Abstract
Background:
Co-infection of hepatitis virus is common in human immunodeficiency virus (HIV) infected adults in China. But little is known about hepatitis virus co-infection in pediatric HIV-infected subjects. The study aimed to investigate the impact of hepatitis B virus (HBV) and/or hepatitis C virus (HCV) co-infection and highly active antiretroviral therapy (HAART) on liver function of pediatric HIV-infected subjects.
Materials and Methods:
A cohort study including 101 pediatric HIV-infected subjects with HBV/HCV co-infection and 44 pediatric comparators with HIV mono-infection was carried out in Henan Province of China from September 2011 to September 2012. All patients received HAART for 1-year. HBV and HCV infection was determined by antibody tests. HIV RNA load, CD4+ T-cell counts and liver function were determined before and after HAART. The Student's t-test or a one-way ANOVA was used for normally distributed values and A Mann-Whitney U-test was performed for values without normal distribution using SPSS statistical package 18.0 (SPSS Inc.).
Results:
After HAART for 1-year, the median levels of viral load were decreased to lower limit of detection in 90.34% pediatric HIV-infected subjects with/without HBV/HCV co-infection (P < 0.001), and CD4+ T-cell counts increased significantly (P < 0.001). Compared with the pre-HAART, mean level of alanine aminotransferase (ALT) in each group had a significant increase after HAART (P < 0.01). The mean levels of ALT and aspartate aminotransferase (AST) in nevirapine (NVP) based HAART group increased significantly after HAART (P < 0.01). Mean change values of ALT and AST were significantly higher in the NVP based regimen group than in the efavirenz (EFV) based regimen group (P < 0.01). For HIV/HBV/HCV co-infected patients, mean change values of ALT and AST in NVP-based HAART group was significantly higher than that in EFV-based HAART group (P < 0.01).
Conclusion:
Highly active antiretroviral therapy can damage liver function in pediatric HIV-infected subjects, especially in those with HBV/HCV co-infection. NVP was more harmful to liver function of pediatric HIV-infected subjects than EFV.
Keywords: Co-infection, hepatitis B virus, hepatitis C virus, highly active antiretroviral therapy, human immunodeficiency virus, liver function, pediatric
INTRODUCTION
Human immunodeficiency virus (HIV) and hepatitis virus share similar modes of transmission, for example, transmission through blood transfusion, vertical/perinatal transmission, and sexual contact. Hence it is common that HIV-infected patients are co-infected with hepatitis B virus (HBV) and/or hepatitis C virus (HCV).[1] Approximately, 5-10% of HIV-infected persons worldwide have chronic HBV co-infection,[2] and in high HIV prevalence areas, rate of HBV co-infection was as large as 25%.[3] And even in areas where HBV is less endemic such as North America, Europe, and Australia, up to half of injection drug users (IDU) infected with HIV are co-infected with HBV.[4] It is estimated that 4-5 million HIV-infected patients are co-infected with HCV in the world,[5] and the prevalence of HIV-HCV co-infection is even up to 90% in persons injecting drugs.[6]
As more new medicines for highly active antiretroviral therapy (HAART) were developed in the recent years, mortality from AIDS is reduced dramatically by HAART. HIV-infected patients are now faced with other co-morbidity including liver damage from HIV-viral hepatitis co-infections and its therapy.[7] Studies on HIV-1 infected adults reveal that the rate of liver cirrhosis and even hepatocellular carcinoma increased significantly in HIV/AIDS patients after receiving effective HAART, and the survival rate of HIV co-infection with HBV/HCV was lower than that of HIV alone,[8,9] which brought a new challenge to the treatment of AIDS.
Pediatric HIV-infected subjects are different from adult patients in that they are in the growth stage, and the liver is more susceptible.[10] Children may acquire all three viral infections by perinatal transmission and blood transfusion. Sexual exposure and IDU are also factors in older children.[11] More than 90% of newborns who are exposed to HBV and HIV from co-infected mothers who do not receive postexposure prophylaxis for HBV develop chronic HBV infection.[12] However, transmission risk of HCV is relatively lower, since it is about 5% for a woman who is positive for viral RNA at the end of her pregnancy, but at least 10% if the woman is positive for HIV.[13] HIV co-infection with HBV/HCV is associated with the failure to seroconvert following acute infection and a greater risk of developing chronic HBV/HCV infection compared to HIV-seronegative children.[14] Nevertheless, studies on liver damage in pediatric HIV-infected patients with HBV/HCV co-infection are rare in China to date. Therefore, this study aimed to investigate the impact of HBV and/or HCV co-infection and HAART on liver function of pediatric HIV-infected subjects.
MATERIALS AND METHODS
Study design and participants
A cohort study including 101 pediatric HIV-infected subjects with HBV/HCV co-infection and 44 pediatric comparators with HIV mono-infection, who were under the age of 16, was carried out in Center for Disease Control and Prevention (CDC) of Shangcai, Henan Province in China from September 2011 to September 2012.
Human immunodeficiency virus-1 infection was diagnosed on the basis of positive results of serological and HIV-RNA detection assays. HCV infection was diagnosed based on the positive results of anti-HCV test when the subjects were enrolled at September 2011. HCV RNA was not detected for the limited conditions. Patients were regarded as having a chronic HBV infection when HBsAg could be detected in plasma on two occasions 6 months before September 2011.
All pediatric HIV-infected subjects received HAART for a minimum of 1-year and had no opportunistic infections diagnosed at the time of being enrolled in the study. Among the entire cohort, 49/145 (33.8%) received efavirenz (EFV)-based HAART and 96/145 (66.2%) nevirapine (NVP)-based HAART. 101 co-infected pediatric HIV-infected subjects were divided into three groups according to various co-infections, HIV/HBV/HCV co-infection groups, HIV/HBV co-infection groups, and HIV/HCV co-infection groups. At the same time, 44 mono-infected age, gender, and ethnically matched pediatric HIV-infected subjects were selected as a control. None of the patients had liver failure during the follow-up period. Informed consent was obtained from the guardians of all subjects. The study was approved by the Ethics Committee of the First Affiliated Hospital, School of Medicine, Zhejiang University (reference number 2011-157).
CD4+ T-lymphocyte count
Whole blood was collected from participants in ethylene diamine tetraacetic acid tubes. Plasma was separated from cells by centrifugation and stored at −70°C for viral load detection. Blood cells were determined by three-color flow cytometry using FACScan (Becton Dickinson, Franklin Lakes, NJ, USA) with a commercial flow cytometry assay (Becton Dickinson), following the manufacturer's protocol.
Quantification of human immunodeficiency virus RNA
Human immunodeficiency virus-1 viral load in the plasma of the AIDS patients was quantitatively detected using a standardized reverse transcriptase-polymerase chain reaction assay (Cobas Amplicor HIV-1 Monitor Test, version 1.5, Ultra Sensitive Specimen Preparation, Roche Diagnostic Systems Inc., Branchburg, NJ, USA). Low detection limit in plasma was defined as 50 HIV-1 RNA copies/ml.
Anti-human immunodeficiency virus antibody
Anti-HCV antibody was detected using the third generation anti-HCV enzyme-linked immunosorbent (ELISA, Beijing Bioneovan Co., Ltd, Beijing, China) kits. Considering the different antiturbulent effect of the third generation anti-HCV ELISA kits, the samples were retested by two kinds of kits from different manufactures, and those both tested positive were considered anti-HCV positive.
Hepatitis B surface antigen
Hepatitis B surface antigen was detected using an ELISA technique (AXSYM Automatic Elisa Detector, Abbott Inc., USA) with third generation assay (HBsAg version 3, Murex Biotech Ltd., Dartford, UK) according to the manufacturer's manual of operation.
Liver enzyme and total bilirubin levels
The alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin levels in peripheral blood were detected using a continuous monitoring assay with fully automatic biochemical analyzer (Hitachi 7600-210, Japan) every 3 months. In this assay, the upper limit of normal was 40 IU/L and values above this were considered “elevated” and graded I to IV according to the AIDS Clinical Trial Group guidelines.[15]
Statistical analysis
Data were analyzed using the SPSS statistical package 18.0 (SPSS Inc., Chicago, IL). Normal distribution was evaluated by the Kolmogorov-Smirnov test and Shapiro-Wilk test. Normally distributed data were shown as mean ± standard deviation, and not normally distributed data were shown as median and quartile. The Student's t-test for independent samples or a one-way ANOVA were used for normally distributed values. The Mann-Whitney U-test was performed for values without normal distribution. A P < 0.05 was considered statistically significant.
RESULTS
Demographic characteristics of pediatric human immunodeficiency virus-infected subjects
Demographic data of all participants and clinical characteristics of pediatric HIV-infected subjects are shown in Table 1. Chronic hepatitis co-infection was present in 101 patients (69.7%), of whom 4 (4.0%) were infected by HCV, 19 (18.8%) were infected by HBV and 78 (77.2%) were HCV/HBV co-infected. The HAART regimens did not differ between patients with and without HBV/HCV. In particular, no statistically significant differences were found among those four groups in CD4+ T-lymphocyte counts and viral load before HAART (P > 0.05).
Table 1.
Demographic data of all participants and clinical characteristics of pediatric HIV-infected subjects
During 1-year of HAART, the median levels of viral load of the HIV mono-infection group, the HIV/HBV/HCV co-infection group, the HIV/HBV co-infection group and the HIV/HCV co-infection group decreased significantly (P < 0.001). The mean level of CD4+ T-lymphocyte counts for the four groups elevated significantly (P < 0.001) and the CDC classes of the patients were improved. 20 of 37 patients in CDC C were improved to CDC B, and 9 to CDC A. 31 of 65 patients in CDC B were improved to CDC A. However, there were no differences among the four groups in viral load and CD4+ T-lymphocyte counts (P > 0.05) [Table 1].
Serum liver enzyme levels before and during therapy
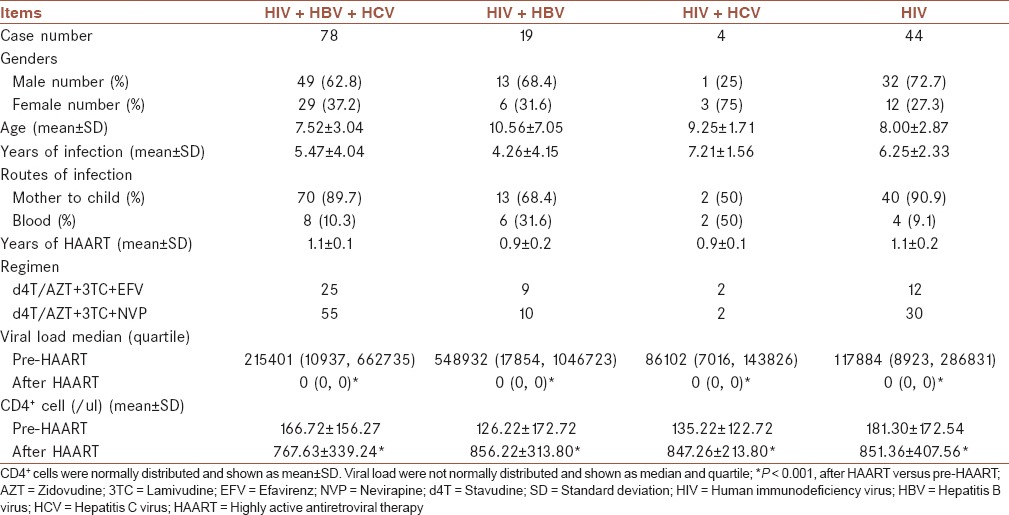
In order to evaluate the change of the transaminase caused by HAART, ALT, AST, and total bilirubin in each of the four groups were analyzed. Compared with pre-HAART, the mean level of ALT in each group had a significant elevation after HAART (P < 0.01). Co-infection groups had a higher increase in ALT compared with HIV mono-infected group after HAART. Moreover, a remarkably higher ALT value was found in HIV/HCV co-infected group than HIV/HBV/HCV co-infected group (P < 0.05). The mean levels of AST and total bilirubin were also increased and much higher than pre-HAART in all the four groups after HAART, especially in HIV/HBV/HCV co-infected and HIV mono-infected group (P < 0.01 and P < 0.05) [Figure 1].
Figure 1.
The comparison of (a) alanine aminotransferase; (b) aspartate aminotransferase; and (c) total bilirubin in human immunodeficiency virus (HIV)/hepatitis B virus (HBV)/hepatitis C virus (HCV) co-infected group, HIV/HBV co-infected group, HIV/HCV co-infected group and HIV mono-infected group before and after highly active antiretroviral therapy (HAART). *P < 0.01, **P < 0.05, after HAART versus pre-HAART
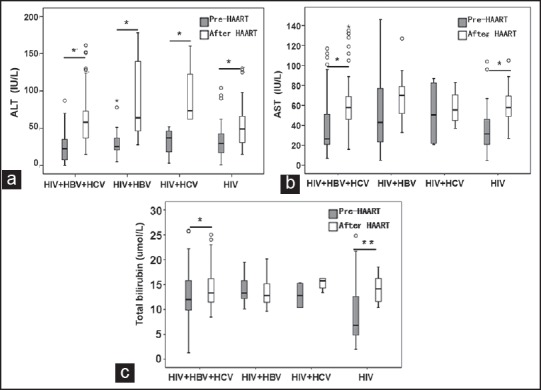
In order to evaluate whether the results were affected by basic liver enzyme elevation (LEE), the change values of ALT, AST, and total bilirubin from baseline were also analyzed. The mean change values from baseline are shown in Figure 2. Co-infected patients, compared to mono-infected patients, had higher mean ALT change values after HAART, but there was no remarkable difference between mono and co-infected patients (P > 0.05). The mean change values of AST and total bilirubin from baseline had no remarkable difference between mono and co-infected patients either (P > 0.05) [Figure 2].
Figure 2.
The comparison of the change values of (a) alanine aminotransferase changes; (b) aspartate aminotransferase changes; and (c) total bilirubin from baseline in human immunodeficiency virus (HIV)/hepatitis B virus (HBV)/hepatitis C virus (HCV) co-infected group, HIV/HBV co-infected group, HIV/HCV co-infected group and HIV mono-infected group before and after HAART. *P < 0.01, after HAART versus pre-HAART
Hepatotoxicity due to efavirenz and nevirapine among patients with/or without co-infection
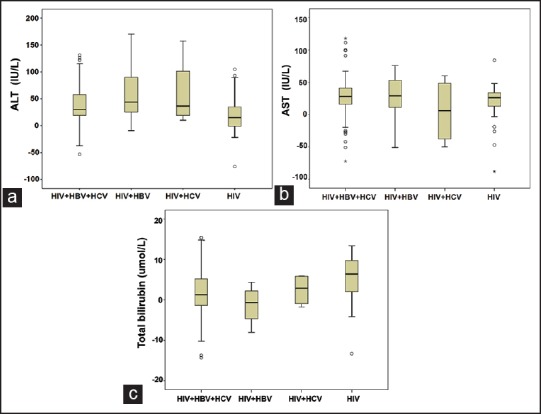
To explore the hepatotoxicity among various antiretroviral drugs, pediatric HIV-infected subjects, including co-infections and mono-infections, were divided into two groups, NVP-based HAART group and EFV-based HAART group, according to their respective therapeutic regimens. The mean levels of ALT and AST in NVP based HAART group increased significantly after HAART (P < 0.01). There was no difference the levels of ALT and AST in EFV-based HAART group before and after HAART. Total bilirubin had no remarkable change in both groups before and after HAART (P > 0.05) [Figure 3].
Figure 3.
The comparison of the mean levels of (a) alanine aminotransferase; (b) aspartate aminotransferase; and (c) total bilirubin in nevirapine-based highly active antiretroviral therapy (HAART) group and efavirenz-based HAART group before and after HAART. *P < 0.01, after HAART versus pre-HAART
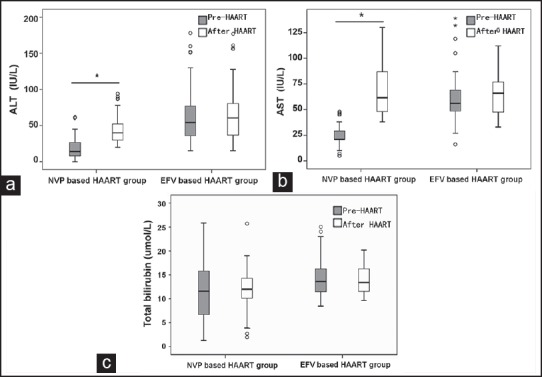
To further investigate the liver toxicity caused by these two antiretroviral drugs, change values of ALT, AST, and total bilirubin from baseline in the EFV based regimen group and the NVP-based regimen group were analyzed. As expected, mean change values of ALT and AST were significantly higher in the NVP based regimen group than in the EFV based regimen group (P < 0.01). However, there was no evidence for a significantly greater change in mean total bilirubin levels in the two groups (P > 0.05) [Figure 4].
Figure 4.
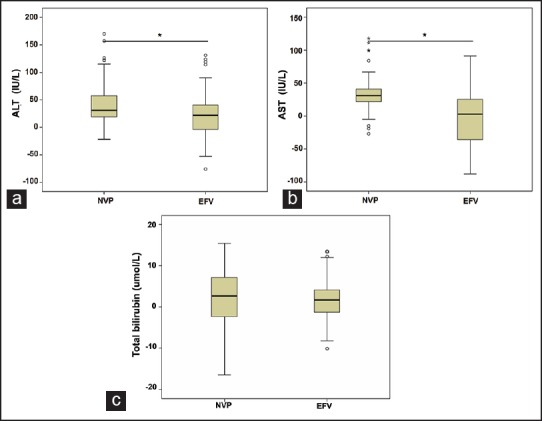
The comparison of the change values of (a) alanine aminotransferase changes; (b) aspartate aminotransferase changes; and (c) total bilirubin from baseline in nevirapine-based highly active antiretroviral therapy (HAART) group and efavirenz-based HAART group before and after HAART. *P < 0.01, after HAART versus pre-HAART
Finally, change values of ALT, AST, and total bilirubin from baseline in both groups were analyzed according to different co-infection. For the HIV/HBV/HCV co-infected patients, the mean change values of ALT and AST in NVP-based HAART group was significantly higher than that in EFV-based HAART group (P < 0.01). There were no remarkable differences in mean ALT, AST, and total bilirubin change values between the two groups for other patients (P > 0.05) [Figure 5].
Figure 5.
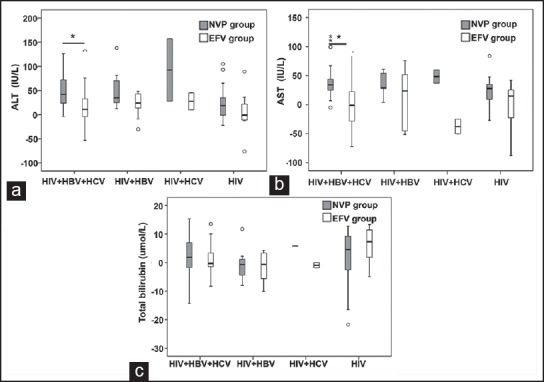
The comparison of the change values of (a) alanine aminotransferase changes; (b) aspartate aminotransferase changes; and (c) total bilirubin from baseline in human immunodeficiency virus (HIV)/hepatitis B virus (HBV)/hepatitis C virus (HCV) co-infected group, HIV/HBV co-infected group, HIV/HCV co-infected group and HIV mono-infected group before and after HAART. *P < 0.01, after highly active antiretroviral therapy (HAART) versus pre-HAART
DISCUSSION
In a place of the usual HIV-associated opportunistic infections, morbidity, and mortality due to the sequelae of HBV and/or HCV co-infection has taken on a leading role in HIV-infected individuals.[16] However, the effect of HBV/HCV co-infection on HAART is still controversial. Whether co-infected with HBV/HCV or not, the viral load of the majority (90.34%) of pediatric HIV-infected subjects in our study was decreased to the lower limit of detection level, while CD4+ T-cell counts increased significantly after receiving effective HAART, and there was no significant difference among co-infection individuals and single infection individuals, which corresponds with the report of Li et al.[16]
Studies previously have demonstrated LEE could be caused by HIV virus, co-infection with HBV/HCV, opportunistic infections, cancer, alcohol abuse, drug interaction, and drug-induced hepatitis and so on.[17,18] Both HBV and HCV are philo-hepatovirus, which would damage the liver when they were replicating, but combined with the hepatotoxic effects of HAART, hepatocyte injury was more severe than mono HIV infection. Cicconi et al.[19] pointed out that co-infection with HBV/HCV was a vital risk factor related with LEE after HAART. Pediatric HIV-infected subjects are different from adult patients in that they are in the growth stage, and the liver is more sensitive to HIV virus, anti-HIV drugs, and HBV/HCV co-infection.[20] LEE was found in our study in all of the four groups of pediatric HIV-infected subjects, and the rise of transaminase in co-infection individuals was higher than in single HIV infection individuals. At the same time, co-infected patients, compared to mono-infected patients, had higher mean ALT change values after HAART, but there was no remarkable difference between mono and co-infected patients
The anti-HIV drugs can also bring about hepatocellular damage.[21,22] Compared to EFV, NVP was more likely to result in hepatotoxicity, which frequently occurred in patients with higher CD4+ T-cell counts.[23] Hepatocytes are more sensitive to the hepatotoxicity of NVP when HIV-infected subjects are co-infected with HBV/HCV. It was shown in our study that the mean levels of ALT and AST in NVP-based HAART group increased significantly after HAART. Moreover, mean changes of ALT and AST in the NVP group were higher than in the EFV group, especially in the HIV/HBV/HCV co-infection group (P < 0.01), which corresponds with some other reports in adults.[19,21,22] Therefore, it is better for HIV-1 infected patients with LEE to choose EFV-based HAART. In spite of several anti-viral drugs having been reported to cause fatal acute hepatitis, they most often result in an asymptomatic elevation of transaminase levels after HAART.[24] Liver tolerability was good in our cohort, and the occurrence of relevant hepatotoxicity was mild in most subjects. According to the handbook of free antiretroviral treatment in China,[25] instead of changing drugs or drug withdrawal, they need only to administer some of the drugs orally, such as glycyrrhizinate capsules, to prevent liver from hepatotoxicity.
There are several limits in our study. The co-infection of HBV and HCV was only tested before HAART, and not followed over time since HCV infection may be cleared by the children during the 1-year of HAART. Because of the limited conditions, HCV RNA was not detected, and HCV infection was diagnosed based on the positive results of HCV antibody test. Another limit of our study is that we did not study the impact of other factors on LEE, such as opportunistic infections, cancer, alcohol abuse, and drug use mentioned above. These limits may bring bias on our results.
CONCLUSION
We showed that regardless of HIV/HBV/BCV co-infections, good immunoviralogical response is achieved by 1-year of HAART in pediatric HIV-1 infected patients. Although the mechanisms underlying this may be multifactorial, our findings most strongly support a significant role for HBV/HCV co-infection as the major contributing factor to liver-related complications. A rational selecting regimen is necessary to both reduce HBV/HCV-related liver disease and maintain antiviral effects. However, the time of HAART for the pediatric AIDS patients is only 1-year in our study, and the long-term effects of HAART on liver function need further follow-up observation.
AUTHOR'S CONTRIBUTION
LW contributed in the conception and design of the work, conducting the study, drafting and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. CJ contributed in the conception and design of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. SB contributed in the conception and design of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. HD contributed in conducting the study, the statistical analysis, approval of the final version of the manuscript, and agreed for all aspects of the work. HR contributed in conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. YL contributed in the statistical analysis, approval of the final version of the manuscript, and agreed for all aspects of the work. NW contributed in the conception and design of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
ACKNOWLEDGMENT
This work was supported by National Key Technologies R&D Program for the 12th Five-year Plan (2012ZX10001-004) and the Grant of Zhejiang Provincial Science & Techology Plan (20l3C37018).
Footnotes
Source of Support: Nil
Conflict of Interest: No conflict of interests.
REFERENCES
- 1.Nikolopoulos GK, Paraskevis D, Hatzitheodorou E, Moschidis Z, Sypsa V, Zavitsanos X, et al. Impact of hepatitis B virus infection on the progression of AIDS and mortality in HIV-infected individuals: A cohort study and meta-analysis. Clin Infect Dis. 2009;48:1763–71. doi: 10.1086/599110. [DOI] [PubMed] [Google Scholar]
- 2.Martín-Carbonero L, Poveda E. Hepatitis B virus and HIV infection. Semin Liver Dis. 2012;32:114–9. doi: 10.1055/s-0032-1316466. [DOI] [PubMed] [Google Scholar]
- 3.Thio CL. Hepatitis B and human immunodeficiency virus coinfection. Hepatology. 2009;49:S138–45. doi: 10.1002/hep.22883. [DOI] [PubMed] [Google Scholar]
- 4.Kourtis AP, Bulterys M, Hu DJ, Jamieson DJ. HIV-HBV coinfection — a global challenge. N Engl J Med. 2012;366:1749–52. doi: 10.1056/NEJMp1201796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambotin M, Barth H, Moog C, Habersetzer F, Baumert TF, Stoll-Keller F, et al. Challenges for HCV vaccine development in HIV-HCV coinfection. Expert Rev Vaccines. 2012;11:791–804. doi: 10.1586/erv.12.52. [DOI] [PubMed] [Google Scholar]
- 6.Deng LP, Gui XE, Zhang YX, Gao SC, Yang RR. Impact of human immunodeficiency virus infection on the course of hepatitis C virus infection: A meta-analysis. World J Gastroenterol. 2009;15:996–1003. doi: 10.3748/wjg.15.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewden C, Salmon D, Morlat P, Bévilacqua S, Jougla E, Bonnet F, et al. Causes of death among human immunodeficiency virus (HIV)-infected adults in the era of potent antiretroviral therapy: Emerging role of hepatitis and cancers, persistent role of AIDS. Int J Epidemiol. 2005;34:121–30. doi: 10.1093/ije/dyh307. [DOI] [PubMed] [Google Scholar]
- 8.Martín-Carbonero L, Teixeira T, Poveda E, Plaza Z, Vispo E, González-Lahoz J, et al. Clinical and virological outcomes in HIV-infected patients with chronic hepatitis B on long-term nucleos(t)ide analogues. AIDS. 2011;25(2):73–9. doi: 10.1097/QAD.0b013e328340fde2. [DOI] [PubMed] [Google Scholar]
- 9.Singal AK, Anand BS. Management of hepatitis C virus infection in HIV/HCV co-infected patients: Clinical review. World J Gastroenterol. 2009;15:3713–24. doi: 10.3748/wjg.15.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin CZ, Zhao Y, Zhang FJ, Yao HP, Wu LJ, Zhao HX, et al. Different plasma levels of interleukins and chemokines: Comparison between children and adults with AIDS in China. Chin Med J (Engl) 2009;122:530–5. [PubMed] [Google Scholar]
- 11.Zhou S, Zhao Y, He Y, Li H, Bulterys M, Sun X, et al. Hepatitis B and hepatitis C seroprevalence in children receiving antiretroviral therapy for human immunodeficiency virus-1 infection in China, 2005-2009. J Acquir Immune Defic Syndr. 2010;54:191–6. doi: 10.1097/QAI.0b013e3181c99226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shepard CW, Finelli L, Fiore AE, Bell BP. Epidemiology of hepatitis B and hepatitis B virus infection in United States children. Pediatr Infect Dis J. 2005;24:755–60. doi: 10.1097/01.inf.0000177279.72993.d5. [DOI] [PubMed] [Google Scholar]
- 13.Mast EE, Hwang LY, Seto DS, Nolte FS, Nainan OV, Wurtzel H, et al. Risk factors for perinatal transmission of hepatitis C virus (HCV) and the natural history of HCV infection acquired in infancy. J Infect Dis. 2005;192:1880–9. doi: 10.1086/497701. [DOI] [PubMed] [Google Scholar]
- 14.Kellerman SE, Hanson DL, McNaghten AD, Fleming PL. Prevalence of chronic hepatitis B and incidence of acute hepatitis B infection in human immunodeficiency virus-infected subjects. J Infect Dis. 2003;188:571–7. doi: 10.1086/377135. [DOI] [PubMed] [Google Scholar]
- 15.Li XF, Kan QC, He Y, Yu ZJ, Li ZQ, Liang HX. The influence of human immunodeficiency virus co-infection with hepatitis C virus and hepatitis B virus on the efficacy of high active anti-retroviral therapy. Zhonghua Nei Ke Za Zhi. 2010;49:951–4. [PubMed] [Google Scholar]
- 16.Jain MK, Seremba E, Bhore R, Dao D, Joshi R, Attar N, et al. Change in fibrosis score as a predictor of mortality among HIV-infected patients with viral hepatitis. AIDS Patient Care STDS. 2012;26:73–80. doi: 10.1089/apc.2011.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterling RK, Chiu S, Snider K, Nixon D. The prevalence and risk factors for abnormal liver enzymes in HIV-positive patients without hepatitis B or C coinfections. Dig Dis Sci. 2008;53:1375–82. doi: 10.1007/s10620-007-9999-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tedaldi E, Peters L, Neuhaus J, Puoti M, Rockstroh J, Klein MB, et al. Opportunistic disease and mortality in patients coinfected with hepatitis B or C virus in the strategic management of antiretroviral therapy (SMART) study. Clin Infect Dis. 2008;47:1468–75. doi: 10.1086/593102. [DOI] [PubMed] [Google Scholar]
- 19.Cicconi P, Cozzi-Lepri A, Phillips A, Puoti M, Antonucci G, Manconi PE, et al. Is the increased risk of liver enzyme elevation in patients co-infected with HIV and hepatitis virus greater in those taking antiretroviral therapy? AIDS. 2007;21:599–606. doi: 10.1097/QAD.0b013e328013db9c. [DOI] [PubMed] [Google Scholar]
- 20.Ashir GM, Rabasa AI, Gofama MM, Bukbuk D, Abubakar H, Farouk GA. Study of hepatic functions and prevalence of hepatitis B surface antigenaemia in Nigerian children with human immunodeficiency virus infection. Niger J Med. 2009;18:260–2. doi: 10.4314/njm.v18i3.51171. [DOI] [PubMed] [Google Scholar]
- 21.van Leth F, Phanuphak P, Ruxrungtham K, Baraldi E, Miller S, Gazzard B, et al. Comparison of first-line antiretroviral therapy with regimens including nevirapine, efavirenz, or both drugs, plus stavudine and lamivudine: A randomised open-label trial, the 2NN Study. Lancet. 2004;363:1253–63. doi: 10.1016/S0140-6736(04)15997-7. [DOI] [PubMed] [Google Scholar]
- 22.Sulkowski MS. Drug-induced liver injury associated with antiretroviral therapy that includes HIV-1 protease inhibitors. Clin Infect Dis. 2004;38(Suppl 2):S90–7. doi: 10.1086/381444. [DOI] [PubMed] [Google Scholar]
- 23.Ocama P, Castelnuovo B, Kamya MR, Kirk GD, Reynolds SJ, Kiragga A, et al. Low frequency of liver enzyme elevation in HIV-infected patients attending a large urban treatment centre in Uganda. Int J STD AIDS. 2010;21:553–7. doi: 10.1258/ijsa.2010.010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puoti M, Torti C, Ripamonti D, Castelli F, Zaltron S, Zanini B, et al. Severe hepatotoxicity during combination antiretroviral treatment: Incidence, liver histology, and outcome. J Acquir Immune Defic Syndr. 2003;32:259–67. doi: 10.1097/00126334-200303010-00004. [DOI] [PubMed] [Google Scholar]
- 25.Zhang F, Wang A, Sun Y, Li T, Zhao H, Lu H, et al. Beijing: People Health Press; 2005. Handbook of Free Antiretroviral Treatment in China; p. 186. [Google Scholar]


