Abstract
Background:
We conducted our study on 1110 patients with breast masses in order to investigate different aspects of power Doppler sonography (PDS) for differentiating between benign and malignant breast lesions and their prognostication.
Materials and Methods:
This study was conducted on the women who were referred to the sonography units of University Hospitals for breast sonography and had a BIRADS-3 mass or higher in gray scale sonography. Then, PDS was performed for all the patients. Vascularization, number of vessels, resistance index (RI), pulsatility index (PI), and vascularization patterns were evaluated for all the lesions. We compared our radiologic findings concerning different histopathologic and hormonal aspects of the lesions.
Results:
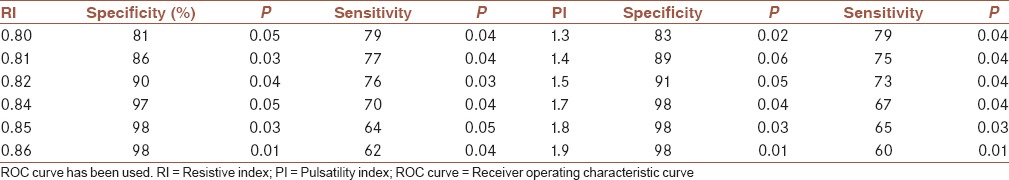
The differences between mean vascular density in malignant lesions concerning size of the tumor, histological grade, stage, and hormone receptor status were statistically significant. Although, there was an overlap between benign and malignant values. A resistive index (RI) value higher than 0.83 as a sign for malignancy had sensitivity equal to 75% and specificity equal to 97% (P = 0.04 and 0.03, respectively). A PI value higher than 1.6 has a sensitivity and specificity value of 70% and 98%, respectively, as a malignancy sign (P = 0.02 and 0.04, respectively).
Conclusion:
It seems that while malignant tumors have significantly higher number of vessels in comparison to benign one, since the number of vessels overlap between benign and malignant tumors, this aspect has little clinical usefulness in distinguishing or prognostication of breast masses. In contrast RI, PI, and vascularization pattern have an ability to differentiate and predict the prognosis of breast lesions.
Keywords: Breast cancer, Doppler sonography, vascular density, vascularization
INTRODUCTION
Breast cancer, the most common cancer in the women's population, is the second mortality cause of cancer in this group and the most important mortality factor among women aged 45-55 years.[1,2,3]
These statistics emphasize the necessity of screening and early diagnosis of breast cancer.[3,4,5,6] Nowadays, sonography is one of the main methods for diagnosing breast diseases and is included in lot of procedures to detect and evaluate breast lesions.[6,7,8,9] The criteria for differentiating between benign and malignant lesions from gray scale sonography are widely accepted.[10,11,12] Although tumor vascularization is becoming highly important in prognostic, diagnostic, and possibly therapeutic terms, there have always been controversies in different studies to define acceptable criteria for differentiating benign and malignant breast lesions by Doppler sonography (DS).[8,10,13,14,15,16,17] Some studies have even doubted its usefulness.[17,18,19]
We conducted a prospective study on 1100 patients with breast masses in order to investigate different aspects and the usefulness of power doppler sonography (PDS) for differentiating between benign and malignant breast lesions. We also compared our radiologic findings concerning different histopathologic and hormonal aspects of the lesions (stage, grade, tumor hormonal receptors). Furthermore, we investigated the possibility of defining criteria to predict the malignancy of breast masses by PDS.
MATERIALS AND METHODS
This is a cross-sectional study conducted between February 2010 and April 2013 with women referred to the sonography unit of two main hospitals of Isfahan (Isfahan Seyed Alshohada and Alzahra Hospital) for breast sonography and having a mass reported in their gray scale sonography. After conducting gray scale sonography, patients with masses classified as BIRADS-3 and higher entered the study until selection of the expected sample size (n = 1110). Those with breast masses categorized lower than BIRADS-3, did not cooperate in administration of core needle biopsy, excisional biopsy or follow-up sonography and the patients whose lesions fulfilled the criteria for simple breast cysts were excluded (189 patients).
Patients with BIRADS-4 and BIRADS-5 masses underwent core needle biopsy or excisional biopsy, and they were categorized into benign or malignant lesions according to their pathologic report. For BIRADS-3, patients were followed up by sonography 6 and 12 months after the first sonography.[6,7,20] If there was no change in the gray scale sonography images after 6 and 12 months, the mass was considered benign; and in case of any suspicious changes in the gray scale sonography image, the mass was considered a BIRADS-4 or 5. This would require a complementary core needle biopsy or excisional biopsy to have a differential diagnosis of a benign or malignant mass based on the results.
Since color power PDS is 3-5 times more sensitive than DS, we performed PDS instead of DS.[10,21] Between 10 and 15 days after the last gray scale sonography, PDS was performed by a fixed and experienced radiologist who was blinded to the pathologic and previous radiologic reports of the patients. All of the radiologic studies were done by a G40 SIMENSE ultrasound machine with a 5-10 MHz linear transducer. The entire lesion was carefully examined from side to side and from top to bottom. We were careful to apply as little pressure as possible on the transducer to prevent collapsing the small tumor vessels. We considered our exploration positive if at least one vessel with arterial flow pattern was detected inside the tumor. The entire examination was recorded with a video recorder.
The total numbers of tumor vessels were recorded, and vascularization density was calculated from

For lesions detected to have vascularization, resistive index (RI), and pulsatility index (PI) were obtained for up to 5 vessels according to accepted standards.[10] For each lesion, the mean RI and PI values were calculated and considered the RI and PI value.
The pattern of detectable tumor vessels was analyzed and classified on the basis of the course, morphology and size of the vessels. We considered non vascular tumors as type 1, those with single, approximately similar size, monomorphic vessels which the vessels gently curved along the margin of the masses as benign type or type 2, and those with irregular or tortuous courses in which vessels penetrated the tumor and varying in size and shape as malignant type or type 3.[19,22]
Regular histologic analyses of breast tumors were assessed by 2 fixed pathologists. For all cancers, the histologic types were classified according to World Health Organization criteria.[24,29] Tumor grading was performed according to modified previously published criteria.[23,24] Tumor stage (Tumor size, nodal status, and metastasis) was also evaluated after surgical removal of the masses. The tumor hormonal receptor status (estrogen receptor [ER], progesterone receptor [PR], and HER-2neu expression) was determined immunohistochemically.
This was a descriptive analytical study. The Chi-square test was conducted to compare qualitative sonographic values; and the independent t-test and the ANOVA tests were adopted to compare quantitative sonographic values between two groups. The receiver operating characteristic curve was used to reach a cutoff point for each quantitative criterion in the diagnosis of mass type (benign or malignant).
RESULTS
We studied 1110 breast masses (343 malignant and 767 benign). The patients age ranged between 33 and 79 (mean age of 50 years, standard deviation 18 years). All of our patients were female.
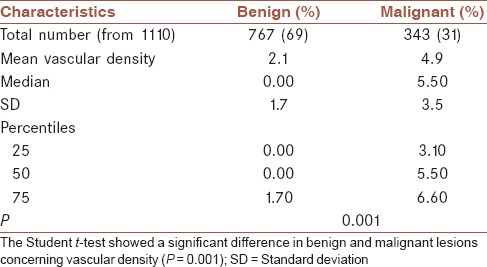
The difference between mean vascular density in benign and malignant lesions was statistically significant (P = 0.001) [Table 1].
Table 1.
Vascular density in malignant and benign tumors
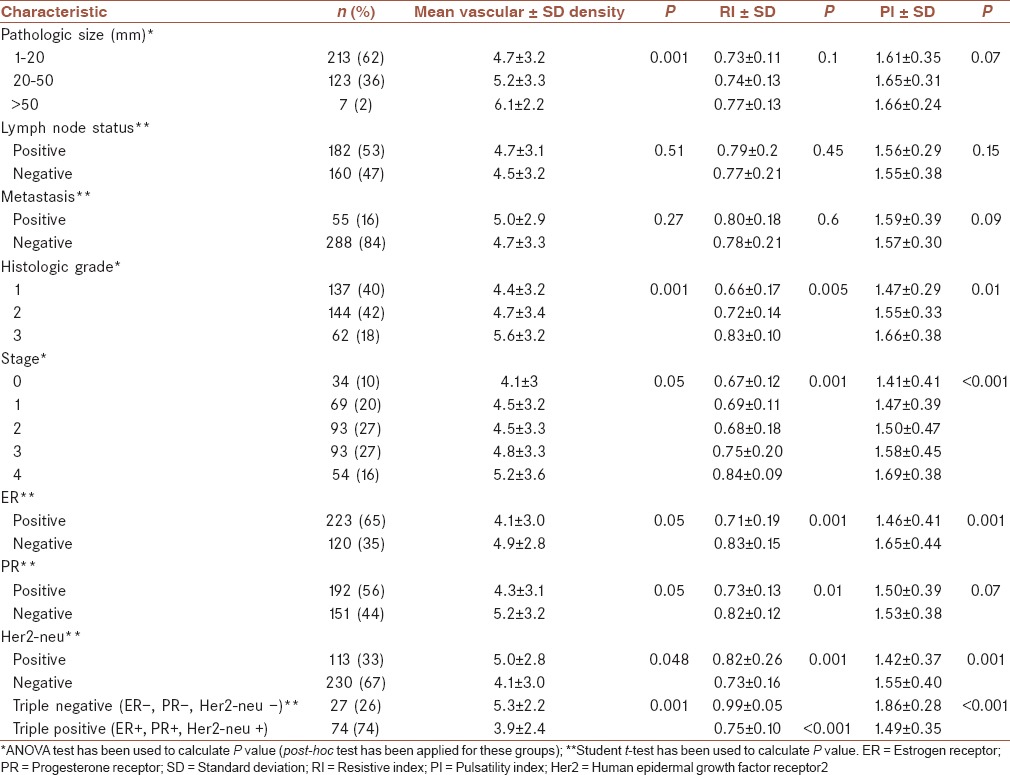
The differences between mean vascular density in malignant lesions concerning size of the tumor, histological grade, stage, and hormone receptor status were statistically significant [Table 2]. On the other hand, differences between mean vascular density in malignant lesions concerning lymph node invasion and metastasis were not statistically significant [Table 2].
Table 2.
Association of mean vascular density, RI and PI with histopathologic and clinical characteristics in malignant tumors
The results of Tukey post-hoc test for groups which have shown statistically significant difference according to ANOVA have been demonstrated in Table 3.
Table 3.
Results of Tukey post-hoc test for groups which have shown statistically significant difference according to ANOVA

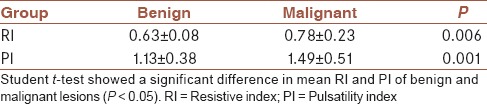
In masses where vessels were detected and for which it had been possible to obtain Doppler spectral waveforms, RI and PI were both significantly higher in malignant tumors [Table 4].
Table 4.
Mean RI and PI of benign and malignant tumors
It was revealed that an RI value higher than 0.83 as a sign for malignancy had sensitivity equal to 75% and specificity equal to 97%. Furthermore, a PI value higher than 1.6 has a sensitivity and specificity value of 70% and 98%, respectively, as a malignancy sign [Table 5]. We have chosen these cut of points due to their highest specificity and moderate sensitivity in comparison to their nearby values. Furthermore, the PI value of 0.9 has the sensitivity of 89%, however, the specificity was equal to 51%. The RI value of 0.64 has a sensitivity of 88%, however, the specificity was equal to 59%. Sensitivity and specificity for other cut-off points are shown in Table 6.
Table 5.
Diagnostic value of various findings on Doppler Sonography as a sign of malignancy
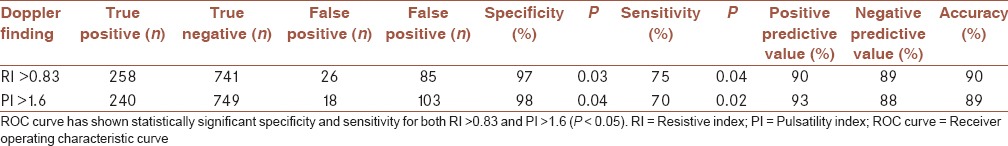
Table 6.
Specificity and sensitivity of other values of RI and PI
Considering different patterns of vascularization, 81% of malignant lesions revealed type 3 pattern while only 5% of benign lesions possessed this pattern [Table 7].
Table 7.
Patterns of vascularization in benign and malignant lesions
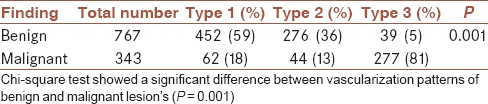
Sensitivity for type 3 was 81% while its specificity was 95%, positive predictive value for type 3 pattern was 89%, and negative predictive value 90%.
DISCUSSION
Since the role of angiogenesis was revealed in the growth and expansion of malignant lesions, lot of efforts have been made to differentiate malignant masses from benign ones, predicting the prognosis of the lesions and even treating them using this characteristic.[10,17,18,19,23,25,26] DS is a fast, cheap, and noninvasive method to evaluate vascularization.[10] DS can be especially useful for breast masses because of their superficial location;[12,18,19,23] hence lot of studies have been conducted to evaluate the efficiency of this method in discrimination of benign and malignant lesions and predicting the prognosis.[17,26,27,28] Until now, lot of parameters have been evaluated like number of vessels, blood flow, RI, PI, vascularization pattern, etc.[8,24] However, there have been always considerable controversies in the results.[10,17,18,19,26,28] This can be due to different methods of collecting patients, assessing the lesions and the small number of patients in the majority of these studies. Although some studies have suggested that other methods such as magnetic resonance imaging or contrast enhanced DS will increase the sensitivity and specificity of detection,[19] but these methods are more time-consuming, expensive, and relatively invasive.[10] Therefore, it seems valuable to find clinically useful parameters for differentiating or prognosticating breast cancer by DS.
To our knowledge, this study is by far the largest study which has been conducted on this topic and also the first one in the Middle East. Since breast cancer has different characteristics in different regions of the world, it is important to compare findings from different regions with each other in order to reach a better understanding of the subject.[29]
To evaluate the potential of PDS in differentiating between malignant and benign breast lesions, we studied different parameters. First, we studied the mean vascular density (number of vessels per cm2 of lesion). According to our results, mean vascular density was significantly higher in malignant versus benign breast masses. Since other studies have suggested that the tumors’ size may be associated with a higher detection of vessels in malignant lesions,[10,15,16,30] logistic regression was performed to control the effect of the tumor size. It was revealed that higher detection rates of vessels on breast cancers are correlated with the malignant nature of the tumor itself. However, the values of malignant and benign lesions overlap significantly [Figures 1–3]. Hence, like many other studies, it was confirmed in our study that the number of vessels has little clinical usefulness in distinguishing between benign and malignant tumors.[17,18,19,23]
Figure 1.
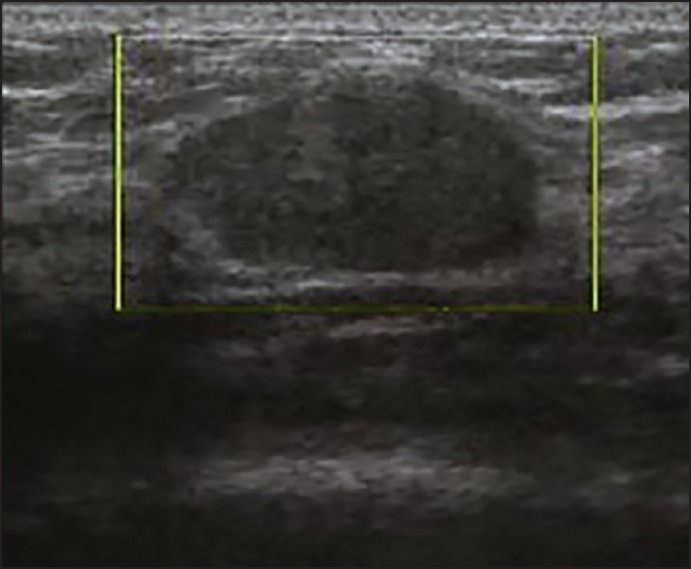
View of a benign lesion in color Doppler sonography without vascularization
Figure 3.
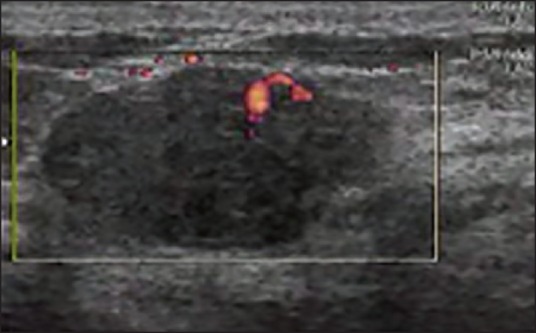
View of a malignant lesion in color Doppler sonography with penetration of vessels
Figure 2.
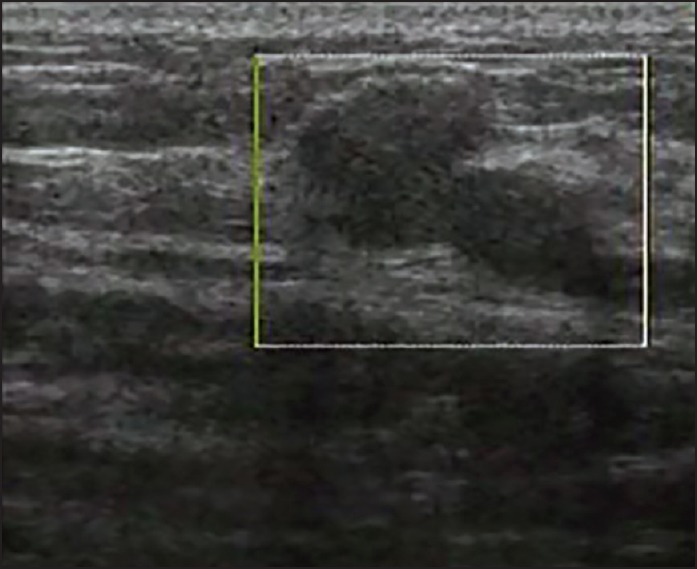
View of a malignant lesion in color Doppler sonography without vascularization
Resistive index and PI were other parameters that were evaluated. In those lesions in which we were able to obtain RI and PI, both were significantly higher in malignant tumors in comparison to benign ones. The reason may be the abnormal structure of vessels in malignant tumors, especially as this was suggested in other studies.[10,31] However, this needs further investigation. Although RI and PI values for benign and malignant tumors overlap, making their interpretation difficult, we were successful in defining a cut-off point for PI and RI to differentiate between benign and malignant lesions. As a sign of malignancy, the detection of RI >0.83 presented a specificity of 97% and sensitivity of 75%, respectively. Furthermore, a PI value >1.6 presented a specificity of 98%, positive and sensitivity of 70%. Other studies reported different results for PI and RI as a sign of malignancy.[10,32] This may be due to differences between the population of the studies or a different methodology.[10,32] For instance, Del Cura et al. reported the highest PI or RI, which they had calculated for each lesion while we reported the mean.[10] Again it seems further investigation is necessary to reach acceptable cut of points for PI and RI.
We also studied vascularization patterns of malignant and benign lesions. Although there are different factors which can be analyzed separately concerning the pattern of vessels, it seems the combination of size, course, and shape is more practical and may lead to more accurate results.[19] Our results showed type 3 (malignant type) has a sensitivity of 81%, specificity of 95%, positive predictive value of 89%, and negative predictive value of 90% as a sign of malignancy. It seems this parameter can be useful in differentiating between benign and malignant lesions. Our findings are consistent with other studies.[19] However, it is necessary to describe these patterns further in other studies in order to make them more precise and applicable.
There have been controversies between different studies about the usefulness of DS in predicting the prognosis and response to the treatment of breast cancer.[10,30,33] Some authors have found a correlation between DS findings and different prognostic factors like Lymph node metastasis, size of the tumor, pathologic grade, hormone receptor status etc.,[23,34] while others have not found any relationships.[10,30] But lot of these studies have been conducted on very small population, and their evaluation may be not accurate. To evaluate these features of PDS, we studied mean vascular density, RI and PI. We found a positive correlation between mean vascular density and tumor size, pathologic grade, stage, ER status, PR status, HER 2 neu status, and combination of PR, ER, and HER 2 neu status, while for lymph node and metastasis status, we did not find any correlation.
But once more, because of the overlap of the values, there seems to be little clinical usefulness. Furthermore, a positive relationship was revealed between RI and PI with pathologic grade, stage, ER status, PR status, HER 2 neu status, and combination of PR, ER, and HER 2 neu status. But again, there was the overlap problem between values, except for one prognostic factor. Triple-negative breast cancers (ER−, PR−, and HER2 neu-) are considered tumors with one of the poorest prognoses since they have commonly high pathologic grade, high stages, and poor response to systematic treatments. In this study, we find out that a RI value equal or higher than 0.92 and PI value equal or higher than 2.21 can be a sign of triple negative tumors with a sensitivity of 98% and 99% and specificity of 99% and 99%, respectively. By our knowledge, this is the first research in which a positive correlation between triple-negative tumors and PDS findings has been confirmed. Thus, it needs to be confirmed by other studies. But the fact that we were able to find a cut-off point with high sensitivity and specificity shows that PDS have a high potential in prognostication of breast cancers as some other authors have mentioned it.[23,34,35]
To conclude, it seems that while malignant tumors have significantly higher number of vessels in comparison to benign one, since the number of vessels overlap between benign and malignant tumors, this aspect has little clinical usefulness in distinguishing or prognostication of breast masses. In contrast, RI, PI, and vascularization pattern have a good ability to differentiate and predict the prognosis of breast lesions. Hence, further studies should be conducted to evaluate these parameters.
AUTHOR'S CONTRIBUTION
MS contributed to the conception of the work, performed ultrasound studies, reported mammograms, reviewed the final manuscript. RS contributed to the conception of the work, contributed to data collecting and data analyzing, wrote and revised the final manuscript, contributed to review of the references. FKh contributed to the data collecting, writing the final manuscript, contributed to review of the references. ER contributed to the conception of the work, contributed to data collecting, contributed to review of the references. MM contributed to the conception of the work, contributed to data collecting.
ACKNOWLEDGMENTS
This project is funded by a grant from Isfahan University of Medical Sciences (Project number: 188067).
Footnotes
Source of Support: This study is supported by a grant from Isfahan University of medical sciences
Conflict of Interest: None declared.
REFERENCES
- 1.Edwards BK, Brown ML, Wingo PA, Howe HL, Ward E, Ries LA, et al. Annual Report to the Nation on the Status of Cancer, 1975-2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005;97:1407–27. doi: 10.1093/jnci/dji289. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin 2010;60:277-300. Erratum in: CA Cancer J Clin. 2011;61:133–4. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Miller AB, Baines CJ, To T, Wall C. Canadian national breast screening study: 1. Breast cancer detection and death rates among women aged 40-49 years. Can Med Assoc J. 1993;148:718. [PMC free article] [PubMed] [Google Scholar]
- 4.Miller AB, To T, Baines CJ, Wall C. Canadian National Breast Screening Study-2: 13-year results of a randomized trial in women aged 50-59 years. J Natl Cancer Inst. 2000;92:1490–9. doi: 10.1093/jnci/92.18.1490. [DOI] [PubMed] [Google Scholar]
- 5.Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784–92. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 6.Bassett LW, Jackson VP, Fu KL. 2nd ed. USA: Elsevier Inc; 1997. Bassett: Diagnosis of Diseases of the Breast; pp. 185–355. [Google Scholar]
- 7.Heywang-Koebrunner SH, Schreer I, David Dershaw D. 2nd ed. UK: Thieme Medical Publishers, Incorporated; 2001. Diagnostic Breast Imaging; pp. 11–263. [Google Scholar]
- 8.Gokalp G, Topal U, Kizilkaya E. Power Doppler sonography: Anything to add to BI-RADS US in solid breast masses? Eur J Radiol. 2009;70:77–85. doi: 10.1016/j.ejrad.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Dixon JM, Walsh J, Paterson D, Chetty U. Colour Doppler ultrasonography studies of benign and malignant breast lesions. Br J Surg. 1992;79:259–60. doi: 10.1002/bjs.1800790325. [DOI] [PubMed] [Google Scholar]
- 10.Del Cura JL, Elizagaray E, Zabala R, Legórburu A, Grande D. The use of unenhanced Doppler sonography in the evaluation of solid breast lesions. AJR Am J Roentgenol. 2005;184:1788–94. doi: 10.2214/ajr.184.6.01841788. [DOI] [PubMed] [Google Scholar]
- 11.Cosgrove DO, Bamber JC, Davey JB, McKinna JA, Sinnett HD. Color Doppler signals from breast tumors. Work in progress. Radiology. 1990;176:175–80. doi: 10.1148/radiology.176.1.2191364. [DOI] [PubMed] [Google Scholar]
- 12.McNicholas MM, Mercer PM, Miller JC, McDermott EW, O’Higgins NJ, MacErlean DP. Color Doppler sonography in the evaluation of palpable breast masses. AJR Am J Roentgenol. 1993;161:765–71. doi: 10.2214/ajr.161.4.8372754. [DOI] [PubMed] [Google Scholar]
- 13.Kwak JY, Kim EK, Kim MJ, Choi SH, Son E, Oh KK. Power Doppler sonography: Evaluation of solid breast lesions and correlation with lymph node metastasis. Clin Imaging. 2008;32:167–71. doi: 10.1016/j.clinimag.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Ozdemir A, Ozdemir H, Maral I, Konus O, Yücel S, Isik S. Differential diagnosis of solid breast lesions: Contribution of Doppler studies to mammography and gray scale imaging. J Ultrasound Med. 2001;20:1091–101. doi: 10.7863/jum.2001.20.10.1091. [DOI] [PubMed] [Google Scholar]
- 15.Yang WT, Metreweli C, Lam PK, Chang J. Benign and malignant breast masses and axillary nodes: Evaluation with echo-enhanced color power Doppler US. Radiology. 2001;220:795–802. doi: 10.1148/radiol.2203001545. [DOI] [PubMed] [Google Scholar]
- 16.Cha JH, Moon WK, Cho N, Kim SM, Park SH, Han BK, et al. Characterization of benign and malignant solid breast masses: Comparison of conventional US and tissue harmonic imaging. Radiology. 2007;242:63–9. doi: 10.1148/radiol.2421050859. [DOI] [PubMed] [Google Scholar]
- 17.Kalmantis K, Dimitrakakis C, Koumpis Ch, Tsigginou A, Papantoniou N, Mesogitis S, et al. The Contribution of Three-Dimensional Power Doppler Imaging in the Preoperative Assessment of Breast Tumors: A Preliminary Report. Obstetrics and Gynecology International. 2009:8. doi: 10.1155/2009/530579. Article ID 530579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho N, Jang M, Lyou CY, Park JS, Choi HY, Moon WK. Distinguishing benign from malignant masses at breast US: Combined US elastography and color doppler US — influence on radiologist accuracy. Radiology. 2012;262:80–90. doi: 10.1148/radiol.11110886. [DOI] [PubMed] [Google Scholar]
- 19.Stuhrmann M, Aronius R, Schietzel M. Tumor vascularity of breast lesions: Potentials and limits of contrast-enhanced Doppler sonography. AJR Am J Roentgenol. 2000;175:1585–9. doi: 10.2214/ajr.175.6.1751585. [DOI] [PubMed] [Google Scholar]
- 20.Masroor I, Afzal S, Sakhawat S, Khan N, Beg MA, Kawal D. Negative predictive value of mammography and sonography in mastalgia with negative physical findings. J Pak Med Assoc. 2009;59:598–601. [PubMed] [Google Scholar]
- 21.Kook SH, Park HW, Lee YR, Lee YU, Pae WK, Park YL. Evaluation of solid breast lesions with power Doppler sonography. J Clin Ultrasound. 1999;27:231–7. doi: 10.1002/(sici)1097-0096(199906)27:5<231::aid-jcu2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 22.Kedar RP, Cosgrove D, McCready VR, Bamber JC, Carter ER. Microbubble contrast agent for color Doppler US: Effect on breast masses. Work in progress. Radiology. 1996;198:679–86. doi: 10.1148/radiology.198.3.8628854. [DOI] [PubMed] [Google Scholar]
- 23.Yang WT, Tse GM, Lam PK, Metreweli C, Chang J. Correlation between color power Doppler sonographic measurement of breast tumor vasculature and immunohistochemical analysis of microvessel density for the quantitation of angiogenesis. J Ultrasound Med. 2002;21:1227–35. doi: 10.7863/jum.2002.21.11.1227. [DOI] [PubMed] [Google Scholar]
- 24.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer, I: The value of histological grades in breast cancer: Experience from a large study with long term follow-up. Histopathology. 1991;19:403–10. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 25.Kedar RP, Cosgrove DO, Bamber JC, Bell DS. Automated quantification of color Doppler signals: A preliminary study in breast tumors. Radiology. 1995;197:39–43. doi: 10.1148/radiology.197.1.7568851. [DOI] [PubMed] [Google Scholar]
- 26.Chatterjee S, Ray AK, Karim R, Biswas A. Image processing of ultrasound color Doppler to characterize malignant breast lesion. Adv Mater Res. 2012;403-408:830–4. [Google Scholar]
- 27.Athanasiou A, Tardivon A, Ollivier L, Thibault F, El Khoury C, Neuenschwander S. How to optimize breast ultrasound. Eur J Radiol. 2009;69:6–13. doi: 10.1016/j.ejrad.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 28.Svensson WE, Pandian AJ, Hashimoto H. The use of breast ultrasound color Doppler vascular pattern morphology improves diagnostic sensitivity with minimal change in specificity. Ultraschall Med. 2010;31:466–74. doi: 10.1055/s-0028-1109478. [DOI] [PubMed] [Google Scholar]
- 29.Leong SP, Shen ZZ, Liu TJ, Agarwal G, Tajima T, Paik NS, et al. Is breast cancer the same disease in Asian and Western countries? World J Surg. 2010;34:2308–24. doi: 10.1007/s00268-010-0683-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehta TS, Raza S. Power Doppler sonography of breast cancer: Does vascularity correlate with node status or lymphatic vascular invasion? AJR Am J Roentgenol. 1999;173:303–7. doi: 10.2214/ajr.173.2.10430124. [DOI] [PubMed] [Google Scholar]
- 31.Less JR, Skalak TC, Sevick EM, Jain RK. Microvascular architecture in a mammary carcinoma: Branching patterns and vessel dimensions. Cancer Res. 1991;51:265–73. [PubMed] [Google Scholar]
- 32.Peters-Engl C, Medl M, Leodolter S. The use of colour-coded and spectral Doppler ultrasound in the differentiation of benign and malignant breast lesions. Br J Cancer. 1995;71:137–9. doi: 10.1038/bjc.1995.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cosgrove DO, Kedar RP, Bamber JC, al-Murrani B, Davey JB, Fisher C, et al. Breast diseases: Color Doppler US in differential diagnosis. Radiology. 1993;189:99–104. doi: 10.1148/radiology.189.1.8372225. [DOI] [PubMed] [Google Scholar]
- 34.Holcombe C, Pugh N, Lyons K, Douglas-Jones A, Mansel RE, Horgan K. Blood flow in breast cancer and fibroadenoma estimated by colour Doppler ultrasonography. Br J Surg. 1995;82:787–8. doi: 10.1002/bjs.1800820622. [DOI] [PubMed] [Google Scholar]
- 35.Kalra N, Ojili V, Gulati M, Prasad GR, Vaiphei K, Suri S. Metastatic choriocarcinoma to the breast: Appearance on mammography and Doppler sonography. AJR Am J Roentgenol. 2005;184(3 Suppl):S53–5. doi: 10.2214/ajr.184.3_supplement.01840s53. [DOI] [PubMed] [Google Scholar]


