Abstract
Chronic inflammation contributes to the development of prostate cancer in humans. Here, we show that male ApcMin/+ mice also develop prostate carcinoma with increasing age, mimicking that seen in humans in their 5th or 6th decade of life. Proinflammatory cytokines were significantly linked with cancer and increasing age in our mouse model; however, prostate and bowel tissues lacked evidence of inflammatory cell infiltrates other than mast cells. Lymphocytes protected against cancer, and protection from prostate cancer resided in antiinflammatory CD4+CD25+ regulatory (TREG) cells that downregulated inflammatory cytokines. Supplementation with syngeneic TREG cells collected from wild-type mice reduced the levels of interleukin (IL)-6 (p < 0.05) and IL-9 (p < 0.001) and lowered prostate cancer risk (p < 0.05). Depletion of CD25+ cells in 2-month-old animals increased the expression of IL-6 (p < 0.005) within prostate and increased the frequency of high-grade prostatic intraepithelial neoplasia (p < 0.05) and microinvasive prostatic carcinoma (p < 0.05) in dorsolateral prostate. Depletion of CD25+ cells in young animals also increased the frequency of intestinal cancer in Min mice. Taken together, chronically elevated proinflammatory cytokines promoted carcinoma in ApcMin/+ mice. TREG lymphocytes downregulated inflammation-associated carcinogenic processes and contributed to immune and epithelial homeostasis.
Keywords: CD25+, TREG, Min mice, prostate cancer
Prostate cancer is the 3rd leading cause of cancer deaths in American men.1 Histological features consistent with prostate neoplasia increase in frequency with increasing age, such that prevalence of high-grade prostatic intraepithelial hyperplasia (HGPIN) rises to levels of 60% by the 6th decade of life.2 Clearly, it is of great interest to further our understanding of exogenous and endogenous risk factors that promote prostate cancer. One such potentially important risk factor is chronic inflammation. On the basis of epidemiology, molecular pathology and sequellae resulting from chronic inflammation, it has been proposed that recurrent or chronic inflammation may be responsible for the increased risk of prostate cancer with increasing age in men.3 Here, we have directly addressed this possibility, and we indeed find that conditions that promote inflammation increase the frequency and severity of prostate cancer.
A link between chronic inflammation and cancer was originally described more than a century ago.4 Although acute inflammation can help destroy tumor cells, extensive epidemiologic data from human patients show that chronic inflammation is associated with disruptive changes to tissues that predispose susceptible individuals to cancer in many tissues throughout the body.5–9 For example, a link between inflammation and cancer has been demonstrated in lower bowel with colitis10 and prostate in individuals with prostatitis11,12 who are at increased risk for cancer. Furthermore, individuals on chronic NSAID therapy including cyclooxygenase (Cox)-2 inhibitors have reduced colonic and prostate cancer risk.13–15 Along these lines, several studies have observed increased expression of Cox-2 within prostate cancer.5 With the growing appreciation of the importance of inflammation and Cox-2 in cancer development, there is a need for improved animal models that allow dissection of the molecular underpinnings that connect chronic inflammation and cancer.16
The adenomatosis polyposis coli (APC) gene is a critical tumor suppressor gene that prevents malignancies of the colon17,18 and prostate19,20 in humans. Indeed, families that carry a mutation in one copy of this gene suffer multiple intestinal neoplasia (Min) because of the spontaneous loss of the remaining wild-type copy.21,22 Loss of function of both copies of the Apc gene leads to disruptions in the critical β-catenin and the wnt signaling pathway in the prostate.23 Nearly one-quarter of advanced prostate cancers in humans have alterations in β-catenin expression, which is consistent with an important role of Apc in preventing prostate cancer.24,25 Indeed, inactivation of the Apc gene in mouse prostate leads to the development of prostate carcinoma.25 In mice that are heterozygous for the Apc gene (ApcMin/+), cells completely lacking Apc frequently arise spontaneously because of the loss of heterozygosity. Consequently, by 3 months of age, ApcMin/+ mice suffer a large number of intestinal polyps that are associated with alterations in β-catenin expression and upregulation of Cox-2.26 Interestingly, ApcMin/+ mice that lack functional lymphocytes (Rag2−/−) are at increased risk for intestinal polyps and submucosal invasion, possibly because of a deficiency or malfunction in cells that normally suppress inflammation.27
T lymphocytes are thought to play an important role in suppressing inflammation, and we hypothesized that they may therefore play an important role in suppressing prostatitis and subsequently prostate cancer. Roles for T lymphocytes in immune homeostasis have been evident for several decades, as first observed in mice undergoing neonatal thymectomy.28 In the mid-1990s, Sakaguchi et al.29 showed that T cells expressing CD25 prevent immune disorders by preventing proliferation of autoreactive T lymphocytes. Likewise, Powrie isolated CD4+ T regulatory (TREG) cells with potent antiinflammatory properties that prevent inflammatory bowel disease30 and promote systemic immune homeostasis.30 Since that time, adoptive transfer or selective lymphocyte depletion techniques have been widely utilized in mice to elucidate roles for CD4+CD25+ cells in immune homeostasis. Adoptive transfer studies reveal that CD4+CD45RBloCD25−TREG31 and CD4+ CD45loCD25+ TREG32–36 cells are potent inhibitors of intestinal inflammatory responses. Relationships between TREG cells and cancer are less clear. CD4+ CD25+ TREG have been shown to inhibit intestinal polyposis in ApcMin/+ mice37,38; however, CD4+CD25+ TREG cells may also undermine beneficial inflammatory responses such as for vaccination purposes.39–42
Here, we combined our ability to manipulate the levels of CD25+ cells in vivo with the prostate-cancer prone ApcMin/+ mice to test the possibility that TREG cells help suppress endogenously arising prostate cancer. In the ApcMin/+ mice, we observed age-associated inflammatory cytokine-dependent development of prostate carcinoma, replete with mast cells but otherwise lacking overt inflammation. Interestingly, we not only found that CD4+ TREG cells downregulate inflammatory cytokines but also observed suppression of malignancy, thus opening doors to new possibilities for the prevention and possibly the treatment of prostate cancer.
Material and methods
Experimental animals
All animals were housed in AAALAC approved facilities and maintained according to protocols approved by the IACUC at Massachusetts Institute of Technology. ApcMin/+ mice on a C57BL/6J background were originally obtained from the Jackson labs and bred in house as (heterozygous X wildtype) crosses to provide ApcMin/+ mice and wildtype littermates for experimental recipients and donors.
Experimental design
A total of 160 mice were used for these experiments. Eighty-four ApcMin/+ mice were included in various treatment regimens or as experimental controls. Sixty C57BL/6 wild-type littermates were used as experimental animals or as cell donors for adoptive transfer experiments. Sixteen Rag2-deficient ApcMin/+ mice housed under identical conditions were used for comparisons of prostate pathology. Experiments were conducted using separate trials with 5–8 mice each. Trials were then run in duplicate or triplicate, as noted.
Adoptive transfer of TREG cells
A total of 10 (5 mice per trial, 2 trials) ApcMin/+ mice aged 3.5–4 months were dosed with 3 × 105 TREG cells. CD4+CD45RBloCD25+ (TREG) lymphocytes were isolated from spleen and mesenteric lymph nodes and adoptively transferred as previously described.37 Upon necropsy, tissues of these mice were compared to 10 (2 trials of 5 mice per trial) untreated 6-month-old ApcMin/+ mice. In a 2nd experiment, a total of 8 Rag2-deficient ApcMin/+ mice aged 3.5–4 months were dosed with 3 × 105 TREG cells. These mice were compared to 8 untreated Rag2-deficient Min mice at 6 months of age. The donor mice for TREG cells included male and female C57BL/6 wild-type littermate mice. The recipients used in this study were all male, in order to study prostate lesions.
Depletion of CD25+ cells
A total of 16 (2 trials of 8 mice each) male Min mice aged 4–6 weeks were treated with anti-CD25 antibody (clone PC-61; Bio-Express, West Lebanon, NH) at 150 µg intraperitoneally per mouse 2 times weekly for 4–6 weeks. Treated mice were compared to age-matched Min mice that received sham isotype antibody alone (N = 16). Depletion of CD25+ cells was confirmed by undetectably low fractions of CD25+ cells in spleens of 4 mice treated with anti-CD25 antibody compared to 4 sham-treated controls using flow cytometry upon completion of the study. In addition, depletion was confirmed by absence of Foxp3+ cells in target tissues, including prostatic and mesenteric lymph nodes at 42 days (N = 8) after onset of the depletion protocol. Depletion results were then confirmed in an additional 8 anti-CD25-treated and 8 sham-treated mice at 10 days (N = 4 mice per time point per group) and 24 days (N = 4 mice per time point per group) after onset of treatment.
Quantitation of intestinal tumors
Location of tumors was recorded using a stereomicroscope at 10× magnification. Location of tumors in the small intestine was recorded as distance from the pylorus with duodenum, jejunum and ileum comprising one-third of small intestine each and in the colon as distance from cecocolic junction.37
Detection of systemic cytokine protein expression
Serum cytokine levels of 5 animals in each experimental group were analyzed using the Bioplex assay system (BioRad, Hercules, CA) according to the manufacturers protocol. Briefly, serum samples were diluted using the sample diluent kit, incubated with antibody coated beads followed by a secondary antibody incubation and analyzed in duplicates on a Bio-Plex 200 system (BioRad, Hercules, CA). The levels of TNF-alpha, IL-1b, IL-6, IL-9, IL-13, IL-17, IFN-gamma and G-CSF were assessed. Statistical analysis was performed using 2-tailed student’s t-test; a p-value of <0.05 was considered as statistically significant.
Histologic evaluation and Immunohistochemistry
As described previously,38 the formalin-fixed tissues were processed, and tissue-sections were stained with H&E, toluidine-blue or IHC-evaluated by a veterinary pathologist blinded to sample identity. The frequency of invasive adenocarcinoma lesions in prostate43 or bowel44 was recorded for the various experimental groups. The preneoplastic and early neoplastic prostate lesions, which were detected based on recent consensus report criteria,43 were quantitatively assessed as follows: From each group of mice, 30 high-power fields (20×) containing the glandular profiles that show the most advanced preneoplastic and early neoplastic lesions were captured using a Nikon eclipse 50i microscope and a Nikon DS-5 M-L1 digital camera. Ten images were randomly selected per treatment group. Lesions were then quantified and recorded as the number of low-grade PIN, high-grade PIN and microinvasive carcinoma foci counted per image.
Primary antibodies used for immunohistochemistry included rabbit anti-Apc, rabbit anti-β-catenin (ThermoFisher Scientific/Lab Vision, Fremont, CA), rabbit anti-E-cadherin (Santa Cruz Biotechnology, Santa Cruz, CA) and mouse anti-SMA (Dako, Glostrup, Denmark). Antigens were retrieved with Trypsin (ThermoFisher Scientific/Lab Vision) for Apc detection. Heat-induced antigen retrieval was performed with citrate buffer, pH 6, for β-catenin and E-cadherin or with EDTA buffer, pH 8, for SMA detection. Primary antibody binding was detected with goat antirabbit IgG Poly-HRP (Chemicon International, Temecula, CA) or the mouse-to-mouse detection system and goat anti-mouse IgG Poly-HRP (Chemicon). Signal was detected with diaminobenzidine, and tissues were counterstained with haematoxylin.
Detection of cytokine mRNA expression
Total RNA from ileum tumors of ApcMin/+ mice was prepared using Trizol reagent according to the supplier’s instruction (Invitrogen, Carlsbad, CA). Five micrograms of the respective RNA was used to generate cDNA using the High Capacity Archive Kit from A/B Applied Biosystems according to the supplier’s procedure. Levels of Cox-2, TNF-α and IL-6 transcripts in the cDNA samples were quantified with the corresponding commercial primers and probes in the ABI Prism Sequence Detection system 7700 (A/B Applied Biosystems). The levels of these cytokines among the samples were normalized by the level of the GAPDH transcripts and compared between the CD4+CD25+ TREG cells-treated and -untreated tumors by the ΔΔCt method described by A/B Applied Biosystems (User Bulletin #2).
Statistical analyses
Total prostate lesion counts and intestinal tumor counts were analyzed by unpaired t-test with Welch’s correction. For all statistical analyses, Graphpad Prism version 4.0 for windows, Graph-Pad software, San Diego, CA, USA was used.
Results
Prostate carcinoma arises spontaneously in ApcMin/+ (Min) mice
Mutations of the Apc gene have been associated with prostate cancer development in both humans24,45 and mice.25 Here, we examined male mice that undergo spontaneous loss of heterozygosity of Apc (ApcMin/+) and found that unmanipulated ApcMin/+ mice between 5 and 6 months of age develop significantly (p < 0.01) more frequent prostate cancer when compared to age-matched wildtype (wt) littermate controls housed under the same conditions (∼40% in ApcMin/+ vs. 0% for the wt controls; see Table I). Furthermore, histological features of both preneoplastic and neoplastic lesions were evident among the ApcMin/+ mice (Fig. 1), while being completely absent among the wild-type controls (Table I). Lesions seen in ApcMin/+ mice were consistent with prostate carcinoma according to criteria of Shappell et al. in a consensus report entitled “Prostate Pathology of Genetically Engineered Mice: Definitions and Classification” published in Cancer Research,43 and also matched prostate cancer previously described in mice with inducible inactivation of the Apc gene.25
TABLE I.
Pre–Neoplastic Findings (In Dorsolateral Prostate) of Male ApcMin/+ (Min) Mice (Mean ± SE Per 10 20 × Images)
| Treatment group | Low grade PIN | High grade PIN | Microinvasive carcinoma | ||||||
|---|---|---|---|---|---|---|---|---|---|
| wt (control; age 6 months) | 0.4 ± 0.1 |
|
0.0 ± 0.0 |
|
|
0.0 ± 0.0 |
* |
||
| Min (age 6 months) | 1.5 ± 0.31 | 1.2 ± 0.20 | 1.0 ± 0.21 |
|
|
||||
| Min (age 6 months) + anti-TNF | 1.6 ± 0.30 | 1.1 ± 0.31 | 0.6 ± 0.16 | ||||||
| Min (age 6 months) + TREG | 1.7 ± 0.30 | 1.1 ± 0.31 | 0.4 ± 0.16 | ||||||
| Min (age 3 months) untreated | 1.6 ± 0.30 | 0.9 ± 0.34 | 0.2 ± 0.13 | ||||||
| Min (age 3 months) + sham IgG | 1.4 ± 0.22 | 0.5 ± 0.16 |
|
0.3 ± 0.15 |
|
||||
| Min (age 3 months) + anti-CD25 IgG | 1.5 ± 0.30 | 1.6 ± 0.16 | 1.0 ± 0.21 | ||||||
Frequency and features of prostate lesions are given in this table. CD25+ cells are important in impeding age-related progression of neoplasia in the prostate of Min mice. Min mice depleted of CD25+ cells (N = 8 mice per trial) from age 1 to 3 months had significantly more HGPIN and microinvasive carcinoma prostate lesions when compared to agematched sham antibody treated (N = 8 mice per trial) control Min mice. Min mice that received supplemental CD25+ TREGS (N = 5 mice) showed a statistically significant decrease in the frequency of microinvasive carcinoma lesions when compared to Min mice of matched age = 6 months (N = 5 mice). Grading of neoplastic progression in the DLP of mice and analysis of results was performed as described in Material and methods.
p < 0.05;
p < 0.01;
p < 0.001.
Figure 1.
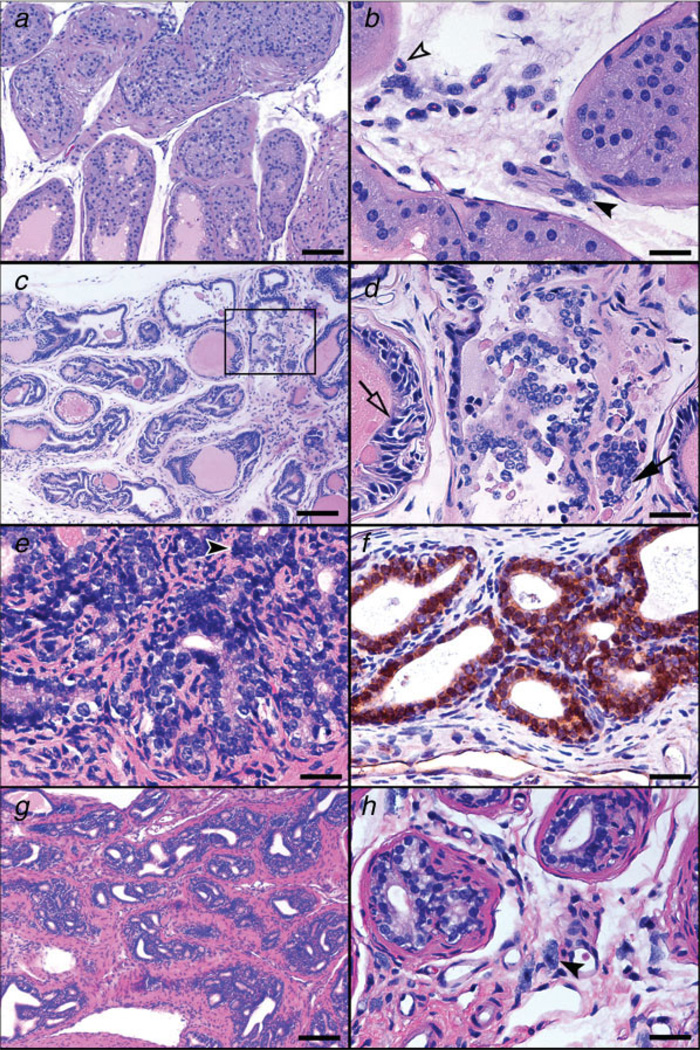
Depletion of CD25+ cells increases the risk of prostate cancer in young mice, but complete lack of lymphocytes and increasing age are the major factors contributing to the progression of prostate neoplasia in ApcMin/+ mice. (a, b) HGPIN lesions in the anterior prostate of CD25-depleted Min mice at 3 months of age. Glands in a are distorted, surrounded by irregular fibromuscular sheaths and filled with atypical epithelial cells. The stroma adjacent to gland profile with PIN lesions in b contains neutrophils (open arrow-head) and mast cells (arrow-head). (c, d) Early invasive neoplastic lesions in the dorsolateral prostate of a RagMin mouse at 6 months of age. Highly irregular glands profiles with tufting epithelia and edematous stromal reaction (c). Higher magnification of the boxed area is shown in D. Early invasion of neoplastic epithelium is found in association with HGPIN. Note the hyperchromasia and the nuclear pleomorphism of the epithelial cells in PIN lesions (open arrow) and the large, euchromatinic nuclei bearing prominent nucleoli of invasive epithelial structures (arrow). (e, f) Prostate adenocarcinoma in the dorsolateral prostate of Min mice at 6 months of age. Moderately differentiated adenocarcinoma with desmoplastic reaction. Abnormal mitotic figure is indicated by arrow-head (e). Nuclear stabilization of β-catenin characterized advanced prostate adenocarcinoma lesions (f). (g, h) Prostate adenocarcinoma in the dorsolateral prostate of Min mice at 6 months of age. Well-differentiated adenocarcinoma with prominent desmoplasia (g). Increased numbers of mast cells (arrow-head) in stroma associate with abnormal glands (h). (a, b, c, d, e, g, h) Hematoxylin and Eosin. (f) β-catenin-specific immunohistochemistry; Hematoxylin counterstain, DAB chromogen. Bars a, c and g: 100 µm; b, d, e, f and h: 25 µm.
Prostate cancer is more frequent with increasing age
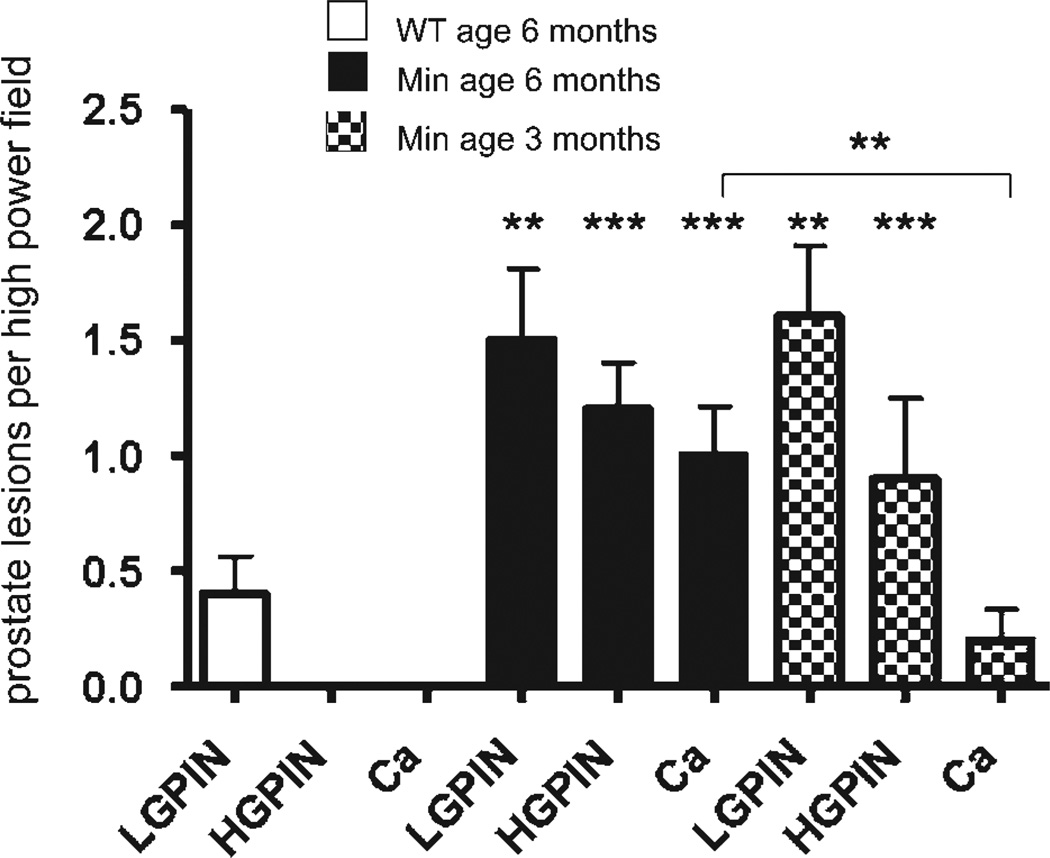
In humans, prostate cancer is more common with increasing age.3 To test whether prostate cancer frequency is associated with aging in this model, we examined male ApcMin/+ mice at 3 or 6 months of age. We discovered that ApcMin/+ male mice of age 5–6 months developed significantly more frequent prostatic microadenocarcinoma lesions than the younger 3-month-old ApcMin/+ males (compare groups in Table I, Figs. 2 and 3), showing that prostate disease is dependent on increasing age of the animals. We analyzed a spectrum of nonneoplastic and neoplastic proliferations, including multifocal hyperplasia with or without atypia and low-grade (LG) to high-grade (HG) prostatic intraepithelial neoplasia (PIN). The HGPIN was occasionally found associating with microinvasive carcinoma. Importantly, only aged Min mice had moderately to well-differentiated adenocarcinoma accompanied by desmoplastic reaction. We also assessed signs of inflammation, and although aggregates of inflammatory cells were not observed, stroma adjacent to preneoplastic and neoplastic epithelia consistently contained mast cells and occasional neutrophils. The seminal vesicles and the anterior prostate were most frequently affected sites, followed by the dorsolateral and the ventral prostate lobes. These data indicated that ApcMin/+ mice have an age-associated predilection to spontaneously arising carcinoma in the prostate. These findings are consistent with prostate carcinoma described in mice with inducible inactivation of the Apc gene.25
Figure 2.
Frequency of prostate carcinoma lesions rises with increasing age. Prostate carcinoma arose spontaneously in male Min mice but not wt mice. Aged (6 months old) Min mice (N = 5 mice per trial) had greater frequency of early neoplastic prostate lesions, when compared to younger (3 months old) Min mice (N = 5 per trial). Pre-neoplastic and early neoplastic prostate lesions, which were detected based on recent consensus report criteria,42 were quantitatively assessed for each group of mice based upon review of 30 high-power fields ×20 (data presented as mean value) containing the glandular profiles low-grade PIN, high-grade PIN and microinvasive carcinoma as shown earlier. The most advanced preneoplastic and early neoplastic lesions were captured using a Nikon eclipse 50i microscope and a Nikon DS-5 M-L1 digital camera. Ten images were randomly selected per treatment group. Lesions were then quantified and recorded as the number of low-grade PIN, high-grade PIN and microinvasive carcinoma foci counted per image.
Figure 3.
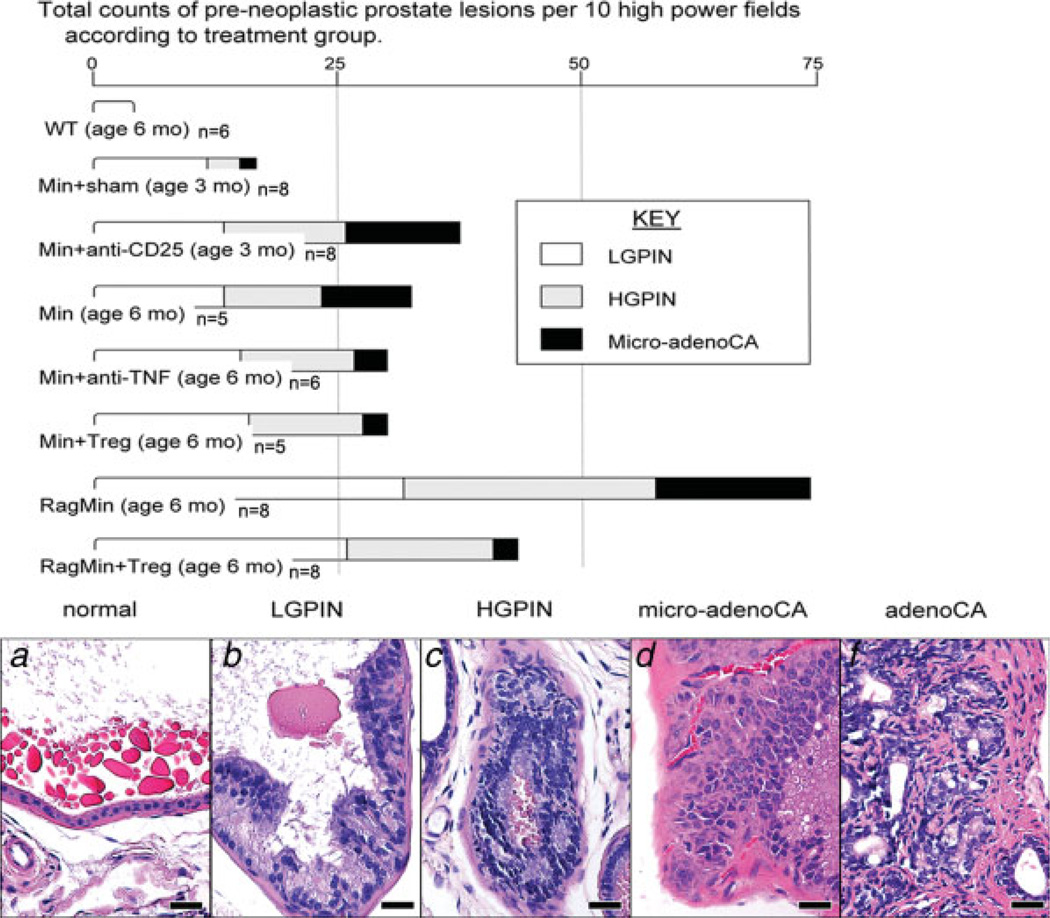
(a) Frequency of prostate cancer lesions in Min and Rag-deficient Min mice. Depletion of CD25+ cells (Min+anti-CD25) (N = 8 mice per trial) accelerated prostate carcinogenesis at age of 3 months, and the frequency of HGPIN and microinvasive adenocarcinoma was significantly higher than those of age-matched (N = 5 mice per trial) or sham-treated (N = 8) mice (p < 0.01 and <0.05, respectively). Compared to the age-matched Min mice (N = 5), RagMin mice (N = 8) had significantly higher frequency of prostate neoplasia at 6 months old (p < 0.05). Assays used tissues from 5 to 8 mice per treatment group (as shown) with review of microscopic fields as described in Material and methods. (b) Histology of dorsolateral prostate illustrating selected key intermediate steps in progression of carcinogenesis in ApcMin/+ mice. (a) Normal-appearing prostate gland. (b) Low-grade prostatic intraepithelial neoplasia (PIN) with epithelial tufting and nuclear stratification. Cellular atypia is evidenced by the presence of nuclear enlargement, increased but not severe nuclear pleomorphism, hyperchromasia and occasional prominent nucleoli. (c) High-grade PIN. Note the irregular contour of the prostate gland. Highly atypical cells with severe nuclear pleomorphism and hyperchromasia fill the lumen. (d) Microinvasive carcinoma. Epithelial cells with notably large, euchromatinic nuclei bearing prominently enlarged nucleoli with penetration through the basement membrane into surrounding stroma. (e) Invasive adenocarcinoma. Moderately differentiated small, irregular malignant glands are bounded by desmoplastic stroma. Hematoxylin and Eosin. Bars: 25 µm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Interleukin-6 is elevated in mice with prostate cancer
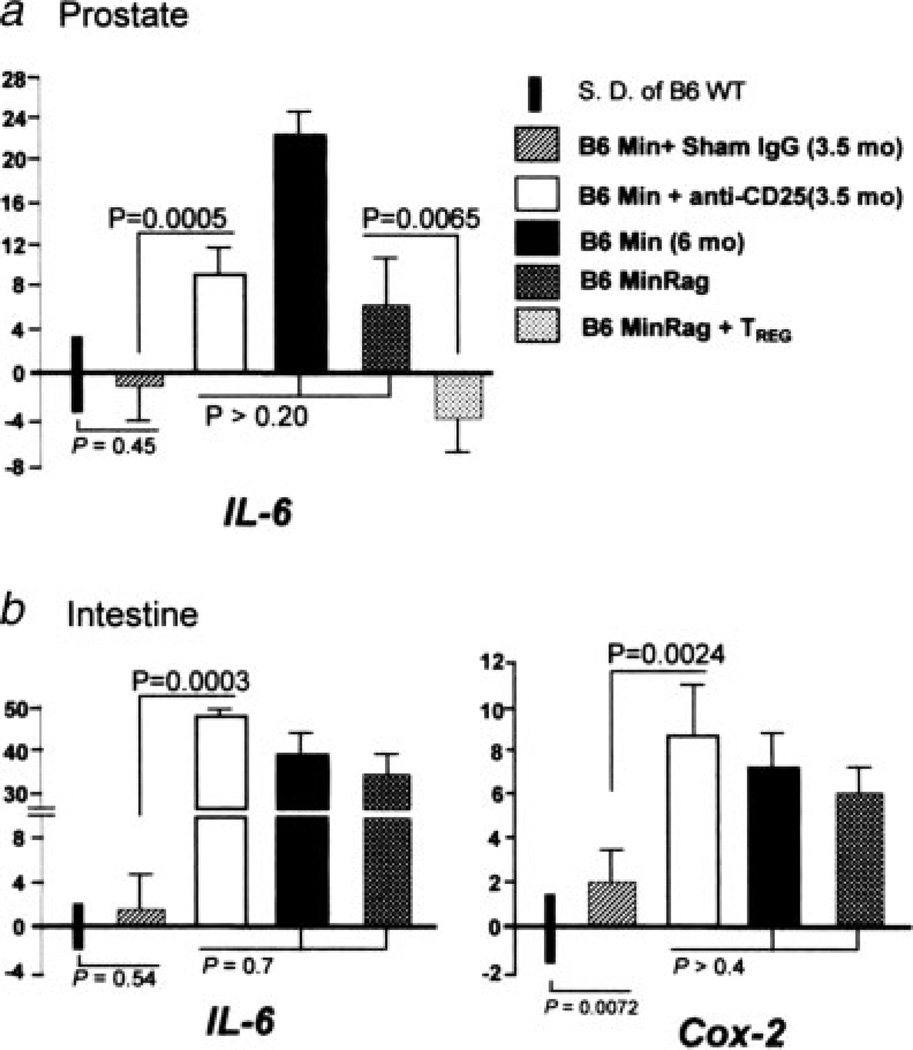
The pleiotropic cytokine IL-6 has been linked with the development of prostate cancer in humans. To determine whether prostate carcinoma is associated with changes in the levels of IL-6 and other cytokines in this murine model, we examined sera of ApcMin/+ mice and wt mice. We found that levels of certain cytokines in sera were indeed significantly higher in ApcMin/+ mice, a group that exhibited higher risk of carcinoma. Specifically, we observed significant elevations of IL-6 (p < 0.01) and IL-9 (p < 0.001) in aged ApcMin/+ mice (Table II; compare wt and Min mice). Additional analysis of gene expression within the prostate tissue revealed a clear increase in IL-6 (p < 0.005) in groups of ApcMin/+ mice also prone to carcinoma, but not in wt animals (Fig. 4a). Elevations in TNF-α and Cox-2 expression in prostate tissue did not reach significance (data not shown).
TABLE II.
Serum Cytokine Levels in Pg/Ml
| TNF-a | IL-1b | IL-6 | IL-17 | IFN-g | G-CSF | IL-9 | IL-13 | |
|---|---|---|---|---|---|---|---|---|
| wt 6 months vs. APCMin/+ 6 months | ||||||||
| WT | 638 (135) | 225 (22) | 18 (6) | 45 (7) | 142 (12) | 81 (101) | 138 (40) | 108 (23) |
| APCmin/+ | 153 (359) | 314 (38) | 90 (16) | 52 (11) | 136 (45) | 229 (93) | 591 (77) | 12 (35) |
| p-value | ns | ns | 0.027 | ns | ns | ns | 0.00002 | ns |
| wt 6.months vs.RagAPCmin/+ 6 months | ||||||||
| WT | 638 (135) | 228 (22) | 18 (6) | 45 (7) | 142 (12) | 81 (101) | 138 (40) | 108 (23) |
| RagAPCmin/+ | 699 (56) | 32 (153) | 113 (17) | 66 (16) | 190 (58) | 238 (114) | 538 (74) | 128 (14) |
| p-value | ns | ns | 0.039 | 0.034 | ns | ns | 0.00002 | ns |
| Apcmin/+ 6 months vs.Rag APCmin/+ 6 months | ||||||||
| APCmin/+ | 1537 (359) | 314 (38) | 90 (16) | 52 (11) | 136 (45) | 229 (93) | 591 (77) | 120 (35) |
| RagAPCmin/+ | 699 (56) | 327 (153) | 113 (17) | 66 (16) | 190 (58) | 238 (114) | 538 (74) | 128 (14) |
| p-value | ns | ns | ns | ns | ns | ns | ns | ns |
| Rag Apcmin/+ 6 months untreated vs.Treg | ||||||||
| Untreated | 699 (56) | 327 (153) | 113 (17) | 66 (16) | 190 (58) | 238 (114) | 538 (74) | 128 (14) |
| Treg | 500 (114) | 225 (28) | 20 (3) | 46 (13) | 115 (7) | 60 (20) | 151 (74) | 89 (11) |
| p-value | 0.016 | ns | 0.043 | ns | 0.021 | 0.010 | 0.00032 | 0.002 |
| Apcmin/+ 3 months vs.APCmin/+ 6 months | ||||||||
| 3 months | 619 (55) | 228 (22) | 16 (3) | 44 (7) | 143 (11) | 57 (29) | 112 (17) | 112 (6) |
| 6 months | 1537 (359) | 314 (38) | 90 (16) | 52 (11) | 136 (45) | 229 (93) | 591 (77) | 120 (35) |
| p-value | ns | ns | 0.023 | ns | ns | 0.013 | 0.00006 | ns |
Regulatory T cells restore immune homeostasis. Serum levels of cytokines IL-6 and IL-9 were significantly increased in aged Min mice at high risk of intestinal polyposis and prostate cancer. Supplementation with wt TREG cells restored immune homeostasis, prevented cancer development and decreased serum cytokine levels of aged Rag2−/−Min mice to those of wt mice or young adult Min mice. Serum cytokine levels in pg/ml; assay used sera of n = 5 mice per group. Standard error is presented in parentheses.
Statistics calculated using 2-tailed Student’s t-test ns, not significant (p = 0.05).
Figure 4.
mRNA expression levels of IL-6 and Cox-2 in the murine prostate and bowel. There were significant increases in expression of IL-6 in (a) prostate and (b) ileum of Min mice treated with anti-CD25 antibody, and also in Rag-deficient Min mice, when compared to sham-treated Min counterparts. Supplementation with TREG cells from wt donor mice decreased expression of IL-6 in prostate tissue, whereas depletion of CD25+ cells increased IL-6 gene expression in prostate tissue. Assay used tissues from 5 to 8 mice per treatment group. For comparison of mRNA levels, the target mRNA was normalized to that of the housekeeping gene GAPDH. Numbers on the y-axis represent mean fold change of target mRNA levels in reference to the control levels (B6 wt, defined as 0, standard deviation represented by solid bars). mos, age in months upon necropsy.
Although both the serum cytokine levels and the tissue cytokine mRNA levels point to an association between prostate cancer and cytokines, we wished to learn more about the possibility that cytokines may actually promote cancer development. To test whether prostate cancer depends on inflammatory cytokines in ApcMin/+ mice, we neutralized a proinflammatory cytokine, tumor necrosis factor (TNF)-α, which is involved in initiation of immune activation and also amplification of other cytokines, using intraperitoneal injection of anti-TNF-α-antibodies for 10 days starting at age 20 weeks, as previously described.27,38 We observed, in particular, a reduction in features of neoplastic invasion (Table I, rows 3 and 6) in treated mice when compared to age-matched controls. This requirement of sustained inflammation for progression of neoplastic invasion has been previously shown in other murine models.38,46
Lymphocytes suppress the risk of developing prostate carcinoma
We next sought to determine whether or not lymphocytes, which have roles in both promoting and suppressing cancer, may be needed for prostatic neoplasia in ApcMin/+ mice. To address this question, we exploited Rag2-deficient C57BL/6 mice to create Rag2-defcient ApcMin/+ mice that completely lack functional lymphocytes. The lymphocyte compartment harbors cells with both pro- and anticancer functions, so it was unknown at the outset whether lack of lymphocytes would promote or suppress prostate cancer development. Interestingly, we found that Rag2-deficient ApcMin/+ mice aged 5–6 months developed more frequent neoplastic features in the prostate gland (Fig. 1) when compared to age-matched ApcMin/+ controls that were housed under the same conditions (Table I; Fig. 3).
Qualitatively, however, the spectrum of prostatic lesions tabulated in aged Rag2-deficient ApcMin/+ mice was similar to that of ApcMin/+ mice (shown in Fig. 1). One exception was that Rag2-deficient ApcMin/+ prostate stroma had a more prominent edematous alteration and a greater frequency of mast cells. Mast cells (Figs. 1e and 1f) were a frequent feature of malignancy in this model. We have previously shown that TNF-α-dependent activities of mast cells are essential for intestinal polyp development in ApcMin/+ mice.47 Significant elevations of IL-6 (p < 0.05) and IL-9 (p < 0.001) were observed in ApcMin/+ mice (Table II). Use of the Rag2-deficient host highlights the innate immune contributions toward IL-6 in prostate tissue in ApcMin/+ mice and role for these cells in subsequent pathogenic events in humans and mice.48
Taken together, it is clear that lymphocytes are not absolutely required for prostate tumorigenesis in the ApcMin/+ mouse model, an observation that is consistent with earlier studies.27 The observation of increased invasive prostate lesions in Rag2-deficient ApcMin/+ mice when compared to their ApcMin/+ counterparts (Table I; Fig. 3) indicates that lymphocytes are among cells that protect against malignancy. On the basis of prior data derived from ApcMin/+ mice,27,37,46,47 we postulated that CD4+ TREG cells may function to maintain immune homeostasis and inhibit prostate cancer in ApcMin/+ mice.
CD4+ TREG cells inhibit prostate carcinogenesis
Having observed that prostatic neoplasia was exacerbated by a deficiency in lymphocytes, we wondered whether supplementation with TREG cells might inhibit developing prostate cancer in Rag2−/− ApcMin/+ mice. The Rag2−/− ApcMin/+ mice permit direct assessment of the impact of adoptively transferred CD4+CD45RBloCD25+ TREG in the complete absence of endogenous TREG cells or other lymphocytes. Highly purified TREG cells derived from wild-type cell donors were adoptively transferred into 14-week-old Rag2−/−ApcMin/+ mice. We found that recipients of TREG cells examined 8 weeks later, at 5–6 months of age, had a significant (p < 0.01) reduction in prostate pathology (N = 8; Fig. 3), in cytokine levels of TNF-α (p < 0.05), IL-6 (p < 0.05) and IL-9 (p < 0.005) in the serum (Table II), and in cytokine expression levels in the prostate tissue (p < 0.01) (Fig. 4a) when compared to untreated Rag2−/−ApcMin/+ mice. Taken together, these data provide evidence that TREG cells alone, when collected from immune-competent donors, are sufficient for inhibition of prostate cancer.
We next examined whether supplementation with TREG cells might inhibit progression of developing prostate cancer in ApcMin/+ mice that have endogenous lymphocytes. To test this possibility, 10 4- to 5-month-old male ApcMin/+ mice underwent adoptive transfer of TREG cells from syngeneic wildtype (wt) donors. Despite the fact that the recipient mice already had TREG cells present, we nevertheless observed a significant (p < 0.05) reduction in prostate pathology in TREG-treated mice (N = 10, with 2 trials of 5 mice per trial; see Table I, Fig. 3). Examination of bowel from the same treated and untreated ApcMin/+ mice showed a significant (p < 0.01) reduction in intestinal adenoma multiplicity (N = 10; µ = 18 ± 3.68) in ApcMin/+ recipients of TREG cells when compared to untreated ApcMin/+ mice (N = 10; µ = 36 ± 5.40). These data, taken together with prior studies,27,46,49 support an essential protective role for TREG cells of immune-competent animals in maintenance of epithelial homeostasis and prevention of cancer.
Depletion of CD25+ cells accelerates progression of prostate carcinogenesis
Finally, we tested whether depletion of TREG cells may alter development of prostate cancer. We reasoned that if TREG are critical in preventing development of prostate cancer, then removing them from young mice should increase cancer risk later in life.37,38 Our lab38 and others31 have previously shown that both CD4+CD25+ and CD4+CD25+ subsets of TREG cells exhibit potent antiinflammatory properties in mice. For these experiments, we performed depletion of the CD25+ subset of TREG cells using antibodies against CD25. We depleted CD25+ cells in ApcMin/+ mice (2 trials; total N = 16) using anti-CD25 antibody injection starting at 6–8 weeks of age, before immune dysregulations are known to arise.50 Upon completion, we confirmed depletion by determining the fraction of CD25+ cells among splenocytes using flow cytometry, as well as inability to detect Foxp3+ cells in target tissues (prostatic lymph and mesenteric nodes) in situ using IHC (data not shown). Afterward, comparisons of CD25+ depleted and sham IgG-treated animals showed that there was a significant (p < 0.01) increase in prostate lesion frequency after depletion of CD25 when compared to either the sham antibody-treated or the unmanipulated age-matched ApcMin/+ mice (Table I). Severity and progression of PIN and microinvasive carcinoma in male Min mice that had undergone CD25 depletion matched those of ApcMin/+ mice of much more advanced age (compare in Table I: 3-month-old ApcMin/+ undergoing CD25 depletion versus 6-month-old ApcMin/+ mice).
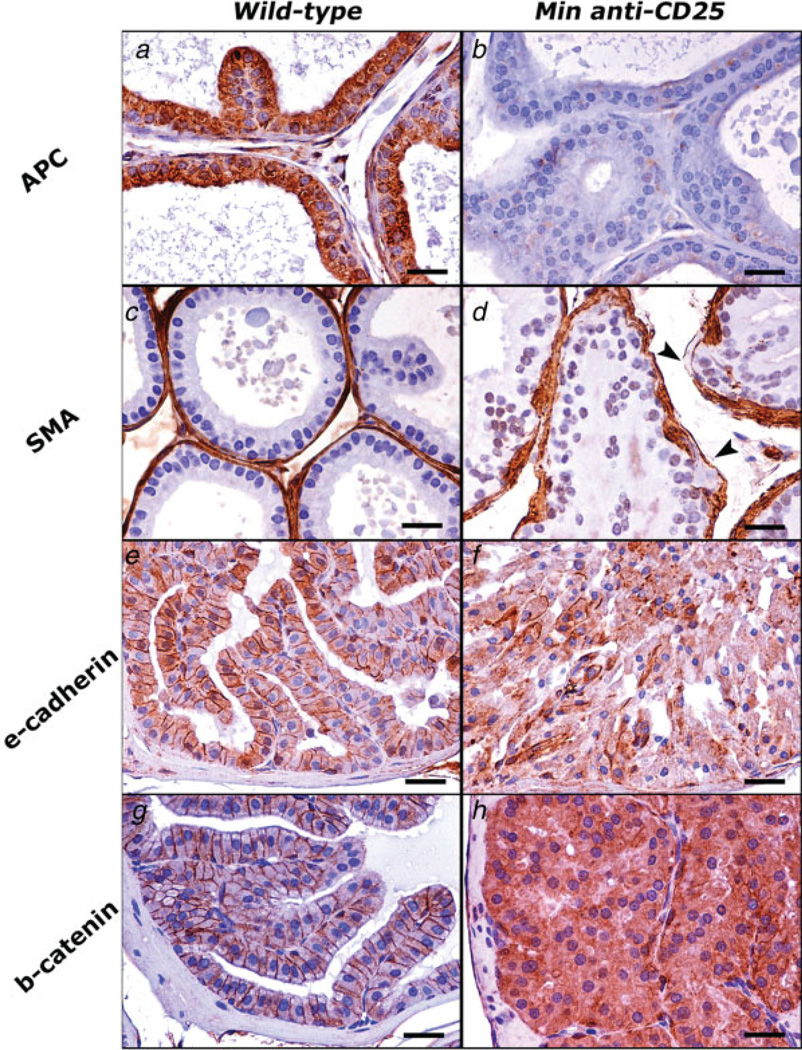
The frequency of preneoplastic foci within prostate tissue arose rapidly after depletion of CD25+ cells (Table I) and were clearly correlated with disruption of β-catenin and E-cadherin (Fig. 5). In general, loss of the Apc gene predisposed to decline in the normal lateral epithelial cell membrane staining pattern of E-cadherin (as shown in Fig. 5e) and β-catenin (as shown in Fig. 5g). The immunohistochemical signal of E-cadherin progressively attenuated (Fig. 5f) as the lesions progressed from LGPIN to prostate adeno-carcinoma. The abnormal and intense cytoplasmic (as with PIN, Fig. 5h), or both cytoplasmic and nuclear (as with adenocarcinoma, Fig. 1f) stabilization of β-catenin typified prostate neoplasia in ApcMin/+ mice. Gene expression analyses of prostate tissue after depletion of CD25+ cells also revealed increased expression of IL-6 when compared to age-matched sham-treated mice (p < 0.0001) (Fig. 4a).
Figure 5.
Immunohistochemical staining illustrates critical steps of carcinogenesis in the Min mouse model of prostate carcinoma. The aberrant immunostaining pattern observed in PIN lesions of Min mice that were depleted of CD25+ cells (right row) can be appreciated by comparison with normal prostate epithelia of wildtype mice (left row). (a–d) Dorsolateral prostate. (e–h) Anterior prostate. (a, b) The normal cytoplasmic expression of Apc (A) is absent in Min mouse prostate shown in b. (c, d) SMA immunohistochemistry highlights the fibromuscular sheath of prostate glands. Compare the smooth and continuous contour of normal prostate gland in c with the irregular and discontinuous (arrow-heads) sheath of prostate gland in d. (e, f) Normal lateral epithelial cell membrane staining pattern of E-cadherin in normal prostate (e) is by large lost in HGPIN shown in f. (g, h) Normal immunolabeling of β-catenin is largely restricted to adherence junctions in the prostate of wildtype mice (g). HGPIN lesions (h) instead of normal staining pattern show intense cytoplasmic staining, which indicates accumulation of β-catenin in the cytoplasm of abnormal prostate epithelial cells. Hematoxylin counterstain, DAB chromogen. Bars: 25 µm.
On the basis of these findings, we constructed a continuum of inflammation-associated prostate pathology in ApcMin/+ mice (see Fig. 3b–3e), progressing from LGPIN to prostatic adenocarcinoma. Upon comparison between all groups, it was Rag2-deficient ApcMin/+ mice that developed the highest frequency of advanced adenocarcinoma and invasive lesions (Table I and Fig. 3). The most advanced types of adenocarcinoma lesions were infrequent in 3-month-old ApcMin/+ mice, even after they had undergone CD25 depletion for 30–45 days. In general, however, carcinogenesis, including high-grade PIN (p < 0.01) and microinvasive adenocarcinoma (p < 0.05), was accelerated by depletion of antiinflammatory CD25+ cells in ApcMin/+ mice (Table I and Fig. 3).
Depletion of CD25+ cells increased frequency of intestinal ampullary tumors in the duodenal papilla of Vater
ApcMin/+ mice are a widely applied and well-established model for intestinal carcinogenesis because of their inherent susceptibility to intestinal tumorigenesis. The Apc mutation is an early event in 80% of sporadic intestinal cancers in humans,51,52 and the ApcMin/+ mouse has been frequently used for study of familial intestinal adenomatous polyposis (FAP) in humans.21,22 To examine whether the carcinogenic effect of CD25+ cell depletion might also affect other types of cancers, we inspected intestinal tissues of the same ApcMin/+ mice used for prostate cancer analyses: 8 males (2 trials; total N = 16, as described earlier) with the depletion protocol starting at 6–8 weeks of age. As previously described for the study of prostate, mice had been treated with anti-CD25 antibodies for 4 weeks starting at 4–6 weeks of age, alongside sham IgG-treated age-matched controls.
As we found for prostate cancer, ApcMin/+ mice treated with anti-CD25 antibody had an increased susceptibility to development of intestinal carcinoma. Specifically, we observed an increased frequency of ampullary cancer of the duodenal papillae of Vater in 25% (2/8) of mice treated with anti-CD25 (Fig. 6). This type of ampullary cancer was not observed even after extensive examination of untreated ApcMin/+ mice of all ages. Lesions were comprised of variably sized duct profiles with abnormal contours that infiltrated duodenal mucosa and the muscular coat of the pancreatic duct. Ductal epithelia lacking goblet cells were pseudostratified and highly atypical. Also, we found that mice that had been treated with anti-CD25 antibody showed signs of increased inflammation; we observed a desmoplastic and inflammatory reaction, characterized mainly by neutrophils and macrophages prominent within ampullary cancer lesions. Histologic features of the tumor matched those of human patients53 and also those previously described in compound mutant ApcMin/+ and SMAD4-deficient mice.54 Mast cells were a frequent feature in invasive intestinal lesions (Fig. 6b). We hypothesized that accelerated carcinogenesis was due to unmitigated inflammatory response and upregulation of inflammatory cytokines in intestinal tissues after depletion of CD25+ cells.
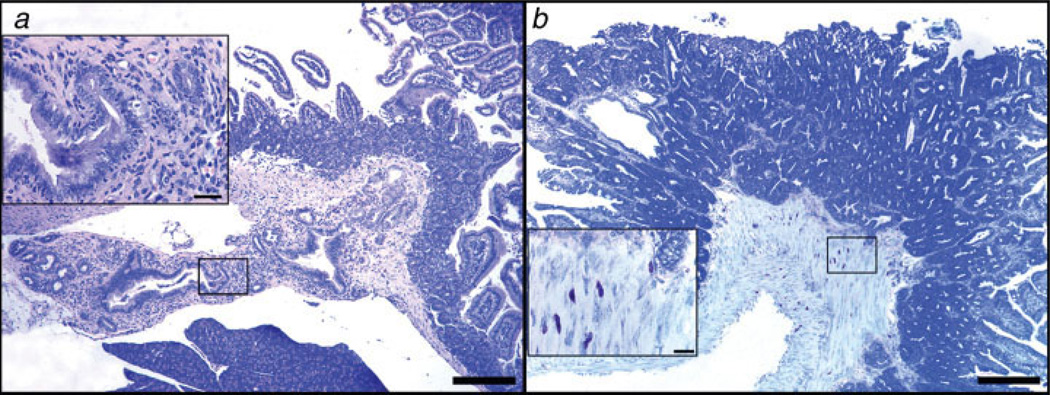
Figure 6.
Depletion of lymphocytes correlates with an invasive neoplastic phenotype in the small intestine of Apcmin/+mice. (a) Ampullary cancer arising from pancreatic duct epithelium in 25% (2/8) of 3-month-old ApcMin/+ mice after undergoing depletion of CD25+ cells (N = 8 mice per trial). High magnification (inset in a) reveals features of the abnormal pancreatic duct epithelium including pseudostratification and nuclear pleomorphism with associated inflammation. (b) Ileum of Rag2−/− ApcMin/+ mouse at 6 months of age revealing submucosal and incipient tunica muscularis invasion of neoplastic glands in an adenomatous polyp. Large numbers of mast cells infiltrate the submucosa and muscle layers at the base of the polyp. The higher magnification (inset) reveals the close topographic association of mast cells with the invasive front of the tumor. (a) Hematoxylin and Eosin. (b) Toluidine Blue. Bars a and b: 250 µm; insets: 25 µm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
We next examined whether proinflammatory cytokines were upregulated in bowel tissue after depletion of CD25+ cells. Upon analysis of snap frozen ileum tissue, we found that anti-CD25-treated ApcMin/+ mice had significantly (p < 0.01) increased levels of IL-6 gene expression in tissue after depletion of CD25+ cells when compared to tissue of sham-treated age-matched controls (Fig. 4b). Gene expression of Cox-2 (Fig. 4b) was also significantly increased when compared to sham IgG-treated counterparts. These findings match data from humans showing that cancer risk in the prostate and bowel is reduced after Cox-2 inhibitor therapy.13–15 Taken together, the results of the CD25-depletion studies support that antiinflammatory CD25+ TREG cells significantly inhibit carcinogenesis in ApcMin/+ mice.
Discussion
These studies demonstrate for the first time to our knowledge that male ApcMin/+ mice exhibit features of neoplasia in the prostate. Untreated ApcMin/+ mice developed age-associated prostate lesions with a high frequency of HGPIN and microadenocarcinoma resembling prostate cancer in humans. These findings were consistent with prostate carcinoma described in mice with inducible inactivation of the Apc gene.25 Overt inflammatory disease was not a feature of cancer; however, factors previously linked with prostate cancer in men, i.e., IL-6, were significantly associated with development of carcinoma in this mouse model. In this model, CD4+ TREG cells inhibited development of prostate carcinoma, at least in part because of downregulation of IL-6 and IL-9 cytokine expression. Not only did supplementation of purified CD4+ Treg cells from immune-competent donors serve to inhibit cancer development, but also the inverse was demonstrated when carcinoma frequency was increased after depletion of CD25+ cells in young mice. Data shown in Table I and Figure 5 after depletion of CD25+ cells were consistent with observations of Bruxvoort et al. showing that loss of the Apc gene leads to disruption of β-catenin and E-cadherin during development of prostatic adenocarcinoma in mice.25
Among genetic factors contributing to cancer, nearly 85% of sporadic colon cancers and 15% of prostate cancers in humans have been linked with inactivation of the Apc gene. Alterations in β-catenin and the wnt signaling pathway have also been correlated with advanced prostate cancer in humans.24,55 Development of cancer was coincident with abnormalities in β-catenin and E-cadherin in prostatic epithelia (see Results Figs. 1 and 5). These findings support data from other mouse models implicating β-catenin25,56 and E-cadherin57 in prostate carcinogenesis, and match earlier findings in humans.58,59 Disruption of cytoskeletal complexes because of reduction in E-cadherin is postulated to contribute to the loss of tissue architecture60 and has relevancy to human prostate health and disease.25
Although recent data involving protective versus destructive roles for TREG cells in cancer appear contradictory on the surface,39–42 we postulate that function of TREG depends on the age of the animal and context within the microenvironment of the animal. In young and immune-competent animals, TREG cells actually serve an important protective role against cancer by maintaining immune homeostasis and constructively regulating levels of TNF-α, IL-6, IL-17 and IL-9 that when dysregulated may contribute to cancer growth. In support of this, data in Table II show that levels of TNF-α, IL-6 and IL-9 are significantly lowered when exogenous TREG cells are added in young mice. Likewise, targeted depletion of CD25 leads to upregulation of cytokines such as IL-6 (p < 0.001) in prostate (see Fig. 4; groups undergoing CD25 cell depletion) and contribute to onset of cancer. Inability to properly regulate IL-6 was shown in other models to divert homeostatic functions of CD4+ cells toward a destructive Th17 pathway that may contribute to cancer growth.48,61 We propose that this process can be interrupted to benefit the host by downregulation of inflammation (as with anti-TNF) or by supplementation with exogenous TREG cells collected from immune competent wt hosts.27,38,46,62 Reenforcement of the antiinflammatory arm of immunity with TREG cells significantly (p < 0.05) reduces levels of IL-6 in mice (see Table II).
Depletion of CD25+ cells in 2- to 3-month-old mice was shown here to increase the rate of prostatic neoplasia that arose spontaneously at a later age of 5–6 months in untreated ApcMin/+ mice (see Table I). One interesting question raised by these data is whether ApcMin/+ mice have an intrinsic defect that diminishes ability of resident TREG cells to prevent prostatic and bowel cancer. One possible explanation is that the growing intestinal tumor burden increases levels of TNF-α and IL-6 that subsequently accelerate a Th-17-driven spiral of TREG-mediated events that subsequently contribute to cancer growth.49,50,63 An alternative interpretation in this “chicken-and-egg” scenario is that thymic depletion, evident at 90 days of age in ApcMin/+ mice,50 may lead to widespread immune dysfunction predisposing to elevated IL-6 that increases cancer risk. By this 2nd way of thinking, immune impairment arises first and then allows for increased levels of TNF-α and IL-6 that impede TREG homeostatic capabilities.48 In either event, maintenance of immune homeostasis is disrupted in the ApcMin/+ mice, providing an opportunity to dissect roles for cytokines that may contribute to cancer growth. In this setting, supplemental CD4+ TREG cells collected from immune competent donors would, at least initially, be untainted by high levels of IL-6 within the tumor microenvironment. Our experimental depletion of CD25+ cells started at 30–45 days of age, preceding the natural onset of thymic involution in ApcMin/+ mice.50 Importantly, at 30– 45 days of age, prostatic neoplasia was otherwise nearly nonexistent in untreated male ApcMin/+ mice (Table I). Thus, removal of CD25+ cells greatly hastened the onset of cancer in an animal otherwise predisposed to preneoplastic pathology.
Our depletion of CD25+ cells matched data of mice undergoing neonatal thymectomy that were found to subsequently develop prostatitis.64 Likewise, cytokine expression was increased in prostate and bowel tissue after CD25 cell depletion (Fig. 4a), and conversely decreased after TREG cell supplementation (Fig. 4a, Table II). It is important to note that the anti-CD25 treatment approach29 may also deplete other nonregulatory cell CD25+ populations, such as activated effector T cells,65 that may also contribute to experimental outcome; although, large numbers of activated cells bearing CD25 are unlikely in young mice lacking tumors and without targeted infections. Rapid repopulation of CD25+ cells after depletion has been previously described.65 In addition, CD4+CD25-TREG cells are previously shown by our lab38 and others31 to downregulate inflammation in mice. These data do not invalidate a key role for CD25-TREG cells in prevention of cancer. Indeed, cotransfer of CD4+CD45RBloCD25+ cells together with CD4+CD45RBhiCD25−cells38 was previously shown to provide the most robust enhancement of longevity and protection from cancer.
The pivotal relationship between TREG cells and IL-6 was previously highlighted in wound repair after epithelial injury in the bowel of mice.62 It was postulated in that model that antiinflammatory efficiency of TREG cells dictates outcome of wound healing. The normal aging process contributes to increased susceptibility to IL-6-mediated disruptions in TREG efficacy manifesting as delayed wound healing and dysregulated wound repair.62 This progression of IL-6-mediated events was found to be most evident in male animals.62 It is interesting that aged male ApcMin/+ mice supplemented with CD4+CD25+ cells in our study exhibited not only decreased levels of TNF-α and IL-6 but also reduction in cancer cachexia, along with improved longevity and fecundity, when compared to age-matched untreated ApcMin/+ mice (data not shown). The ApcMin/+ mouse has been proposed elsewhere66 as a model for IL-6-associated wasting disease for cancer and aging in men, highlighting broader relevancy for our findings.
Another noteworthy observation is the absence of overt inflammatory disease associated with prostate carcinoma development in this model. In humans, there is controversy over a role for inflammation in prostate cancer, although molecular pathogenesis of prostate cancer is characterized by somatic alterations of genes involved in inflammatory defenses and tissue recovery.3 Interestingly, lymphocytes and macrophages were infrequent within prostate tissue in this model. Only mast cells were clearly linked with neoplastic lesions and invasive features (see Figs. 1 and 6). The absence of inflammatory cell infiltrate in ApcMin/+ mice matches prior data of TNF-α-dependent bowel neoplasia in mice lacking overt IBD.27,46 These findings raise the possibility that the interplay between TNF-α, IL-6, IL-17 and TREG cells may have broader relevancy beyond cancers that are clearly linked with overt inflammatory disease.
Inflammatory cytokine IL-9 was significantly elevated in mouse sera (Table II) and correlated with the development of prostatic neoplasia in our study. IL-9 is a growth factor linked with recruitment and activation of mast cells.67,68 It is possible that IL-9 promoted prostate carcinogenesis in ApcMin/+ mice through activation of mast cells; indeed, mast cells may be important for creating a prostatic environment conducive to cancer development.12,69 Mast cells and IL-9 are previously linked with activated TREG cells in maintenance of immune tolerance68; thus, it is interesting that supplementation with exogenous TREG cells serves to lower levels of IL-9 (see Table II) and also effectively suppresses carcinogenesis in ApcMin/+ mice. TNF-α-dependent mast cell accumulations were previously shown to be an essential feature of intestinal polyposis in mice,47 and mast cells were a frequent feature in invasive intestinal lesions in our study (see Figs. 6c and 6d). Alternatively, carcinoma may be due to a direct carcinogenic effect of IL-9 in prostate epithelia, although thus far IL-9 has proven oncogenic activities only in lymphoid cell malignancies of mice and humans.70
Although similar prostate pathology was reported in male mice with inducible inactivation of the Apc gene,25 it was unclear from the present studies whether intestinal polyposis contributed in any way to prostate cancer growth. In many murine models, bowel pathology depends on pathogenic bacterial infections that trigger a local and systemic proinflammatory carcinogenic host response. We have previously shown that infections with pathogenic intestinal bacteria may upregulate proinflammatory cytokines, such as TNF-α34,37 and IL-6,62 that subsequently contribute to carcinoma development and malignancy in distant sites. Infections with pathogenic bacteria have previously been linked with prostatitis in humans71 and in rats.12 Interestingly, in an immune-competent host, pathogenic bacteria or their toxins may also induce potent IL-10-dependent CD4+ TREG cells that are highly protective against inflammation-associated cancer.27,46,72 Preliminary data using experimental bacterial challenges reveal similar dual harmful and beneficial roles for microbiota in prostate cancer in male ApcMin/+ mice (data not shown).
The appearance of ampullary carcinomas in the duodenal papilla of Vater after depletion of CD25+ cells in 25% (2/8) of young ApcMin/+ mice is noteworthy. No such carcinomas were found after extensive examination of bowel tissues from age-matched or older ApcMin/+ mice. In humans with FAP, ampullary tumors are a serious cause of comorbidity.73 Duodenal tumors in FAP patients are often clustered in the ampullary region and frequently become malignant. Compound mutant mice with disruption of Apc and SMAD4 suffer from a similar tumor phenotype. This invasive phenotype mirrors mice lacking Tgf-β1 and SMAD3,74–76 as well as murine recipients of dysfunctional TREG cell,62 suggesting that dysregulation of Tgf-β signaling may be a shared feature in all of these pathological syndromes.
In conclusion, spontaneous prostate cancer arose in untreated male ApcMin/+ mice with a high frequency of HGPIN and microadenocarcinoma resembling age-associated prostate cancer in humans. Factors previously found with human prostate cancer, i.e., IL-6, were significantly associated with development of carcinoma in this model. Although accumulated epidemiological and medical data support a link between chronic inflammation and prostate cancer, this is the first report providing evidence that TREG cells reduce development of preneoplastic and neoplastic findings in the prostate. Inflammatory cytokines were significantly elevated and linked with prostate cancer development, but without the presence of overt inflammatory disease in the prostate. In particular, serum IL-6 levels were correlated with cancer in these mice and may serve as a useful biomarker in humans. Future prophylactic and therapeutic strategies may focus upon reinforcing relevant immune fortification for protection against cancer development.
Acknowledgements
The authors thank Ms. Kathy Cormier and Mr. Chakib Boussahamain for help with histology and immunohistochemistry and Mr. Glenn Paradis and Ms. Michele Perry for assistance with cell sorting. They also thank Mr. Brian D. Morrison and Ms. Elaine Robbins for help with figures. W.O. is recipient of an APART fellowship of the Austrian Academy of Sciences.
Grant sponsor: National Institutes of Health; Grant numbers: R01CA108854, P30ES02109, T32RR07036.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Sakr WA, Grignon DJ, Crissman JD, Heilbrun LK, Cassin BJ, Pontes JJ, Haas GP. High grade prostatic intraepithelial neoplasia (HGPIN) and prostatic adenocarcinoma between the ages of 20–69: an autopsy study of 249 cases. In Vivo. 1994;8:439–443. [PubMed] [Google Scholar]
- 3.Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349:366–381. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- 4.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 5.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karin M, Lawrence T, Nizet V. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell. 2006;124:823–835. doi: 10.1016/j.cell.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Lawrence T. Inflammation and cancer: a failure of resolution? Trends Pharmacol Sci. 2007;28:162–165. doi: 10.1016/j.tips.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Karin M. Rsk Tolls the bell for endocytosis in DCs. Nat Immunol. 2007;8:1197–1199. doi: 10.1038/ni1107-1197. [DOI] [PubMed] [Google Scholar]
- 10.Riddell R, Petras RE, Williams GT, Sobin LH. Dysplasia and cancer in inflammatory bowel disease. Washington, DC: AFIP Press; 2003. pp. 189–201. [Google Scholar]
- 11.Krieger JN, Nyberg L, Jr., Nickel JC. NIH consensus definition and classification of prostatitis. JAMA. 1999;282:236–237. doi: 10.1001/jama.282.3.236. [DOI] [PubMed] [Google Scholar]
- 12.Palapattu GS, Sutcliffe S, Bastian PJ, Platz EA, De Marzo AM, Isaacs WB, Nelson WG. Prostate carcinogenesis and inflammation: emerging insights. Carcinogenesis. 2005;26:1170–1181. doi: 10.1093/carcin/bgh317. [DOI] [PubMed] [Google Scholar]
- 13.Gupta S, Adhami VM, Subbarayan M, MacLennan GT, Lewin JS, Hafeli UO, Fu P, Mukhtar H. Suppression of prostate carcinogenesis by dietary supplementation of celecoxib in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2004;64:3334–3343. doi: 10.1158/0008-5472.can-03-2422. [DOI] [PubMed] [Google Scholar]
- 14.Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356:2131–2142. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- 15.Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer. 2001;1:11–21. doi: 10.1038/35094017. [DOI] [PubMed] [Google Scholar]
- 16.Vykhovanets EV, Resnick MI, MacLennan GT, Gupta S. Experimental rodent models of prostatitis: limitations and potential. Prostate Cancer Prostatic Dis. 2007;10:15–29. doi: 10.1038/sj.pcan.4500930. [DOI] [PubMed] [Google Scholar]
- 17.Schneikert J, Behrens J. The canonical Wnt signalling pathway and its APC partner in colon cancer development. Gut. 2007;56:417–425. doi: 10.1136/gut.2006.093310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Segditsas S, Tomlinson I. Colorectal cancer and genetic alterations in the Wnt pathway. Oncogene. 2006;25:7531–7537. doi: 10.1038/sj.onc.1210059. [DOI] [PubMed] [Google Scholar]
- 19.Brewster SF, Browne S, Brown KW. Somatic allelic loss at the DCC APC, nm23-H1 and p53 tumor suppressor gene loci in human prostatic carcinoma. J Urol. 1994;151:1073–1077. doi: 10.1016/s0022-5347(17)35186-8. [DOI] [PubMed] [Google Scholar]
- 20.Phillips SM, Morton DG, Lee SJ, Wallace DM, Neoptolemos JP. Loss of heterozygosity of the retinoblastoma and adenomatous polyposis susceptibility gene loci and in chromosomes 10p, 10q and 16q in human prostate cancer. Br J Urol. 1994;73:390–395. doi: 10.1111/j.1464-410x.1994.tb07602.x. [DOI] [PubMed] [Google Scholar]
- 21.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 22.Corpet DE, Pierre F. Point: from animal models to prevention of colon cancer. Systematic review of chemoprevention in min mice and choice of the model system. Cancer Epidemiol Biomarkers Prev. 2003;12:391–400. [PMC free article] [PubMed] [Google Scholar]
- 23.Chesire DR, Ewing CM, Sauvageot J, Bova GS, Isaacs WB. Detection and analysis of b-catenin mutations in prostate cancer. Prostate. 2000;45:323–334. doi: 10.1002/1097-0045(20001201)45:4<323::aid-pros7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 24.Yardy GW, Brewster SF. Wnt signalling and prostate cancer. Prostate Cancer Prostatic Dis. 2005;8:119–126. doi: 10.1038/sj.pcan.4500794. [DOI] [PubMed] [Google Scholar]
- 25.Bruxvoort KJ, Charbonneau HM, Giambernardi TA, Goolsby JC, Qian CN, Zylstra CR, Robinson DR, Roy-Burman P, Shaw AK, Buckner-Berghuis BD, Sigler RE, Resau JH, et al. Inactivation of Apc in the mouse prostate causes prostate carcinoma. Cancer Res. 2007;67:2490–2496. doi: 10.1158/0008-5472.CAN-06-3028. [DOI] [PubMed] [Google Scholar]
- 26.Taketo MM. Wnt signaling and gastrointestinal tumorigenesis in mouse models. Oncogene. 2006;25:7522–7530. doi: 10.1038/sj.onc.1210058. [DOI] [PubMed] [Google Scholar]
- 27.Rao VP, Poutahidis T, Ge Z, Nambiar PR, Boussahmain C, Wang YY, Horwitz BH, Fox JG, Erdman SE. Innate immune inflammatory response against enteric bacteria Helicobacter hepaticus induces mammary adenocarcinoma in mice. Cancer Res. 2006;66:7395–7400. doi: 10.1158/0008-5472.CAN-06-0558. [DOI] [PubMed] [Google Scholar]
- 28.Taguchi O, Nishizuka Y. Experimental autoimmune orchitis after neonatal thymectomy in the mouse. Clin Exp Immunol. 1981;46:425–434. [PMC free article] [PubMed] [Google Scholar]
- 29.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 30.Powrie F. T cells in inflammatory bowel disease: protective and pathogenic roles. Immunity. 1995;3:171–174. doi: 10.1016/1074-7613(95)90086-1. [DOI] [PubMed] [Google Scholar]
- 31.Kullberg MC, Jankovic D, Gorelick PL, Caspar P, Letterio JJ, Cheever AW, Sher A. Bacteria-triggered CD4(+) T regulatory cells suppress Helicobacter hepaticus-induced colitis. J Exp Med. 2002;196:505–515. doi: 10.1084/jem.20020556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erdman SE, Poutahidis T, Tomczak M, Rogers AB, Cormier K, Plank B, Horwitz BH, Fox JG. CD4+ CD25+ regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice. Am J Pathol. 2003;162:691–702. doi: 10.1016/S0002-9440(10)63863-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erdman SE, Rao VP, Poutahidis T, Ihrig MM, Ge Z, Feng Y, Tomczak M, Rogers AB, Horwitz BH, Fox JG. CD4(+)CD25(+) regulatory lymphocytes require interleukin 10 to interrupt colon carcinogenesis in mice. Cancer Res. 2003;63:6042–6050. [PubMed] [Google Scholar]
- 35.Annacker O, Pimenta-Araujo R, Burlen-Defranoux O, Barbosa TC, Cumano A, Bandeira A. CD25+ CD4+ T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J Immunol. 2001;166:3008–3018. doi: 10.4049/jimmunol.166.5.3008. [DOI] [PubMed] [Google Scholar]
- 36.Maloy KJ, Salaun L, Cahill R, Dougan G, Saunders NJ, Powrie F. CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med. 2003;197:111–119. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erdman SE, Sohn JJ, Rao VP, Nambiar PR, Ge Z, Fox JG, Schauer DB. CD4+CD25+ regulatory lymphocytes induce regression of intestinal tumors in ApcMin/+ mice. Cancer Res. 2005;65:3998–4004. doi: 10.1158/0008-5472.CAN-04-3104. [DOI] [PubMed] [Google Scholar]
- 38.Rao VP, Poutahidis T, Ge Z, Nambiar PR, Horwitz BH, Fox JG, Erdman SE. Proinflammatory CD4+ CD45RB(hi) lymphocytes promote mammary and intestinal carcinogenesis in Apc(Min/+) mice. Cancer Res. 2006;66:57–61. doi: 10.1158/0008-5472.CAN-05-3445. [DOI] [PubMed] [Google Scholar]
- 39.Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E, Vieweg J. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 41.Khazaie K, von Boehmer H. The impact of CD4+CD25+ Treg on tumor specific CD8+ T cell cytotoxicity and cancer. Semin Cancer Biol. 2006;16:124–136. doi: 10.1016/j.semcancer.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 42.Degl’Innocenti E, Grioni M, Capuano G, Jachetti E, Freschi M, Bertilaccio MT, Hess-Michelini R, Doglioni C, Bellone M. Peripheral T-cell tolerance associated with prostate cancer is independent from CD4+CD25+ regulatory T cells. Cancer Res. 2008;68:292–300. doi: 10.1158/0008-5472.CAN-07-2429. [DOI] [PubMed] [Google Scholar]
- 43.Shappell SB, Thomas GV, Roberts RL, Herbert R, Ittmann MM, Rubin MA, Humphrey PA, Sundberg JP, Rozengurt N, Barrios R, Ward JM, Cardiff RD, et al. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res. 2004;64:2270–2305. doi: 10.1158/0008-5472.can-03-0946. [DOI] [PubMed] [Google Scholar]
- 44.Boivin GP, Washington K, Yang K, Ward JM, Pretlow TP, Russell R, Besselsen DG, Godfrey VL, Doetschman T, Dove WF, Pitot HC, Hal-berg RB, et al. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology. 2003;124:762–777. doi: 10.1053/gast.2003.50094. [DOI] [PubMed] [Google Scholar]
- 45.Abate-Shen C, Shen MM. Molecular genetics of prostate cancer. Genes Dev. 2000;14:2410–2434. doi: 10.1101/gad.819500. [DOI] [PubMed] [Google Scholar]
- 46.Rao VP, Poutahidis T, Fox JG, Erdman SE. Breast cancer: should gastrointestinal bacteria be on our radar screen? Cancer Res. 2007;67:847–850. doi: 10.1158/0008-5472.CAN-06-3468. [DOI] [PubMed] [Google Scholar]
- 47.Gounaris E, Erdman SE, Restaino C, Gurish MF, Friend DS, Gounari F, Lee DM, Zhang G, Glickman JN, Shin K, Rao VP, Poutahidis T, et al. Mast cells are an essential hematopoietic component for polyp development. Proc Natl Acad Sci USA. 2007;104:19977–19982. doi: 10.1073/pnas.0704620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 49.Faubion WA, De Jong YP, Molina AA, Ji H, Clarke K, Wang B, Mizoguchi E, Simpson SJ, Bhan AK, Terhorst C. Colitis is associated with thymic destruction attenuating CD4+25+ regulatory T cells in the periphery. Gastroenterology. 2004;126:1759–1770. doi: 10.1053/j.gastro.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 50.Coletta PL, Muller AM, Jones EA, Muhl B, Holwell S, Clarke D, Meade JL, Cook GP, Hawcroft G, Ponchel F, Lam WK, MacLennan KA, et al. Lymphodepletion in the ApcMin/+ mouse model of intestinal tumorigenesis. Blood. 2004;103:1050–1058. doi: 10.1182/blood-2003-03-0707. [DOI] [PubMed] [Google Scholar]
- 51.Homfray TF, Cottrell SE, Ilyas M, Rowan A, Talbot IC, Bodmer WF, Tomlinson IP. Defects in mismatch repair occur after APC mutations in the pathogenesis of sporadic colorectal tumours. Hum Mutat. 1998;11:114–120. doi: 10.1002/(SICI)1098-1004(1998)11:2<114::AID-HUMU3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 52.Jaiswal AS, Balusu R, Narayan S. Involvement of adenomatous polyposis coli in colorectal tumorigenesis. Front Biosci. 2005;10:1118–1134. doi: 10.2741/1605. [DOI] [PubMed] [Google Scholar]
- 53.Fenoglio-Preiser C, Noffsinger AE, Stmmermann GN, Lantz PE, Listrom MB, Rilke FO. Neoplastic lesions of the small intestine. Philadelphia, PA: Lippincott Williams & Wilkins; 1999. [Google Scholar]
- 54.Takaku K, Oshima M, Miyoshi H, Matsui M, Seldin MF, Taketo MM. Intestinal tumorigenesis in compound mutant mice of both Dpc4 (Smad4) and Apc genes. Cell. 1998;92:645–656. doi: 10.1016/s0092-8674(00)81132-0. [DOI] [PubMed] [Google Scholar]
- 55.Verras M, Sun Z. Roles and regulation of Wnt signaling and β-catenin in prostate cancer. Cancer Lett. 2006;237:22–32. doi: 10.1016/j.canlet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 56.Shukla S, MacLennan GT, Flask CA, Fu P, Mishra A, Resnick MI, Gupta S. Blockade of β-catenin signaling by plant flavonoid apigenin suppresses prostate carcinogenesis in TRAMP mice. Cancer Res. 2007;67:6925–6935. doi: 10.1158/0008-5472.CAN-07-0717. [DOI] [PubMed] [Google Scholar]
- 57.Kim MJ, Bhatia-Gaur R, Banach-Petrosky WA, Desai N, Wang Y, Hayward SW, Cunha GR, Cardiff RD, Shen MM, Abate-Shen C. Nkx3.1 mutant mice recapitulate early stages of prostate carcinogenesis. Cancer Res. 2002;62:2999–3004. [PubMed] [Google Scholar]
- 58.Isaacs WB, Bova GS, Morton RA, Bussemakers MJ, Brooks JD, Ewing CM. Molecular biology of prostate cancer. Semin Oncol. 1994;21:514–521. [PubMed] [Google Scholar]
- 59.Isaacs WB, Bova GS, Morton RA, Bussemakers MJ, Brooks JD, Ewing CM. Molecular biology of prostate cancer progression. Cancer Surv. 1995;23:19–32. [PubMed] [Google Scholar]
- 60.Umbas R, Schalken JA, Aalders TW, Carter BS, Karthaus HF, Schaafsma HE, Debruyne FM, Isaacs WB. Expression of the cellular adhesion molecule E-cadherin is reduced or absent in high-grade prostate cancer. Cancer Res. 1992;52:5104–5109. [PubMed] [Google Scholar]
- 61.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 62.Poutahidis T, Haigis KM, Rao VP, Nambiar PR, Taylor CL, Ge Z, Watanabe K, Davidson A, Horwitz BH, Fox JG, Erdman SE. Rapid reversal of interleukin-6-dependent epithelial invasion in a mouse model of microbially induced colon carcinoma. Carcinogenesis. 2007;28:2614–2623. doi: 10.1093/carcin/bgm180. [DOI] [PubMed] [Google Scholar]
- 63.Gounari F, Chang R, Cowan J, Guo Z, Dose M, Gounaris E, Khazaie K. Loss of adenomatous polyposis coli gene function disrupts thymic development. Nat Immunol. 2005;6:800–809. doi: 10.1038/ni1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taguchi O, Kojima A, Nishizuka Y. Experimental autoimmune prostatitis after neonatal thymectomy in the mouse. Clin Exp Immunol. 1985;60:123–129. [PMC free article] [PubMed] [Google Scholar]
- 65.Couper KN, Blount DG, de Souza JB, Suffia I, Belkaid Y, Riley EM. Incomplete depletion and rapid regeneration of Foxp3+ regulatory T cells following anti-CD25 treatment in malaria-infected mice. J Immunol. 2007;178:4136–4146. doi: 10.4049/jimmunol.178.7.4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baltgalvis KA, Berger FG, Pena MM, Davis JM, Muga SJ, Carson JA. Interleukin-6 and cachexia in ApcMin/+ mice. Am J Physiol Regul Integr Comp Physiol. 2008;294:R393–R401. doi: 10.1152/ajpregu.00716.2007. [DOI] [PubMed] [Google Scholar]
- 67.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 68.Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, Scott ZA, Coyle AJ, Reed JL, Van Snick J, Strom TB, Zheng XX, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 69.Keith IM, Jin J, Neal D, Jr., Teunissen BD, Moon TD. Cell relationship in a Wistar rat model of spontaneous prostatitis. J Urol. 2001;166:323–328. [PubMed] [Google Scholar]
- 70.Knoops L, Renauld JC. IL-9 and its receptor: from signal transduction to tumorigenesis. Growth Factors. 2004;22:207–215. doi: 10.1080/08977190410001720879. [DOI] [PubMed] [Google Scholar]
- 71.Correa P. Commentary: is prostate cancer an infectious disease? Int J Epidemiol. 2005;34:197–198. doi: 10.1093/ije/dyh403. [DOI] [PubMed] [Google Scholar]
- 72.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 73.Jagelman DG, DeCosse JJ, Bussey HJ. Upper gastrointestinal cancer in familial adenomatous polyposis. Lancet. 1988;1:1149–1151. doi: 10.1016/s0140-6736(88)91962-9. [DOI] [PubMed] [Google Scholar]
- 74.Engle SJ, Ormsby I, Pawlowski S, Boivin GP, Croft J, Balish E, Doetschman T. Elimination of colon cancer in germ-free transforming growth factor β 1-deficient mice. Cancer Res. 2002;62:6362–6366. [PubMed] [Google Scholar]
- 75.Maggio-Price L, Bielefeldt-Ohmann H, Treuting P, Iritani BM, Zeng W, Nicks A, Tsang M, Shows D, Morrissey P, Viney JL. Dual infection with Helicobacter bilis and Helicobacter hepaticus in p-glycoprotein-deficient mdr1a−/− mice results in colitis that progresses to dysplasia. Am J Pathol. 2005;166:1793–1806. doi: 10.1016/S0002-9440(10)62489-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maggio-Price L, Treuting P, Zeng W, Tsang M, Bielefeldt-Ohmann H, Iritani BM. Helicobacter infection is required for inflammation and colon cancer in SMAD3-deficient mice. Cancer Res. 2006;66:828–838. doi: 10.1158/0008-5472.CAN-05-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]