Abstract
Background:
Microsatellite instability (MSI) is a mutational signature that is the hallmark of Lynch syndrome, and MSI testing is a cost-effective method to screen the disease. Since there is no enough data about MSI status and associated clinicopathologic features of hereditary nonpolyposis colorectal cancer (HNPCC) in Iran, our study is a new trial to describe them in center of Iran (Isfahan).
Materials and Methods:
It is a descriptive retrospective study to screen HNPCC families using Amsterdam II criteria in Central Iran within 2000-2013. For MSI testing, we used a commercially available kit evaluating mononucleotide markers (BAT-25, BAT-26, MON0-27, NR-21 and NR-24). After a fluorescent multiplex polymerase chain reaction amplification of the markers, samples were sequenced to fragment analysis. Data analysis was performed using SPSS 16 software (SPSS Inc., Chicago, IL, USA).
Results:
Overall, 31 of 45 screened HNPCC families were eventually included to MSI testing. Totally, 9/31 patients (29.0%) showed MSI in their tumor tissues. BAT-26 was the most instable marker with instability in 7/24 MSI tumors (29.2%). The mean age at diagnosis in microsatellite stable (MSS), MSI-Low (MSI-L), and MSI-High (MSI-H) probands was respectively 44.7 (standard deviation [SD] = 11.83), 51.7 (SD = 16.17), and 36.0 (SD = 3.41) years. The most common tumor sites in MSS, MSI-L, and MSI-H probands were rectosigmoid (∼72.8%), rectum (66.7%) and right colon (50.0%), respectively. Of 186 cancer patients among 31 HNPCC families, 86 patients (46.2%) had colorectal cancer (CRC) and 100 patients (53.8%) had extracolonic cancers. The average of CRC affected members among MSS, MSI-L, and MSI-H groups of our HNPCC families was 2.2 (SD = 1.30), 3.3 (SD = 3.21), and 4.7 (SD = 2.42) patients per family, respectively. Stomach with 18.3% and 26.7% of all extracolonic cancers were most common involved organ in MSS and MSI-H families, respectively.
Conclusion:
Our different molecular results could be suggested to describe HNPCC families based on some new molecular mechanisms leading to MSS HNPCC phenotypes. Meanwhile, more evaluations within our population are recommended.
Keywords: Clinicopathologic, Iran, Lynch syndrome, microsatellite instability
INTRODUCTION
Both genetic and epigenetic alterations have the essential role to evolve tumorigenesis phenomena. Chromosomal instability, microsatellite instability (MSI) and CpG island methylator phenotype pathways are responsible for genetic instability in colorectal cancer (CRC).[1,2]
Microsatellite instability is genomic instability of short tandem repeats. These are stretches of repeating units of 1-5 base pairs with 25-60 bp in length and are commonly in the form of CA (bp) that are distributed throughout the genome.[3] Actually, MSI is a molecular fingerprinting[4] or a mutational signature that is the hallmark of CRC. It has evolved as a result of inactivation of the DNA mismatch repair (MMR) system.[5] MSI creates novel alleles detectable as a change in allele size between tumor and normal DNA. Expansion or contraction of these repeating sequences due to increase or decrease in the repeat units gives rise to what is referred to as MSI. Almost 15-24% of all CRCs show MSI[6] while in Lynch syndrome, including nearly 3% of all CRCs, MSI is presented in almost all of the cases[5] containing 20-25% of all MSI-CRCs. The rest CRCs (75-80%) are due to the acquired loss of DNA MMR activity caused by promoter hypermethylation of the hMLH1 gene.[7] MSI is identified by polymerase chain reaction (PCR) amplification of specific microsatellite repeats. The existence of instability is determined by comparing the length of nucleotide repeats in tumor cells and normal cells.[4]
One of the major clinical implications of finding MSI tumors is that it is a cost-effective method to screen for Lynch syndrome. Once MSI tumors are detected, they can then be tested for the germ-line mutation of the missing MMR protein. It has a great impact on survival outcomes for patients and their families through early introduction of genetic counseling and cancer screening practices.[8]
Colorectal cancer is the third most prevalent and the third leading cause of cancer-related deaths in Iran[9] and its incidence has been increased over the last three decades in the Country.[10] Since there is no enough data about MSI status and associated clinicopathologic features of hereditary nonpolyposis colorectal cancer (HNPCC) in Iran, our study is a new trial to describe them in center of Iran (Isfahan).
MATERIALS AND METHODS
Screening method
We used a descriptive retrospective study to identify CRC patients at risk Lynch syndrome in Central Iran. Our samples were screened from 1659 CRC patients registered in Poursina Hakim Research Center (PHRC), an referral gastroenterology clinic in Central part of Iran, Isfahan, during about 14 years, from 2000 to end of 2013. We called patients who met Amsterdam II criteria contain: Three or more relatives with an associated cancer (CRC, or cancer of the endometrium, small intestine, ureter or renal pelvis, hepatobiliary tract, brain, skin, breast, and etc); two or more successive generations affected; one or more relatives diagnosed before the age of 50 years; one should be a first-degree relative of the other two; familial adenomatous polyposis should be excluded in cases of CRC; tumors should be verified by pathologic examination.[11]
Microsatellite instability testing
For MSI analysis, we need both tumor and healthy tissue DNA. The National Cancer Institute recommended a panel included two mononucleotide markers (BAT25 and BAT26) and three dinucleotide markers (D2S123, D5S346 and D17S250) to analyze MSI.[12,13] According to some studies, mononucleotide markers have higher specificity and similar or better sensitivity than dinucleotide markers to identify an MSI-High (MSI-H) phenotype.[14,15]
We used a commercially kit from Promega Corporation (MSI Analysis System, Version 1.2, Madison, USA) that evaluates just mononucleotide markers (BAT-25, BAT-26, MON0-27, NR-21 and NR-24). Moreover, it contains two pentanucleotide markers (Penta C and Penta D) as specimen identification markers to detect specimen mix-ups. After a fluorescent multiplex PCR amplification of the markers, samples are sequenced to fragment analysis. In this process, allelic profiles of amplified microsatellite markers are compared from matching pairs of test samples, which may be MMR-deficient, and normal tissue samples. If new alleles are observed in the tumor sample without their presence in the corresponding normal sample, MSI is confirmed. Tumors are considered as MSI-H if at-least two markers of the quintet show instability and MSI-Low (MSI-L) if only one marker is unstable.
Data analysis
We analyzed the obtained data by SPSS 16 software package (SPSS Inc., Chicago, IL, USA).
Ethical approval
Ethical approval was received from the medical ethics committee of Shahrekord University of Medical Sciences (Research project Number: 91-01-70-1364). The research was carried out according to principles set out in the Declaration of Helsinki 1964 and all subsequent revisions, informed consent was obtained, and the privacy and confidentiality were observed throughout the study.
RESULTS
Altogether 1659 CRC patients had been registered in PHRC within a 14-year period (2000-2013). Of these 413 patients (24.9%) were ≤50 years at diagnosis. Among 219/413 successful calls, 45 HNPCC families were eventually selected using Amsterdam II criteria for molecular testing stage of which 14 probands were omitted due to lack of their tumor tissues or being unwilling for incorporation.
Of 31 studied HNPCC probands, 9 patients (∼29%) showed MSI in their tumor tissues (6 patients [19.4%] with MSI-H). The proportion of male to female in microsatellite stable (MSS) probands was 11/11 = 1, and in MSI probands it was 6/3 = 2. Among quasimononucleotide markers used in MSI testing, BAT-26 was the most instable marker with instability in 7/31 MSI tumors (22.6%), both BAT-25 and NR-24 markers showed instability in 6/31 (19.3%), and both MONO-27 and NR-21 markers were instable in 5/31 (16.1%) MSI-CRC tumors.
In 4/6 MSI-H patients all markers were instable (66.6%), and among two rest patients, one showed instability in four markers (except MONO-27) and the other showed instability in two of them (BAT-26 and NR-24) [Figures 1 and 2].
Figure 1.
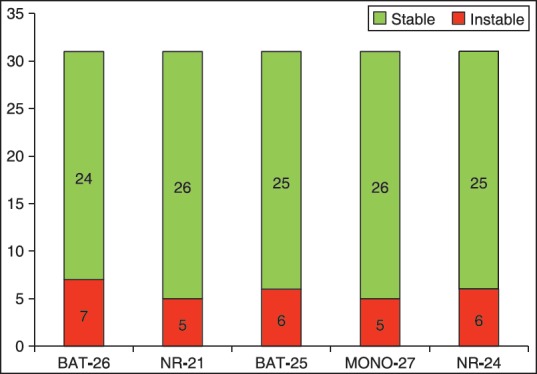
Frequency of five quasimononucleotide markers in 31 HNPCC tumors according to instability state. HNPCC = Hereditary non-polyposis colorectal cancer
Figure 2.
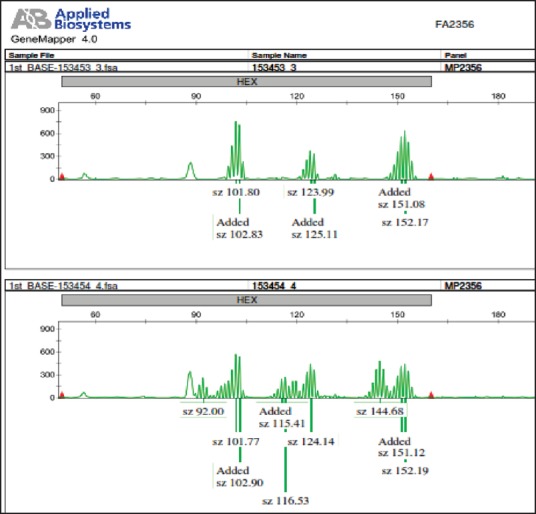
An example of microsatellite instability in one of our DNA electropherogram Traces: It is related to three quasimononucleotide markers including NR-21, BAT-25, and MONO-27, indicating normal stability state in upper sample (153453 3) as normal healthy tissue, and instability state in every three markers in lower sample (153454 4) as tumor tissue
The mean age at diagnosis in MSS, MSI-L, and MSI-H probands was respectively 44.7, 51.7, and 36.0 years (P = 0.123).
The most common tumor sites in CRC probands with MSS, MSI-L, and MSI-H phenotype were rectosigmoid (~72.8%), rectum (∼66.7%), and right colon (50.0%), respectively. Although in MSS probands, just 18.2% of the CRC tumors were located in the right side of the splenic flexure, in MSI-H tumors it was about 70%.
Overall, there was history of 186 cancer patients within our 31 HNPCC families (103 men or 55.4%) of which 86 patients (46.2%) had been affected by CRC tumors and 100 patients (53.8%) by extracolonic cancers. The average of cancer patients among MSS, MSI-L, and MSI-H groups of the HNPCC families was 5.4, 7.7, and 6.0 patients per family, respectively (P = 0.12). Moreover, the average of CRC affected members among MSS, MSI-L, and MSI-H groups of our HNPCC families was 2.2, 3.3, and 4.7 patients per family, respectively (P = 0.014). Meanwhile, the average of affected members by extracolonic cancers in these three groups was 3.2, 4.3, and 1.3 patients per family (P = 0.045). Moreover, the proportion of CRC patients to all cancer patients was 40.3%, 41.7%, and 65.1% among MSS, MSI-L, and MSI-H groups of these families, respectively.
Among extracolonic cancers, stomach, lung, and breast with respectively: 18.3, 15.5, and 11.3% were most common involved organs in MSS group of the studied families. In males of this group, lung and stomach with 28.9 and 23.7%, and among their females, breast and brain with 24.2 and 15.2%, respectively, were the most prevalent involved organs by extracolonic cancers. Moreover in MSI-L families, breast and brain equally with 21.4%, and hepatobiliary tract with 14.3%, and in MSI-H families, stomach and hematopoietic system with 26.7 and 20.0%, respectively, were evaluated as the most common involved organs. Among the males of MSI-H families, stomach and hematopoietic system with 25.0 and 33.3% were the most prevalent involved organs by extracolonic cancers. While in females of this group, 84.2% of cancers were CRC, and then breast, uterus, and hepatobiliary tract equally with 33.3% of all extracolonic cancers were the most common involved organs [Table 1].
Table 1.
Percentage of different types of cancer among Amsterdam-II positive families according to MSI status and gender
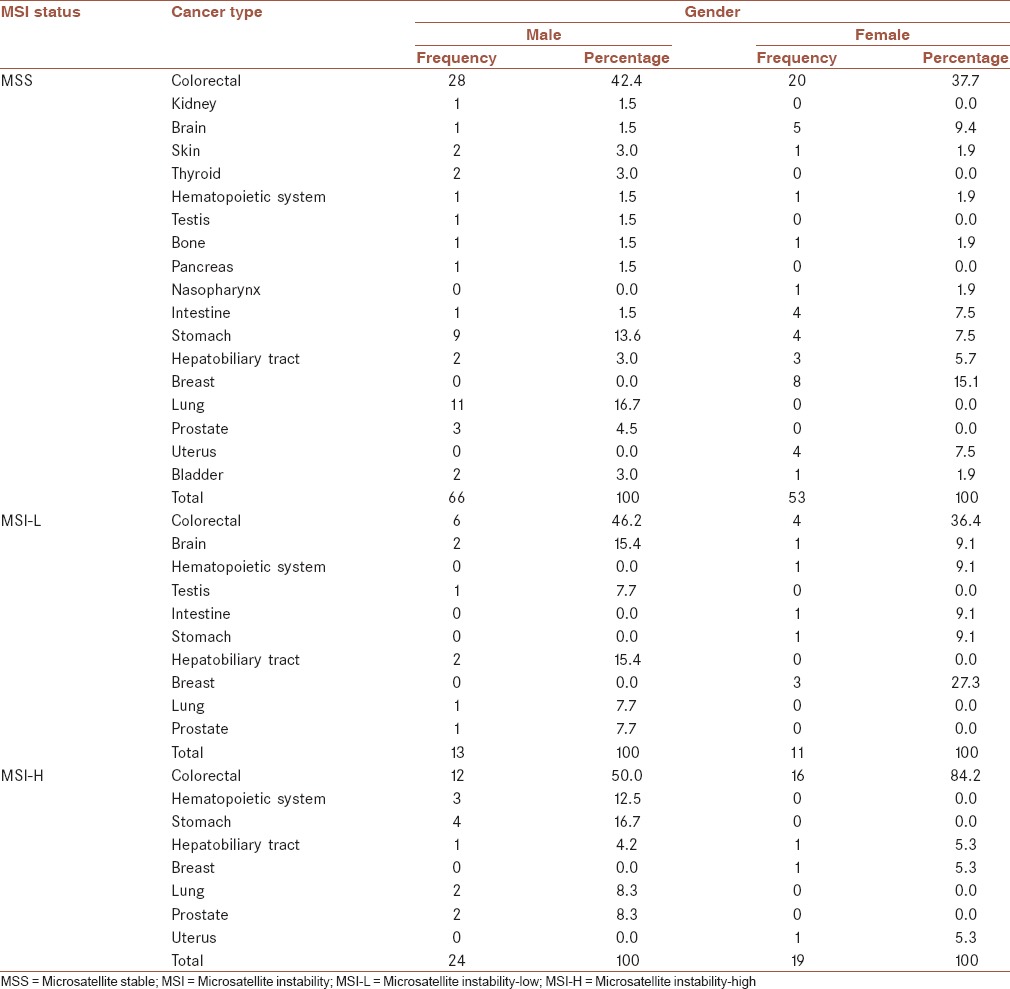
Overall, respectively, 8, 10, and 18 different organs were affected within MSI-H, MSI-L, and MSS families.
According to pathologic reports of the probands’ tumors, the most common pathologic phenotype in MSS and MSI-L CRC tumors was “well differentiated Adenocarcinoma” with 31.8 and 66.7%, respectively; while in MSI-H CRC tumors, “moderate differentiated Adenocarcinoma” with 50.0% was evaluated as the most common pathologic phenotype.
Moreover, 63.6% of MSS CRC tumors of the probands have been diagnosed in late pathologic TNM stages (stages III or IV) while 100% and 83.3% of MSI-L and MSI-H CRC tumors, respectively were diagnosed in these late stages.
Overall 12/31 (38.7%) of the probands had been deceased at our study time. The proportion of the deceased probands among MSS, MSI-L, and MSI-H groups was 31.8%, 100%, and 33.3%, respectively.
The survival period of the probands, the interval between diagnosis time and date of death or date of analyses were averagely calculated for MSS, MSI-L, and MSI-H group of the probands as: 6.1, 2.0, and 5.8 years, respectively (P = 0.341).
DISCUSSION
We evaluated in this study, tumor MSI state in index CRC patients among 31 identified HNPCC families within Central Iran for the 1st time. Screening process was performed according to genetic counseling of at-risk families for HNPCC given the Amsterdam II criteria. Somewhat high prevalence of familial CRC among our population and the limitation of our financial resources made us using these more specific criteria. Meanwhile, we had not an explicit visage of molecular aspects in our HNPCC families to establish a systematic, population-based screening program in our population. So, running this study could help us to elucidate partially this vague state.
Microsatellite instability prevalence
Sole 29% of screened CRC probands showed MSI in their tumor tissues of which 19.4% demonstrated MSI-H. Apparently, it is lower than similar studies on different populations around the world. For example, in a recent Spanish study, 80% of CRC patients with Amsterdam or Bethesda criteria had MSI positive tumors.[16] In addition in another study, 59.4% of Amsterdam I-positive CRC probands had MSI tumors.[17] Although some Iranian studies have shown high relative frequency of MSI among sporadic CRC tumors,[18,19] given the lack of enough data regard to MSI status among Iranian HNPCC patients, it seems more evaluation is necessary. Meanwhile, more molecular analyses are advisable to explore possible, unknown DNA loci among MSS patients.
As above, the frequency of instability among 5 quasimononucleotide markers of pentaplex Promega panel in our MSI-CRC tumors was between 19.2% (MONO-27 and NR-21) and 29.2% (BAT-26). In other words, all of these five markers were determinant to diagnose MSI status of our HNPCC probands. Nevertheless, different studies have presented variable molecular phenotypes for these markers. For example, in a recent Iranian study on 80 sporadic CRC patients, the most instable markers were NR-21, NR-24 with 45%. In this study, NR-27 with zero and BAT-26 with 6.7% instability were the most stable markers.[20] Other similar studies among different ethnic populations around the world have shown various results.[21,22,23] Apparently, instability feature of mononucleotide markers differs among different populations due to an ethnic variation in frequency of different mononucleotide markers. Moreover, may be it differs in sporadic CRCs compared to HNPCC CRCs, an issue about which more evaluations are necessary.
Clinicopathologic features
Microsatellite instability-H group of our probands had been affected averagely 7 years earlier than MSS group. Moreover, the proportion of CRC tumors localizing in the right colon among MSI-H probands to MSS group was near 4-fold (70% vs. 18%), while all of MSI-L tumors localized in distal colon. This is like to other similar studies among different populations in which there are findings for early onset and proximal localization of majority of MSI-H tumors.[17,24,25,26]
The average of CRC patients among the MSI-H families was significantly >2 other groups (4.7 patients vs. 3.3 and 2.2 in MSI-L and MSS families, respectively). Meanwhile, the average of extracolonic cancer patients among the MSS families was about 2.5-fold of MSI-H families (3.2 vs. 1.3). Similar previous studies have presented more relative frequency of CRC patients among Lynch syndrome-affected families compared to those affected by familial colorectal cancers.[17,27,28,29]
The most prevalent extracolonic cancers among males of MSS families were in lung and stomach, and among their females in breast and brain, respectively. Although gastric and breast cancers have been the most common cancers among Iranian population in recent decade,[30] apparently some unknown inheritable molecular changes are accused for familial aggregation of less prevalent cancers like brain in these families. Meanwhile, similar environmental conditions in interaction with some particular genotypes may lead to familial aggregation of some less inheritable cancers including lung and hematopoietic system.[31,32] The most prevalent extracolonic cancers among males and females of MSI-H families were in stomach and breast, respectively, compatible with current epidemiologic pattern of cancers among Iranian general population.[30] It again could determine significance of the environmental factors in tumorgenesis among these families in interaction with some molecular phenotypes.
As we mentioned, of 18 different organs involved in cancer patients among the families, just 8 organs were affected within MSI-H families. Whereas, 18 and 10 different involved organs were seen among MSS and MSI-L families, respectively. Apparently, familial aggregation of distinct cancers involving multiple organs among MSS HNPCC families is more extensive than MSI group. It could correspond to different underlying molecular pathology of MSS probands compared to MSI group of them, suggesting a new cancer-causing gene or genes responsible for tumorigenesis in multiple organs than only colorectal tract or a few associated organs. It is, apparently, different from some similar studies on other populations, discussing a heterogeneous feature of the disease among various communities.[17] It could also discuss new definitions to describe HNPCC families based on some new molecular phenotypes; an issue around which more evaluations should be administered. Moreover, multiple organ involvement among MSS HNPCC families or “familial CRC type X” families according to some authors[27,29,33] suggests that the current cancer screening guidelines for HNPCC like annual colonoscopy or transvaginal ultrasound is somewhat questionable.
“Well differentiated Adenocarcinoma” was the most prevalent pathologic feature of the MSS CRC tumors; while among MSI-H CRC tumors, the most common was “moderate differentiated Adenocarcinoma”. According to different studies, MSS HNPCC patients have had less often poorly differentiated Adenocarcinoma in comparison to MSI HNPCC patients.[17,27,29]
We found more MSI probands in late pathologic TNM stages than MSS group while there was no statistical difference between them regard to their survival period. It could be corresponded to better survival among MSI group of the probands than MSS group of them; the fact that other similar studies have pointed to it.[6,25,34] Meanwhile, more evaluations within our population are recommended.
AUTHOR'S CONTRIBUTION
MZ is the first author. MHE is corresponding author. MHCh contributed as the main guide master. RS contributed as the main consultant master, and MK contributed as a technical consultant.
ACKNOWLEDGMENT
This article is concluded of a PhD thesis (Project No: 91-01-70-1364) in Shahrekord University of Medical Sciences. We appreciate the helpful cooperation of all health workers in Poursina Hakim Research Institute, genetic department of Isfahan Medicine School and Cellular and Molecular Research Center of Shahrekord University of Medical Sciences to technical consultation.
Footnotes
Source of Support: Nil
Conflict of Interest: No conflict of interests.
REFERENCES
- 1.Armaghany T, Wilson JD, Chu Q, Mills G. Genetic alterations in colorectal cancer. Gastrointest Cancer Res. 2012;5:19–27. [PMC free article] [PubMed] [Google Scholar]
- 2.Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–60. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sameer AS. Colorectal cancer: Molecular mutations and polymorphisms. Front Oncol. 2013;3:114. doi: 10.3389/fonc.2013.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vilar E, Gruber SB. Microsatellite instability in colorectal cancer-the stable evidence. Nat Rev Clin Oncol. 2010;7:153–62. doi: 10.1038/nrclinonc.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boland CR, Koi M, Chang DK, Carethers JM. The biochemical basis of microsatellite instability and abnormal immunohistochemistry and clinical behavior in Lynch syndrome: From bench to bedside. Fam Cancer. 2008;7:41–52. doi: 10.1007/s10689-007-9145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desselle F, Verset G, Polus M, Louis E, Van Daele D. Lynch syndrome and microsatellite instability: A review. Rev Med Liege. 2012;67:638–43. [PubMed] [Google Scholar]
- 7.Cunningham JM, Christensen ER, Tester DJ, Kim CY, Roche PC, Burgart LJ, et al. Hypermethylation of the hMLH1 promoter in colon cancer with microsatellite instability. Cancer Res. 1998;58:3455–60. [PubMed] [Google Scholar]
- 8.Wu C, Bekaii-Saab T. CpG island methylation, microsatellite instability, and BRAF mutations and their clinical application in the treatment of colon cancer. Chemother Res Pract 2012. 2012 doi: 10.1155/2012/359041. 359041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mousavi SM, Gouya MM, Ramazani R, Davanlou M, Hajsadeghi N, Seddighi Z. Cancer incidence and mortality in Iran. Ann Oncol. 2009;20:556–63. doi: 10.1093/annonc/mdn642. [DOI] [PubMed] [Google Scholar]
- 10.Haghighi MM, Javadi GR, Parivar K, Milanizadeh S, Zali N, Fatemi SR, et al. Frequent MSI mononucleotide markers for diagnosis of hereditary nonpolyposis colorectal cancer. Asian Pac J Cancer Prev. 2010;11:1033–5. [PubMed] [Google Scholar]
- 11.Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999;116:1453–6. doi: 10.1016/s0016-5085(99)70510-x. [DOI] [PubMed] [Google Scholar]
- 12.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: Development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–57. [PubMed] [Google Scholar]
- 13.Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Rüschoff J, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–8. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy KM, Zhang S, Geiger T, Hafez MJ, Bacher J, Berg KD, et al. Comparison of the microsatellite instability analysis system and the Bethesda panel for the determination of microsatellite instability in colorectal cancers. J Mol Diagn. 2006;8:305–11. doi: 10.2353/jmoldx.2006.050092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agostini M, Enzo MV, Morandi L, Bedin C, Pizzini S, Mason S, et al. A ten markers panel provides a more accurate and complete microsatellite instability analysis in mismatch repair-deficient colorectal tumors. Cancer Biomark. 2010;6:49–61. doi: 10.3233/CBM-2009-0118. [DOI] [PubMed] [Google Scholar]
- 16.Wielandt AM, Zárate AJ, Hurtado C, Orellana P, Alvarez K, Pinto E, et al. Lynch syndrome: Selection of families by microsatellite instability and immunohistochemistry. Rev Med Chil. 2012;140:1132–9. doi: 10.4067/S0034-98872012000900005. [DOI] [PubMed] [Google Scholar]
- 17.Valle L, Perea J, Carbonell P, Fernandez V, Dotor AM, Benitez J, et al. Clinicopathologic and pedigree differences in amsterdam I-positive hereditary nonpolyposis colorectal cancer families according to tumor microsatellite instability status. J Clin Oncol. 2007;25:781–6. doi: 10.1200/JCO.2006.06.9781. [DOI] [PubMed] [Google Scholar]
- 18.Mokarram P, Rismanchi M, Alizadeh Naeeni M, Mirab Samiee S, Paryan M, Alipour A, et al. Microsatellite instability typing in serum and tissue of patients with colorectal cancer: Comparing real time PCR with hybridization probe and high-performance liquid chromatography. Mol Biol Rep. 2014;41:2835–44. doi: 10.1007/s11033-014-3138-1. [DOI] [PubMed] [Google Scholar]
- 19.Brim H, Mokarram P, Naghibalhossaini F, Saberi-Firoozi M, Al-Mandhari M, Al-Mawaly K, et al. Impact of BRAF, MLH1 on the incidence of microsatellite instability high colorectal cancer in populations based study. Mol Cancer. 2008;7:68. doi: 10.1186/1476-4598-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esmailnia G, Montazer-Haghighi M, Javadi G, Parivar K, Zali M. Microsatellite instability markers status in colorectal cancer. Zahedan J Res Med Sci (ZJRMS) 2014;16:25–8. [Google Scholar]
- 21.Faghani M, Fakhrieh Asl S, Mansour-Ghanaei F, Aminian K, Tarang A, Seighalani R, et al. The Correlation between microsatellite instability and the features of sporadic colorectal cancer in the North part of Iran. Gastroenterol Res Pract 2012. 2012 doi: 10.1155/2012/756263. 756263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ichikawa A, Sugano K, Fujita S. DNA variants of BAT-25 in Japanese, a locus frequently used for analysis of microsatellite instability. Jpn J Clin Oncol. 2001;31:346–8. doi: 10.1093/jjco/hye066. [DOI] [PubMed] [Google Scholar]
- 23.Pyatt R, Chadwick RB, Johnson CK, Adebamowo C, de la Chapelle A, Prior TW. Polymorphic variation at the BAT-25 and BAT-26 loci in individuals of African origin. Implications for microsatellite instability testing. Am J Pathol. 1999;155:349–53. doi: 10.1016/S0002-9440(10)65131-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bapat B, Lindor NM, Baron J, Siegmund K, Li L, Zheng Y, et al. The association of tumor microsatellite instability phenotype with family history of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:967–75. doi: 10.1158/1055-9965.EPI-08-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kloor M, Staffa L, Ahadova A, von Knebel Doeberitz M. Clinical significance of microsatellite instability in colorectal cancer. Langenbecks Arch Surg. 2014;399:23–31. doi: 10.1007/s00423-013-1112-3. [DOI] [PubMed] [Google Scholar]
- 26.Oh JR, Kim DW, Lee HS, Lee HE, Lee SM, Jang JH, et al. Microsatellite instability testing in Korean patients with colorectal cancer. Fam Cancer. 2012;11:459–66. doi: 10.1007/s10689-012-9536-4. [DOI] [PubMed] [Google Scholar]
- 27.Lindor NM, Rabe K, Petersen GM, Haile R, Casey G, Baron J, et al. Lower cancer incidence in Amsterdam-I criteria families without mismatch repair deficiency: Familial colorectal cancer type X. JAMA. 2005;293:1979–85. doi: 10.1001/jama.293.16.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Renkonen E, Zhang Y, Lohi H, Salovaara R, Abdel-Rahman WM, Nilbert M, et al. Altered expression of MLH1, MSH2, and MSH6 in predisposition to hereditary nonpolyposis colorectal cancer. J Clin Oncol. 2003;21:3629–37. doi: 10.1200/JCO.2003.03.181. [DOI] [PubMed] [Google Scholar]
- 29.Llor X, Pons E, Xicola RM, Castells A, Alenda C, Piñol V, et al. Differential features of colorectal cancers fulfilling Amsterdam criteria without involvement of the mutator pathway. Clin Cancer Res. 2005;11:7304–10. doi: 10.1158/1078-0432.CCR-05-0965. [DOI] [PubMed] [Google Scholar]
- 30.Kolahdoozan S, Sadjadi A, Radmard AR, Khademi H. Five common cancers in Iran. Arch Iran Med. 2010;13:143–6. [PubMed] [Google Scholar]
- 31.Ridge CA, McErlean AM, Ginsberg MS. Epidemiology of lung cancer. Semin Intervent Radiol. 2013;30:93–8. doi: 10.1055/s-0033-1342949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoder LH. Lung cancer epidemiology. Med Surg Nurs. 2006;15:171–4. [PubMed] [Google Scholar]
- 33.Lynch HT, Lynch PM, Lanspa SJ, Snyder CL, Lynch JF, Boland CR. Review of the Lynch syndrome: History, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin Genet. 2009;76:1–18. doi: 10.1111/j.1399-0004.2009.01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang X, Li J. Era of universal testing of microsatellite instability in colorectal cancer. World J Gastrointest Oncol. 2013;5:12–9. doi: 10.4251/wjgo.v5.i2.12. [DOI] [PMC free article] [PubMed] [Google Scholar]


