the fully adjusted model. It is important to note that this analysis does not address the issue of the temporal association of disease onset and the diagnosis of cancer.
Table 2.
Examination of “risk factors” for cancer and a close cancer-scleroderma interval (+/− 2 years), adjusted relatives odds (95% CI)
| Variable | Cancer* N=1021 |
Close cancer-scleroderma interval** N=161 |
|---|---|---|
| Autoantibody status, ever positive | ||
| RNA polymerase III | 1.07 (0.64, 1.80) | 5.08 (1.60, 16.1) |
| Centromere | 0.96 (0.60, 1.51) | 0.88 (0.24, 3.27) |
| Topoisomerase 1 | 0.79 (0.46, 1.35) | 2.09 (0.59, 7.44) |
| Age at scleroderma onset (years) | 1.04 (1.02, 1.05) | 1.04 (1.00, 1.08) |
| Race: white vs. all others | 2.71 (1.22, 6.04) | 1.73 (0.17, 17.3) |
| Gender: female vs. male | 1.01 (0.64, 1.60) | 0.99 (0.36, 2.74) |
| Smoking: ever vs. never | 1.18 (0.84, 1.67) | 1.28 (0.56, 2.92) |
| Subtype: diffuse vs. limited | 1.14 (0.74, 1.76) | 1.27 (0.44, 3.68) |
Cancer model includes scleroderma patients with and without cancer.
Cancer-scleroderma interval model only includes patients with both cancer and scleroderma.
Older age of scleroderma onset and anti-RNA polymerase III antibodies are independently associated with cancer-associated scleroderma (Model 2)
Our previous work demonstrated an immune response to mutated RNA polymerase III in cancers from pol positive scleroderma patients who had a short cancer-scleroderma interval (11). Therefore, we wondered what other factors may also influence having contemporaneous cancer and scleroderma, suggesting a biological connection. We performed a second analysis to explore the clinical and serological factors that may be associated with a short cancer-scleroderma interval (± 2 years) that would be suggestive of cancer-associated scleroderma (Table 2). Among 163 patients with complete data on both scleroderma onset and cancer diagnosis dates, 22.7% had cancer-associated scleroderma. A detailed representation of the distribution of cancer-scleroderma intervals observed is shown in Supplemental Figure 2.
We examined the association between a short cancer-scleroderma interval (a dichotomous variable with short interval defined as ± 2 years) and autoantibody status, age at scleroderma onset, race, gender, smoking status, and cutaneous subtype (Table 2). The adjusted relative odds of a close cancer-scleroderma interval was 5.08 for anti-pol positive vs. pol negative patients (95% CI 1.60, 16.1) and 1.04 (95% CI 1.00, 1.08) per 1 year increase in age at scleroderma onset. The other factors were not significantly associated with a close cancer-scleroderma interval in the adjusted model. There was not a statistically significant interaction between age at scleroderma onset and pol antibody status (data not shown). Therefore, the relationship between a short cancer-scleroderma interval (± 2 years) and pol antibody status is not likely to be influenced by age at scleroderma onset.
The cancer-scleroderma interval shortens with increasing age at scleroderma onset in the topoisomerase-1 and “Other” antibody subgroups
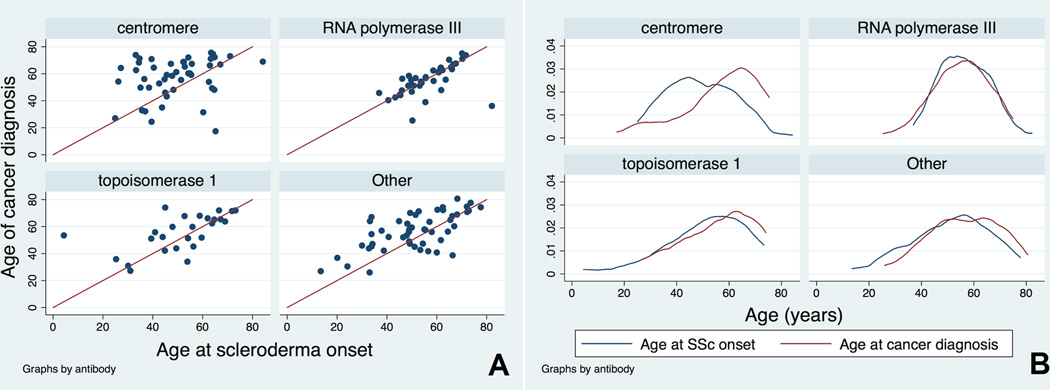
Given the findings of our multivariable logistic regression analyses demonstrating that (i) older age of scleroderma onset associated with an increased risk of cancer and (ii) that pol positivity and older age of scleroderma onset independently associated with a close cancer-scleroderma interval, we wondered what factors might influence the exact interval between onset of cancer and scleroderma. Therefore, for this analysis, we examined the cancer-scleroderma interval as a continuous variable. We found that the cancer-scleroderma interval shortens with increasing age at scleroderma onset, particularly in patients with topo and “Other” antibodies. The mean cancer-scleroderma interval was negatively correlated with older age at scleroderma onset in the overall group of patients with cancer (N=163, p<0.0001) and in patients without pol antibodies (Spearman’s correlation p<0.05 for centromere, topo and “Other” groups). To further study these relationships, we graphically examined age at cancer diagnosis and age of scleroderma onset by autoantibody status (Figure 1). The red line in each scatterplot subgraph denotes perfect concordance between age of cancer diagnosis and age of scleroderma onset, i.e. where the cancer-scleroderma interval equals zero (Figure 1A). One sees that while a small number of patients with anti-centromere antibodies have a short cancer-scleroderma interval, the vast majority of patients have wide intervals often with scleroderma preceding cancer by many years. In contrast, the patients with anti-RNA polymerase III have an almost universal short cancer-scleroderma interval, as shown by the majority of patients clustering along the red line. Among topo positive patients, the cancer-scleroderma interval shortens with increasing age at scleroderma onset. The “Other” subgroup shows features of the other 3 known antibody subsets; there is a increased clustering of cancer with scleroderma among patients with an older age at scleroderma onset, but patients with wider intervals are also present. This subgroup likely consists of patients targeting multiple autoantibody specificities, and this may account for the varied patterns observed. In order to further demonstrate the significant overlap between age of cancer diagnosis and age of scleroderma onset in the pol, topo and “Other” subgroups, we plotted the overall distributions of age at scleroderma onset and age at cancer diagnosis (Figure 1B). Again the distributions of age at scleroderma onset and age at cancer diagnosis are strikingly similar for the pol positive patients, with significant overlap also noted in the topo and “Other” patient subgroups.
Figure 1. The relationship between age at cancer diagnosis and age at scleroderma onset.
A) The red line in each scatterplot denotes perfect concordance between age of cancer diagnosis and age of scleroderma onset, i.e. where the cancer-scleroderma interval equals zero. While a small number of patients with anti-centromere antibodies have a short cancer-scleroderma interval, the vast majority of patients have wide intervals often with scleroderma preceding cancer by many years. In contrast, patients with anti-RNA polymerase III have an almost universal short cancer-scleroderma interval, as shown by the majority of patients clustering along the red line. Among topo positive patients, the cancer-scleroderma interval shortens with increasing age at scleroderma onset. The “Other” subgroup shows features of the other 3 known antibody subsets; there is a increased clustering of cancer with scleroderma among patients with an older age at scleroderma onset, but patients with wider intervals are also present. This subgroup likely consists of patients targeting multiple autoantibody specificities, and this may account for the varied patterns observed.
B) A kernel density function illustrates the distributions of age at scleroderma onset and age at cancer diagnosis. Again the distributions of age at scleroderma onset and age at cancer diagnosis are strikingly similar for the pol positive patients, with significant overlap also noted in the topo and “Other” patient subgroups.
Sensitivity and other analyses
We were concerned that our findings could be secondary to misclassification of cancer status due to an insufficient follow-up duration for the detection of cancer. There was no statistically significant difference in follow-up duration at our Center by cancer status (mean 5.7 years (SD 5.5) in cancer patients vs. 5.6 years (SD 5.4) in the no cancer group; p=0.89). We also examined follow up duration since the onset of scleroderma (rather than follow up time at our Center), and there was no difference in patients with cancer (mean 12.4 years (SD 10.5)) than in those without cancer (mean 11.0 years (SD 8.6); p=0.08). As a cancer detection bias may be present in older individuals developing scleroderma, we also examined whether follow-up duration from scleroderma onset was different by cancer status in various age strata (age at scleroderma onset <40, 41–50, 51–60, 61+). This analysis suggested that patients who developed scleroderma at ≤ 50 years of age and had cancer were followed for a longer period of time than those without cancer in the same age group. This may be consistent with a medical surveillance bias and potential differential misclassification of cancer status among younger individuals (≤ 50 years) developing scleroderma. However, if additional cancer cases were identified with longer duration of follow up in this age group, this would associate with a wide cancer-scleroderma interval amongst younger patients, remaining consistent with our overall findings. There were no differences in follow up duration by cancer status in the older age (51–60, 61+) strata.
Ninety-eight patients had less than 2 years of follow up from the onset of scleroderma. We examined whether exclusion of these patients changed our results, and there was no change in our findings for the regression models in Table 2 (data not shown).
Of note, all 41 skin cancers in this cohort were present in white individuals. When we reanalyzed our cancer model (Model 1) excluding all skin cancers, the association between cancer risk and white race was no longer statistically significant (data not shown).
All of the primary analyses were also performed defining scleroderma onset by the first scleroderma symptom, Raynaud’s or non-Raynaud’s, and there were no changes in any of the primary findings (data not shown).
DISCUSSION
Several important findings were identified in this exploratory survey of a large cohort of well-characterized scleroderma patients. Older age at the clinical onset of scleroderma was a strong predictor of both cancer overall and a shorter cancer-scleroderma interval. Patients with RNA polymerase III antibodies and cancer uniformly have a very close temporal relationship between cancer diagnosis and the development of scleroderma that is independent of age at scleroderma onset (Figure 1; OR for short interval 5.08, 95% CI 1.60, 16.1). This confirms our initial report of the clustering of cancer and scleroderma among anti-RNA polymerase III positive patients,(7) in a larger sample of well-characterized patients. In contrast to the anti-RNA polymerase III group, who had a fairly consistent short cancer-scleroderma interval, patients with antibodies against centromere, topoisomerase 1, or “Other” specificities have a negative correlation between the cancer-scleroderma interval and older age at scleroderma onset. The shortening of the cancer-scleroderma interval with increased age at scleroderma onset is most notable among topo positive patients and those in the “Other” antibody subgroup (Figure 1). These patients with a clustering of cancer with scleroderma onset are similar to those with pol antibodies, where we have demonstrated genetic alterations of POLR3A in tumors that trigger specific immune responses (11). In the context of this prior work, our current data suggest that studying these older patients with a short cancer-scleroderma interval in the topo and “Other” subgroups may provide additional insights into biological mechanisms that link cancer with the development of scleroderma. Defining the autoantigens that are targeted in the “Other” subgroup is an important priority. Studying these newly identified proteins and topoisomerase-1 in tumors from older scleroderma patients with a short cancer-scleroderma interval may allow us to test whether the insights obtained from studying RNA polymerase III in tumors is more broadly applicable to other patients with scleroderma.
The data demonstrating a shortening of the cancer-scleroderma interval with increased age at scleroderma onset in the topo and “Other” antibody groups suggest a few possibilities. First, it may be coincidental that older age at cancer diagnosis tracks with older age at scleroderma onset since cancer incidence increases with age. However, we have demonstrated that age at scleroderma onset is significantly higher in patients with cancer compared to those without cancer in the pol, topo, and “Other” subgroups (Supplement); and, the mean age at cancer diagnosis is the same across antibody groups (Supplement). The older age at scleroderma onset in patients with cancer compared to those without cancer suggests that the tight relationship between age of scleroderma onset and age of cancer diagnosis is not coincidental. One would expect no difference in age of scleroderma onset between the groups if cancer was not influencing the disease process. Secondly, it is possible that different mechanisms may explain the relationship between cancer and scleroderma in younger compared to older individuals in the topo and “Other” groups. Those who develop scleroderma at a young age may have cancer arise as a consequence of immunosuppressive therapies, damage from the disease itself (e.g. lung cancers in the setting of pulmonary fibrosis), or environmental exposures. Instead, older patients who are at risk for developing cancer may be more likely to have a paraneoplastic mechanism whereby an anti-tumor immune response results in autoimmunity and thus scleroderma.(20) This possibility is supported by our recent data demonstrating genetic alterations of POLR3A in scleroderma patients’ cancers that result in mutant-specific T cell immune responses and the generation of autoantibodies that cross-react between mutant and wild type proteins.(11) Lastly, it is possible that aging affects the quality and robustness of immune surveillance of malignancies, with a robust immune response eradicating most malignancies in younger but not older individuals with scleroderma.
Our study did not identify a statistically significantly increased prevalence of cancer in patients with RNA polymerase III antibodies compared to other antibody subgroups, similar to the data in the Australian cohort and in contrast with the UK data (9, 10). While it is clear that pol antibodies identify patients at a high risk of cancer within a few years of scleroderma onset, further study is needed to determine whether pol antibodies are truly a marker of increased cancer risk overall.
The association between an increased risk of cancer with older age at scleroderma onset could reflect a bias that (i) we are more likely to be aware of a cancer diagnosis if a patient develops scleroderma at an older age and (ii) cancer may be misclassified if patients are not followed for a sufficient duration. To address this, we examined follow-up duration by cancer status and age of scleroderma onset. Among patients who developed scleroderma young (≤50), follow-up duration was longer in the cancer group than those without cancer. While this may lead to misclassification of cancer status in individuals developing scleroderma at a young age, the detection of additional cancer cases with longer follow up time would associate with a wide cancer-scleroderma interval among these younger patients. Therefore, our primary findings would remain unchanged. We recognize that while we have always recommended age-appropriate cancer screening to our patients, we have not systematically captured cancer screening data or screened all patients for cancer using pre-defined cancer screening protocols at set time intervals. In addition, the relatively small sample size of patients with cancer precludes our ability to investigate complex relationships and interactions between age and other factors or competing hypotheses that may contribute to cancer risk in scleroderma. Another limitation is that we could not examine the effects of the timing and cumulative dose of immunosuppressive therapy on subsequent cancer development in patients with pre-existing scleroderma, or the effects of prior chemotherapy or radiation therapy on subsequent scleroderma development. Further prospective study is required to better define these relationships and to evaluate for dose effects of previous treatments. Lastly, this investigation does not examine standardized malignancy incidence ratios in older patients developing scleroderma compared to younger patients developing scleroderma. Future studies defining these relationships may provide additional supportive evidence that an older age of scleroderma onset associates with an increased risk of cancer in scleroderma.
This study shows that older age at scleroderma onset is a strong predictor of (i) increased cancer risk overall, and (ii) a temporal clustering of cancer with scleroderma particularly in patients with topo antibodies and those negative for pol, topo, and centromere antibodies. We also confirm a close temporal relationship between cancer diagnosis and scleroderma onset among patients with RNA polymerase III autoantibodies, consistent with our earlier observations made using a small cohort.(7) The recognition that scleroderma and cancer are kinetically clustered at older age suggests the possibility that the same mechanisms underlying the immune responses to Pol3 (somatic mutation in POLR3 in the cancer) (11) might also be more broadly applicable to other immune responses in scleroderma. Mechanistic insights will be obtained by studying topoisomerase-1 and other proteins in tumors from these older patients with a short cancer-scleroderma interval.
Supplementary Material
“Other” refers to the group of patients negative for antibodies against RNA polymerase III, centromere and topoisomerase-1. The numbers listed above each bar on the graph represents the total number of scleroderma patients in each age of scleroderma onset bin.
The interval between cancer diagnosis and the clinical onset of scleroderma is illustrated. Negative values reflect that a cancer diagnosis preceded scleroderma onset, whereas positive values reflect a cancer diagnosis following scleroderma onset. There is a peak of cancer cases clustering around the time of scleroderma onset.
Acknowledgements
The authors would like to thank Adrianne Woods, Margaret Sampedro, and Corrie Poelman for excellent database management support and acquisition of cancer pathology reports. We would also like to thank Dr. Rebecca Manno for careful manuscript review.
Funding Statement: This work was supported by NIAMS/NIH (K23 AR061439 to AAS), the Scleroderma Research Foundation, the Dorothy and Donald Stabler Foundation, and the Catherine Keilty Memorial Fund for Scleroderma Research.
Footnotes
Competing Interests: The authors have no conflicts of interests to declare.
References
- 1.Olesen AB, Svaerke C, Farkas DK, Sorensen HT. Systemic sclerosis and the risk of cancer: a nationwide population-based cohort study. The British journal of dermatology. 2010;163(4):800–806. doi: 10.1111/j.1365-2133.2010.09861.x. [DOI] [PubMed] [Google Scholar]
- 2.Buchbinder R, Forbes A, Hall S, Dennett X, Giles G. Incidence of malignant disease in biopsy-proven inflammatory myopathy. A population-based cohort study. Annals of internal medicine. 2001;134(12):1087–1095. doi: 10.7326/0003-4819-134-12-200106190-00008. [DOI] [PubMed] [Google Scholar]
- 3.Hill CL, Zhang Y, Sigurgeirsson B, Pukkala E, Mellemkjaer L, Airio A, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357(9250):96–100. doi: 10.1016/S0140-6736(00)03540-6. [DOI] [PubMed] [Google Scholar]
- 4.Zantos D, Zhang Y, Felson D. The overall and temporal association of cancer with polymyositis and dermatomyositis. The Journal of rheumatology. 1994;21(10):1855–1859. [PubMed] [Google Scholar]
- 5.Launay D, Le Berre R, Hatron PY, Peyrat JP, Hachulla E, Devulder B, et al. Association between systemic sclerosis and breast cancer: eight new cases and review of the literature. Clinical rheumatology. 2004;23(6):516–522. doi: 10.1007/s10067-004-0940-5. [DOI] [PubMed] [Google Scholar]
- 6.Lu TY, Hill CL, Pontifex EK, Roberts-Thomson PJ. Breast cancer and systemic sclerosis: a clinical description of 21 patients in a population-based cohort study. Rheumatology international. 2008;28(9):895–899. doi: 10.1007/s00296-008-0540-9. [DOI] [PubMed] [Google Scholar]
- 7.Shah AA, Rosen A, Hummers L, Wigley F, Casciola-Rosen L. Close temporal relationship between onset of cancer and scleroderma in patients with RNA polymerase I/III antibodies. Arthritis and rheumatism. 2010;62(9):2787–2795. doi: 10.1002/art.27549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Airo P, Ceribelli A, Cavazzana I, Taraborelli M, Zingarelli S, Franceschini F. Malignancies in Italian patients with systemic sclerosis positive for anti-RNA polymerase III antibodies. The Journal of rheumatology. 2011;38(7):1329–1334. doi: 10.3899/jrheum.101144. [DOI] [PubMed] [Google Scholar]
- 9.Nikpour M, Hissaria P, Byron J, Sahhar J, Micallef M, Paspaliaris W, et al. Prevalence, correlates and clinical usefulness of antibodies to RNA polymerase III in systemic sclerosis: a cross-sectional analysis of data from an Australian cohort. Arthritis research & therapy. 2011;13(6):R211. doi: 10.1186/ar3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moinzadeh P, Fonseca C, Hellmich M, Shah AA, Chighizola C, Denton CP, et al. Association of anti-RNA polymerase III autoantibodies and cancer in scleroderma. Arthritis research & therapy. 2014;16(1):R53. doi: 10.1186/ar4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joseph CG, Darrah E, Shah AA, Skora AD, Casciola-Rosen LA, Wigley FM, et al. Association of the autoimmune disease scleroderma with an immunologic response to cancer. Science. 2013 doi: 10.1126/science.1246886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis and rheumatism. 1980;23(5):581–590. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 13.Steen VD. The many faces of scleroderma. Rheumatic diseases clinics of North America. 2008;34(1):1–15. v. doi: 10.1016/j.rdc.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 14.LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA, Jr, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. The Journal of rheumatology. 1988;15(2):202–205. [PubMed] [Google Scholar]
- 15.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. American journal of respiratory and critical care medicine. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 16.Knudson RJ, Kaltenborn WT, Knudson DE, Burrows B. The single-breath carbon monoxide diffusing capacity. Reference equations derived from a healthy nonsmoking population and effects of hematocrit. The American review of respiratory disease. 1987;135(4):805–811. doi: 10.1164/arrd.1987.135.4.805. [DOI] [PubMed] [Google Scholar]
- 17.Mukerjee D, St George D, Knight C, Davar J, Wells AU, Du Bois RM, et al. Echocardiography and pulmonary function as screening tests for pulmonary arterial hypertension in systemic sclerosis. Rheumatology. 2004;43(4):461–466. doi: 10.1093/rheumatology/keh067. [DOI] [PubMed] [Google Scholar]
- 18.Medsger TA, Jr, Silman AJ, Steen VD, Black CM, Akesson A, Bacon PA, et al. A disease severity scale for systemic sclerosis: development and testing. The Journal of rheumatology. 1999;26(10):2159–2167. [PubMed] [Google Scholar]
- 19.Fiorentino DF, Chung LS, Christopher-Stine L, Zaba L, Li S, Mammen AL, et al. Most patients with cancer-associated dermatomyositis have antibodies to nuclear matrix protein NXP-2 or transcription intermediary factor 1gamma. Arthritis and rheumatism. 2013;65(11):2954–2962. doi: 10.1002/art.38093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah AA, Rosen A. Cancer and systemic sclerosis: novel insights into pathogenesis and clinical implications. Current opinion in rheumatology. 2011;23(6):530–535. doi: 10.1097/BOR.0b013e32834a5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
“Other” refers to the group of patients negative for antibodies against RNA polymerase III, centromere and topoisomerase-1. The numbers listed above each bar on the graph represents the total number of scleroderma patients in each age of scleroderma onset bin.
The interval between cancer diagnosis and the clinical onset of scleroderma is illustrated. Negative values reflect that a cancer diagnosis preceded scleroderma onset, whereas positive values reflect a cancer diagnosis following scleroderma onset. There is a peak of cancer cases clustering around the time of scleroderma onset.