Abstract
There is growing evidence of neurotrophin alterations in neuropsychiatric illnesses such as schizophrenia and further, neurotransmitters known to be adversely affected in schizophrenia (e.g. dopamine) can activate neurotrophin signalling pathways via G protein-coupled receptors. However, it is unclear how the primary therapeutic agents used in schizophrenia affect neurotrophin signalling. This is important given that all currently prescribed antipsychotic drugs serve as ligands at dopamine receptors. In this study, chronic effects of representative conventional and second-generation antipsychotics on nerve growth factor (NGF) receptor levels were assessed in the rat. The results indicated no significant drug effects on TrkA levels in any brain region analysed; however, three of the five antipsychotics analysed significantly decreased phospho-TrkA (i.e. the activated form of the receptor) in the hippocampus. These data indicate that chronic antipsychotic treatment may result in deleterious effects on neurotrophin signalling in an important brain region for information processing and cognition.
Keywords: cognition, nerve growth factor, neurotrophin, schizophrenia, TrkA
Introduction
Neurotrophins are well documented as serving important roles in mammalian neurodevelopment as well as in the support and maintenance of several neuronal phenotypes in the adult. Interestingly, there is growing evidence that neurotrophins including the prototype nerve growth factor (NGF) may be decreased in CNS disorders such as schizophrenia (for review see Buckley et al. 2007). In addition, neurotransmitters such as dopamine that are known to be adversely affected in schizophrenia (i.e. the neurotransmitter levels and/or their receptors are abnormally increased or decreased in a brain-region specific manner) can activate NGF signalling pathways via G protein-coupled receptors (Kobayashi et al. 1997). However, it is unclear how the primary therapeutic agents used in schizophrenia (i.e. the antipsychotics) affect NGF signalling, an important question given that all of the currently prescribed compounds serve as ligands (i.e. antagonists or partial agonists) at dopamine (D2) receptors (see review by Miyamoto et al. 2005).
NGF interacts with two different plasma membrane receptors: the low-affinity neurotrophin receptor p75NTR, and the high-affinity TrkA receptor (Huang & Reichardt, 2003). NGF binding to TrkA causes TrkA dimerization, followed by autophosphorylation of multiple tyrosine residues located within the intracellular domain of TrkA. TrkA phosphorylation generally activates pathways that promote neuron survival and plasticity (Patapoutian & Reichardt, 2001). In contrast, NGF signalling via p75NTR typically turns on pathways leading to cell death (reviewed in Barrett, 2000), although diverse and sometimes opposing effects of p75NTR activation (i.e. the facilitation of cell survival, cell death, or growth inhibition) have been reported depending on the adaptor proteins and co-receptors that are recruited (Barker, 2004; Lu et al. 2005).
The objective of the experiments described here was to evaluate the effects of chronic treatment with representative conventional and second-generation antipsychotics on NGF receptor levels in the rat brain, specifically TrkA and phospho-TrkA (i.e. the activated form of the TrkA receptor). Antipsychotic drugs were delivered in drinking water and dosed on a mg/kg per 24 h basis for 90 d followed by a 2-wk drug-free washout period (to assess potential protracted antipsychotic effects).
Materials and methods
Test subjects and drug dosing
Male albino Wistar rats (Harlan Sprague–Dawley Inc., USA) 2–3 months old were housed individually in a temperature-controlled room (25 °C), maintained on a 12-h light/dark cycle (lights on 18:00 hours), and allowed free access to food (Teklad Rodent Diet 8604 pellets, Harlan, USA). Water was allowed ad libitum for the first week, but then replaced with solutions that contained antipsychotics for the remainder of the study (see below). Beginning on week 2 after arrival, test subjects were treated with vehicle in their drinking water (very dilute acetic acid solution, see below) or one of the following antipsychotic drugs: haloperidol, chlorpromazine, risperidone, olanzapine, or ziprasidone dissolved in vehicle (see Table 1 for doses). In one cohort of rats (n=4–5 per group), antipsychotics were administered for a minimum of 2 wk (to reach steady state), plasma was collected, and then analysed by liquid chromatography coupled with ultraviolet detection (see below). In a separate cohort of rats (n=8–10 per group), animals were administered antipsychotics for 90 d, subsequently given a 14-d drug-free washout period and then sacrificed for ELISA analysis (see below).
Table 1.
Plasma antipsychotic levels at steady state
| Treatment group | Dose/24 h | Compound measured |
Plasma levels (ng/ml)±s.e.m. |
Estimated human therapeutic plasma range (ng/ml)a |
|---|---|---|---|---|
| Haloperidol | 2.0 mg/kg | Haloperidol | 14.7±2.2 | 5.0–20.0 |
| Risperidone | 2.5 mg/kg | Risperidone | 10.9±5.7 | |
| 9-OH-risperidone | 31.0±10.2 | |||
| Risperidone + 9-OH-risperidone |
41.9±15.6 | 20.0–60.0 | ||
| Chlorpromazine | 10.0 mg/kg | Chlorpromazine | 19.7±6.6 | 25.0–350.0 (or unknown) |
| Olanzapine | 10.0 mg/kg | Olanzapine | 28.1±6.6 | 9.0–80.0 |
| Ziprasidone | 12.0 mg/kg | Ziprasidone | 103.6±35.4 | 50.0–130.0 |
Estimated human therapeutic plasma ranges listed are based on: Baldessarini et al. (1988), Balant-Gorgia et al. (1999), Chetty et al. (1996), Perry et al. (2001), Rao et al. (2001), Gex-Fabry et al. (2003) and Vogel et al. (2009).
Oral antipsychotic dosing was based on the following factors: (1) previous rodent studies in our laboratory in which time-dependent behavioural and neurochemical effects were detected and in which the plasma drug levels achieved approximated those associated with antipsychotic effects in humans, (2) the doses selected were expected to achieve comparable and therapeutically relevant D2 receptor occupancy values in vivo (see our previous publications, Terry et al. 2005, 2006, 2007). The antipsychotics were dissolved in 0.1 m acetic acid and subsequently diluted (1:100) with deionized water for daily drug administration in drinking water. Drug dosing was based on the average daily (i.e. 24 h) fluid consumption and the weight of the animals. In the initial 2 wk of drug exposure, liquid consumption was measured every 2 d and averaged. Afterwards, it was measured once per week. The pH of the final solutions (both vehicle and drugs diluted in vehicle) ranged between 4.5 and 4.7. There was a modest decrease in fluid consumption in all of the test groups (when compared to normal water consumption); however, we have successfully used this approach (without health-related problems) in rats for periods up to 320 days (A. V. Terry et al., personal communication, see also our published 180-d treatment studies, e.g. Terry et al. 2007).
All procedures employed during this study were reviewed and approved by the Medical College of Georgia Institutional Animal Care and Use Committee and are consistent with AAALAC guidelines. Measures were taken to minimize pain or discomfort in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996. Significant efforts were also made to minimize the total number of animals used while maintaining statistically valid group numbers (i.e. based on power analyses for neurochemical effects in previous studies).
Plasma antipsychotic analyses
The analysis of antipsychotics from plasma was accomplished using a previously published method from Zhang et al. (2007) without modification. This method was highly sensitive and reliable with quantitation limits of 2.0 ng/ml for these analytes. Briefly, the plasma samples were prepared using liquid–liquid back extraction to isolate the antipsychotics from endogenous substances. The extract was injected into an Agilent Model 1100 high-performance liquid chromatography system (Palo Alto, USA) using a variable wavelength ultraviolet detector for detection.
ELISA
Euthanasia of rats, brain dissections, preparation of brain lysates, and ELISA methods were performed as described in detail previously (see Gearhart et al. 2006), except as noted below. TrkA and phospho-TrkA levels were measured by indirect and sandwich ELISA, respectively. Briefly, the basal forebrain (BF), prefrontal cortex (PFC), and hippocampal formation (Hipp) were dissected and then homogenized in RIPA buffer containing protease inhibitors and glycerol. The Micro BCA™ Protein Assay kit (Pierce Biotechnology, USA) was used to determine the total protein concentration in each brain lysate. The amount of protein analysed per well in each ELISA varied by antigen and brain region (see below). As an internal control for day-to-day variation in the ELISA methods, brain lysates from vehicle and antipsychotic-treated rats were assayed at the same time on the same ELISA plate. The quantity of protein analysed per well was: TrkA ELISA (BF 0.5 μg, PFC and Hipp 0.4 μg); phospho-TrkA ELISA (BF 30 μg, Hipp and PFC 70 μg). For the phospho-TrkA ELISA, we did not use the antiphosphotyrosine, recombinant 4G10 HRP-conjugate (Upstate catalogue #16-184) that was used in Gearhart et al. (2006) due to problems with high background (see Terry et al. 2007). Coating with anti-TrkA (capture antibody), blocking, and sample incubation were the same as our published ELISA protocol for measuring phospho-TrkA (Gearhart et al. 2006). However, the captured phospho-TrkA was detected using the following three steps (in order): (1) a 2-h incubation with mouse anti-phosphotyrosine (Upstate catalogue #05-521, diluted 1:1000 in blocking buffer, 50 μl/well); (2) a 1-h incubation with goat anti-mouse IgG-HRP (Jackson Immunoresearch catalogue #115-035-145, diluted 1:10 000 in blocking buffer, 50 μl/well); (3) tetramethylbenzidine incubation and spectrophotometry (optical absorbance read at 450 nm) were performed as described previously (Gearhart et al. 2006).
Statistical analyses
Statistical comparisons were made by analysis of variance (ANOVA) followed by the Holm–Sidak post-hoc test (p value <0.05 considered significant).
Results
Plasma antipsychotic levels
Plasma antipsychotic concentrations assessed at steady state during continuous oral administration of antipsychotic drugs are provided in Table 1. The combination (i.e. summed levels) of risperidone and the active 9-hydroxyrisperidone (9-OH-Ris) metabolite is also provided since this combination is considered the most clinically relevant measurement (Megens et al. 1994). As indicated in Table 1, plasma drug levels in the rat were within the proposed therapeutic range for humans for haloperidol and the risperidone+9-OH-Ris combination (see Baldessarini et al. 1988; Balant-Gorgia et al. 1999). Similarly, plasma levels were within the proposed therapeutic range for olanzapine (Gex-Fabry et al. 2003; Perry et al. 2001; Rao et al. 2001) and ziprasidone (Vogel et al. 2009). In the case of chlorpromazine, plasma levels were slightly below the therapeutic range published by Curry et al. (1970) and Chetty et al. (1996), although other researchers have indicated that the optimal therapeutic range for chlorpromazine is not well established (Baldessarini et al. 1988).
ELISA data
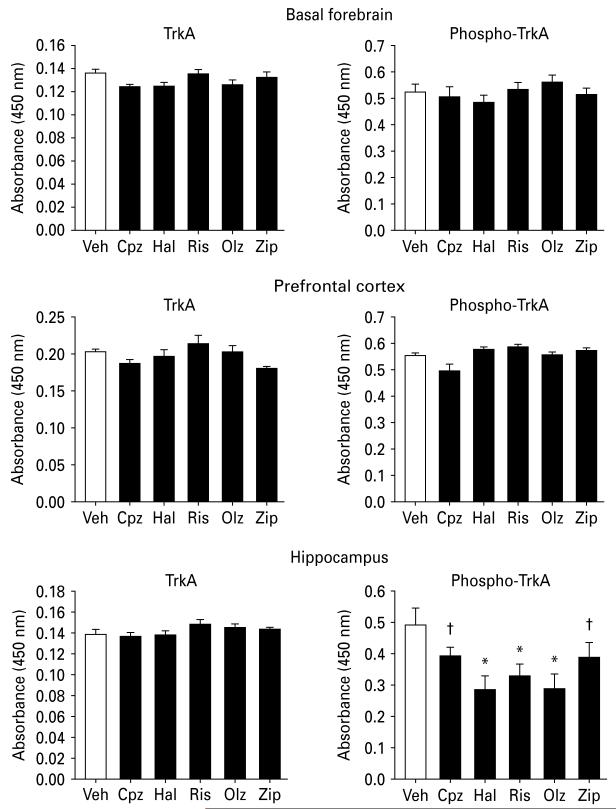
Figure 1 depicts the ELISA results from the three selected brain regions in rats previously treated chronically with antipsychotic drugs. None of the antipsychotic drugs evaluated had notable effects on TrkA levels in the BF, PFC or hippocampus (main effect for treatment: p>0.05 for all three brain regions). While only minor antipsychotic-related effects on phospho-TrkA levels were observed in the BF and PFC (main effect for treatment: p>0.05), all five antipsychotics were associated with significant (or nearly significant) decreases in phospho-TrkA levels in the hippocampus (main effect for treatment: F5,54=2.9, p=0.02).
Fig. 1.
ELISA results for NGF receptor protein levels (mean± s.e.m.) in the brain regions indicated from rats treated with vehicle or antipsychotic drugs for 90 d followed by a 14-d, drug-free washout. Left panels: TrkA; right panels: phospho-TrkA, measured by indirect and sandwich ELISA, respectively. For each ELISA, samples from one brain region for each treatment group were analysed in the same 96-well ELISA plate, and equal amounts of total protein were analysed across treatment groups. Data are expressed as relative levels (absorbance at 450 nm). * p<0.05, significantly different from vehicle control. †p<0.1 compared to vehicle control (n=8–10). Veh, Vehicle; Cpz, chlorpromazine; Hal, haloperidol; Ris, risperidone; Olz, olanzapine; Zip, ziprasidone.
Discussion
The results of this rodent study can be summarized as follows: (1) the plasma antipsychotic values generated during chronic oral administration in rats approximated those generally considered therapeutic in humans and were consistent with previous studies published in our laboratory; (2) the antipsychotics evaluated in this study had little effect on TrkA or phospho-TrkA levels in the BF or PFC. In contrast, while TrkA levels also appeared to be unaffected in the hippocampus by chronic antipsychotic treatment, all five compounds (representing both first- and second-generation antipsychotic classes) were associated with significant (or nearly significant) decreases in phospho-TrkA levels in the hippocampus.
The results described here supplement a relatively small number of published animal studies that were designed to evaluate the effects of antipsychotic drugs on neurotrophins. Most of the studies published to date have evaluated neurotrophins as opposed to their receptors. For example, in rodents, both acute (3 d) and subchronic (21 d) exposures to haloperidol have been associated with decreased BDNF protein levels in the PFC, hippocampus, and amygdala. Decreased BDNF protein levels (Angelucci et al. 2000) and decreased expression of BDNF mRNA levels (Lipska et al. 2001) was also observed after risperidone and clozapine administration. Angelucci et al. (2000) reported that subchronic (29 d) treatment with haloperidol or risperidone in rats increased NGF immunoreactivity in the hypothalamus, but decreased levels in the striatum and hippocampus. These authors (Angelucci et al. 2005) more recently reported that 29 d oral olanzapine treatment increased NGF in the hippocampus, occipital cortex and hypothalamus, but decreased BDNF in the hippocampus and frontal cortex. The results of our published studies indicate that the effects of conventional and second-generation antipsychotics on NGF protein levels are temporally dependent and can be very different depending on the length of time of administration. For example, NGF levels in the hippocampus were either unchanged or up-regulated in rats in response to several antipsychotics during short periods of treatment (7, 14, 45 d); however, NGF levels were significantly decreased by haloperidol, chlorpromazine, olanzapine, and risperidone when continuously administered orally for 90 d or 180 d (Pillai et al. 2006; Terry et al. 2006). We have also published evidence to suggest that chronic treatment with either first- or second-generation antipsychotics can be associated with alterations in several cholinergic markers as well as impairments in memory-related task performance (see Terry et al. 2006, 2007; and for review Terry & Mahadik, 2007).
The results of the present study indicate that chronic treatment with either first- or second-generation antipsychotics can lead to alterations in levels of the activated NGF receptor, phospho-TrkA, in a brain region (i.e. the hippocampus) well documented to be intimately involved in cognitive function. Antipsychotic-related decreases in the levels of NGF and its activated receptors could also explain anomalies in the expression of cholinergic proteins given the known role of NGF in the maintenance and survival of forebrain cholinergic neurons (for review, see Terry & Mahadik, 2007). The basis of the brain-region specific effect of the antipsychotics (i.e. decreases in phospho-TrkA in the hippocampus, but not in the BF or PFC) is unclear. Interestingly, NGF levels in rat brain have been reported (Korsching et al. 1985) to be considerably higher in the hippocampus (1.41 ng/g) when compared to the neocortex (0.53 ng/g) and BF structures (i.e. 0.37, 0.71, and 0.51 ng/gm for the basilar nucleus, diagonal band of Broca and septum, respectively). As noted above, we previously observed time-dependent decreases in NGF in the hippocampus associated with the same antipsychotics evaluated in this study, thus, it is interesting to speculate that chronic antipsychotic exposure leads to decreases in NGF over time which may in turn lead to decreased binding and thus, decreased activation of TrkA receptors. Given that most of the known biological functions of neurotrophins (including NGF) require binding to Trk receptors and their subsequent phosphorylation (Patapoutian & Reichardt, 2001), examining the chronic effects of antipsychotic drugs on the expression and functional activation of neurotrophin receptors is an important step towards understanding their long-term effects on neuronal function.
There are some limitations of this study that should be discussed. While considerable efforts were made in previous studies towards designing dosing protocols that result in therapeutically relevant plasma levels in rats (and we have confirmed these levels in the present study), we have not (to date) evaluated a range of doses to establish important dose–effect relationships on neurotrophins. In addition, our findings may not apply to all of the currently prescribed antipsychotics (e.g. clozapine) given their heterogeneous range of pharmacological actions. Moreover, all of our (neurotrophin-related) studies conducted to date have evaluated antipsychotic effects in young adult (otherwise healthy) rats and thus it is unclear how the drugs would affect neurotrophins in disease-related rat models.
In conclusion, the data described in this report combined with our previously published findings indicate that chronic exposure to either representative first- or second-generation antipsychotics may result in deleterious effects on neurotrophin signalling in a brain region known to be important for information processing and cognition. Such animal data should be considered in light of the fact that antipsychotic drugs have been commonly consumed by patients with schizophrenia and other psychiatric disorders for decades. These data combined with the results of several recent clinical studies such as CATIE (Clinical Antipsychotic Trials of Intervention Effectiveness), CUtLASS (Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study) and EUFEST (European First-Episode Schizophrenia Trial) effectiveness trials (for review, see Leucht, 2009), clearly indicate that (much like the older agents) newer antipsychotics have limitations that should be considered especially in the context of their long-term administration.
Acknowledgements
The authors acknowledge the technical support of Kristy Bouchard, Samantha Warner, and Leah Vandenhuerk. This work was supported in part by grants AG032140 and ES012241 from the National Institutes of Health.
Footnotes
Statement of Interest
None.
References
- Angelucci F, Brene S, Mathe AA. BDNF in schizophrenia, depression and corresponding animal models. Molecular Psychiatry. 2005;10:345–352. doi: 10.1038/sj.mp.4001637. [DOI] [PubMed] [Google Scholar]
- Angelucci F, Luigi A, Gruber SHM, Fiore M, et al. Chronic antipsychotic treatment selectively alters nerve growth factor and neuropeptide Y immunoreactivity and the distribution of choline acetyl transferase in rat brain regions. International Journal of Neuropsychopharmacology. 2000;3:13–25. doi: 10.1017/S1461145700001759. [DOI] [PubMed] [Google Scholar]
- Balant-Gorgia AE, Gex-Fabry M, Genet C, Balant LP. Therapeutic drug monitoring of risperidone using a new, rapid HPLC method: reappraisal of interindividual variability factors. Therapeutic Drug Monitoring. 1999;21:105–115. doi: 10.1097/00007691-199902000-00017. [DOI] [PubMed] [Google Scholar]
- Barker PA. p75NTR is positively promiscuous: novel partners and new insights. Neuron. 2004;42:529–533. doi: 10.1016/j.neuron.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Baldessarini RJ, Cohen BM, Teicher MH. Significance of neuroleptic dose and plasma level in the pharmacological treatment of psychoses. Archives of General Psychiatry. 1988;45:79–91. doi: 10.1001/archpsyc.1988.01800250095013. [DOI] [PubMed] [Google Scholar]
- Barrett GL. The p75 neurotrophin receptor and neuronal apoptosis. Progress in Neurobiology. 2000;61:205–229. doi: 10.1016/s0301-0082(99)00056-8. [DOI] [PubMed] [Google Scholar]
- Buckley PF, Mahadik S, Pillai A, Terry A., Jr. Neurotrophins and schizophrenia. Schizophrenia Research. 2007;94:1–11. doi: 10.1016/j.schres.2007.01.025. [DOI] [PubMed] [Google Scholar]
- Chetty M, Pillay VL, Moodley SV, Miller R. Response in chronic schizophrenia correlated with chlorpromazine, 7-OH-chlorpromazine and chlorpromazine sulfoxide levels. European Neuropsychopharmacology. 1996;6:85–91. doi: 10.1016/0924-977x(95)00047-s. [DOI] [PubMed] [Google Scholar]
- Curry SH, Marshall JH, Davis JM, Janowsky DS. Chlorpromazine plasma levels and effects. Archives of General Psychiatry. 1970;22:289–296. doi: 10.1001/archpsyc.1970.01740280001001. [DOI] [PubMed] [Google Scholar]
- Gearhart DA, Middlemore ML, Terry AV., Jr. ELISA methods to measure cholinergic markers and nerve growth factor receptors in cortex, hippocampus, prefrontal cortex, and basal forebrain from rat brain. Journal of Neuroscience Methods. 2006;150:159–173. doi: 10.1016/j.jneumeth.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Gex-Fabry M, Balant-Gorgia AE, Balant LP. Therapeutic drug monitoring of olanzapine: the combined effect of age, gender, smoking, and comedication. Therapeutic Drug Monitoring. 2003;25:46–53. doi: 10.1097/00007691-200302000-00007. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annual Reviews in Biochemistry. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Ogren SO, Ebendal T, Olson L. Dopamine receptor antagonists block nerve growth factor-induced hyperactivity. European Journal of Pharmacology. 1997;326:1–5. doi: 10.1016/s0014-2999(97)83487-5. [DOI] [PubMed] [Google Scholar]
- Korsching S, Auburger G, Heumann R, Scott J, Thoenen H. Levels of nerve growth factor and its mRNA in the central nervous system of the rat correlate with cholinergic innervation. EMBO Journal. 1985;6:1389–1393. doi: 10.1002/j.1460-2075.1985.tb03791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leucht S, Kissling W, Davis JM. Second-generation antipsychotics for schizophrenia: can we resolve the conflict? Psychological Medicine. 2009;39:1591–1602. doi: 10.1017/S0033291709005455. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Khaing ZZ, Weickert CS, Weinberger DR. BDNF mRNA expression in rat hippocampus and prefrontal cortex: effects of neonatal ventral hippocampal damage and antipsychotic drugs. European Journal of Neuroscience. 2001;14:135–144. doi: 10.1046/j.1460-9568.2001.01633.x. [DOI] [PubMed] [Google Scholar]
- Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nature Reviews Neuroscience. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- Megens AA, Awouters FH, Schotte A, Meert TF, et al. Survey on the pharmacodynamics of the new antipsychotic risperidone. Psychopharmacology (Berlin) 1994;114:9–23. doi: 10.1007/BF02245439. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Molecular Psychiatry. 2005;10:79–104. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- Patapoutian A, Reichardt LF. Trk receptors: mediators of neurotrophin action. Current Opinion in Neurobiology. 2001;11:272–280. doi: 10.1016/s0959-4388(00)00208-7. [DOI] [PubMed] [Google Scholar]
- Perry PJ, Lund BC, Sanger T, Beasley C. Olanzapine plasma concentrations and clinical response: acute phase results of the North American Olanzapine Trial. Journal of Clinical Psychopharmacology. 2001;21:14–20. doi: 10.1097/00004714-200102000-00004. [DOI] [PubMed] [Google Scholar]
- Pillai A, Terry AV, Jr., Mahadik SP. Differential effects of long-term treatment with typical and atypical antipsychotics on NGF and BDNF levels in rat striatum and hippocampus. Schizophrenia Research. 2006;82:95–106. doi: 10.1016/j.schres.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Rao ML, Hiemke C, Grasmäder K, Baumann P, et al. Olanzapine: pharmacology, pharmacokinetics and therapeutic drug monitoring. Fortschritte der Neurologie-Psychiatrie. 2001;69:510–517. doi: 10.1055/s-2001-18381. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr., Gearhart DA, Mahadik SP, Warsi S, et al. Chronic exposure to typical or atypical antipsychotics in rodents: temporal effects on central alpha 7 nicotinic acetylcholine receptors. Neuroscience. 2005;136:519–529. doi: 10.1016/j.neuroscience.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr., Gearhart DA, Warner SE, Zhang G, et al. Oral haloperidol or risperidone treatment in rats: temporal effects on nerve growth factor receptors, cholinergic neurons, and memory performance. Neuroscience. 2007;146:1316–1332. doi: 10.1016/j.neuroscience.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry AV, Jr., Mahadik SP. Time dependent cognitive deficits associated with first and second generation antipsychotics: cholinergic dysregulation as a potential mechanism. Journal of Pharmacology and Experimental Therapeutics. 2007;320:961–968. doi: 10.1124/jpet.106.106047. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr., Parikh V, Gearhart DA, Pillai A, et al. Time dependent effects of haloperidol and ziprasidone on nerve growth factor, cholinergic neurons, and spatial learning in rats. Journal of Pharmacology and Experimental Therapeutics. 2006;318:709–724. doi: 10.1124/jpet.105.099218. [DOI] [PubMed] [Google Scholar]
- Vogel F, Gansmüller R, Leiblein T, Dietmaier O, et al. The use of ziprasidone in clinical practice: analysis of pharmacokinetic and pharmacodynamic aspects from data of a drug monitoring survey. European Psychiatry. 2009;24:143–148. doi: 10.1016/j.eurpsy.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Zhang G, Terry AV, Jr., Bartlett MG. Simultaneous determination of five antipsychotic drugs in rat plasma by high performance liquid chromatography with ultraviolet detection. Journal of Chromatography B. 2007;856:20–28. doi: 10.1016/j.jchromb.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]