Abstract
The CLSI reduced the cefepime Enterobacteriaceae susceptibility breakpoint and introduced the susceptible-dose-dependent (S-DD) category. In this study, MICs were determined for a Gram-negative collection to assess the impact of this change. For Enterobacteriaceae, this resulted in <2% reduction in susceptibility, with 1% being S-DD. If applied to Pseudomonas aeruginosa, the % susceptibility (%S) dropped from 77% to 43%, with 34% being S-DD. The new breakpoints did little to the Enterobacteriaceae %S, but for P. aeruginosa, a profound reduction was seen in %S. The recognition of a S-DD response to cefepime should alert clinicians to the possible need for higher doses.
TEXT
Cefepime is a commonly utilized antimicrobial for empirical and directed therapy against infections involving both Enterobacteriaceae and Pseudomonas aeruginosa. Prior to 2014, the Clinical and Laboratory Standards Institute (CLSI) susceptibility breakpoint for cefepime was ≤8 μg/ml for both Enterobacteriaceae and P. aeruginosa (1). As a result of the changing phenotypic profiles to a variety of antimicrobials, including cefepime, used to treat Enterobacteriaceae, as well as changes in the doses commonly utilized for this agent, the Enterobacteriaceae breakpoint for cefepime was reduced to ≤2 μg/ml in January 2014. Along with this breakpoint revision, the CLSI introduced the Enterobacteriaceae susceptible-dose-dependent (S-DD) category of 4 to 8 μg/ml in order to encourage clinicians to use higher doses for organisms with higher MICs (2). The purpose of this study was to determine the impacts of these new susceptibility criteria on the reported cefepime profile for Enterobacteriaceae, and since these new criteria may subsequently be extended to include P. aeruginosa, we also assessed effect on the % susceptibility (%S) of cefepime for this pathogen.
(This work was presented in part at the 54th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 5 to 9 September 2014 [3].)
A total of 2,596 nonduplicate nonurine isolates of Enterobacteriaceae (Escherichia coli [n = 1,327]; Klebsiella pneumoniae [n = 1,173]; Citrobacter spp., including C. koseri and C. freundii [n = 11]; Proteus spp. [n = 8], including P. mirabilis and P. vulgaris; Morganella morganii [n = 4]; Enterobacter spp., including E. cloacae and E. gergoviae [n = 40]; Klebsiella oxytoca [n = 12]; and Serratia marcescens [n = 21]) and 1,278 P. aeruginosa isolates were obtained from 49 hospitals spread throughout the United States from the period of June 2013 to July 2014. The isolates were identified at each site, transferred to Trypticase soy agar slants, and refrigerated until shipping. Once received at the central processing laboratory (Center for Anti-Infective Research and Development, Hartford Hospital, Hartford, CT, USA), the isolates were transferred onto Trypticase soy agar plates containing 5% blood for MIC determination. Cefepime and the other antibiotics were purchased from Sigma (St. Louis, MO). MIC testing was undertaken using CLSI-defined broth microdilution methods using the quality control strains E. coli 25922 and P. aeruginosa 27853. The MIC trays were prepared using the Biomek 3000 (Beckman Instruments, Inc., Fullerton, CA), and colony counts were performed on each isolate to verify the correct inoculum.
The cefepime %S was assessed using the January 2014 Enterobacteriaceae breakpoints of ≤2 μg/ml for all organisms, as well as the ≤8-μg/ml cutoff value. We also evaluated the distribution of organisms within the S-DD (4 to 8 μg/ml) and resistant categories.
Comparisons of the susceptibility profiles using the old versus new CLSI breakpoints, including the S-DD category, were performed using Pearson's χ2 test for categorical variables. JMP (version 8; SAS Institute, Inc., Japan) was used for the statistical analysis. The level of significance for statistical testing was defined at a P value of <0.05 (2-sided), unless otherwise specified.
All isolates were evaluated phenotypically for the production of extended-spectrum β-lactamases (ESBLs) using methods described by the CLSI (1). Briefly, the ceftazidime and cefotaxime MICs were determined with and without clavulanate; those isolates that exhibited MIC shifts of ≥8-fold in the presence of clavulanate were classified as ESBL producers.
The isolates determined by CLSI microdilution methods to be nonsusceptible to ertapenem, imipenem, or meropenem were evaluated for carbapenemase production using the CarbaNP test (4).
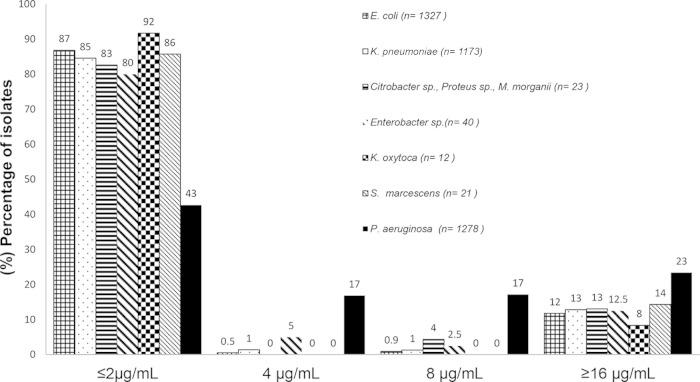
The MIC distribution for all organisms is displayed in Fig. 1. When these new cefepime breakpoints were applied to E. coli and K. pneumoniae, 1% and 2% reductions in %S, respectively, were recognized for these organisms. The majority of Enterobacteriaceae with MICs in the S-DD range were Enterobacter and Citrobacter species. A comparison of the cefepime %S, S-DD, and resistant (≥16 μg/ml) categories for all organisms is shown in Fig. 2. Overall, 9% (n = 14) of the phenotypically confirmed ESBL-positive E. coli isolates (n = 156) and 19% (n = 22) of the ESBL-positive K. pneumoniae isolates (n = 118) had a cefepime MIC of ≤2 μg/ml, whereas the majority of the ESBL-positive E. coli (86%) and K. pneumoniae (62%) isolates had a cefepime MIC of ≥16 μg/ml (Fig. 3). Sixty-five of the 72 CarbaNP-positive Enterobacteriaceae were K. pneumoniae, and only a single K. pneumoniae isolate displayed an MIC of ≤2 μg/ml; all other isolates were defined as resistant (data not shown).
FIG 1.
Cefepime MIC distribution for E. coli, K. pneumoniae, Citrobacter spp., Proteus spp., M. morganii, Enterobacter spp., K. oxytoca, S. marcescens, and P. aeruginosa. The MICs are on the x axis.
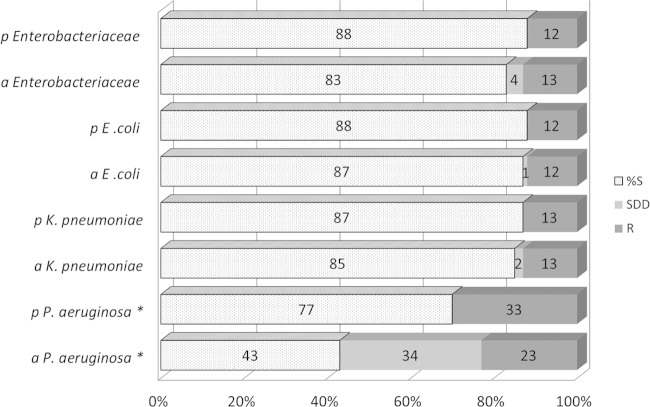
FIG 2.
Comparison of cefepime percent susceptibility (%S), susceptible-dose-dependent (S-DD), and resistant (R) categories for Enterobacteriaceae (n = 2,596) and P. aeruginosa (n = 1,278), applying the 2014 Enterobacteriaceae breakpoints. p, pre-2014; a, after 2014. An asterisk (*) indicates that cefepime susceptibility (%S) was reduced from 77% to 43% (P < 0.01) for only P. aeruginosa.
FIG 3.
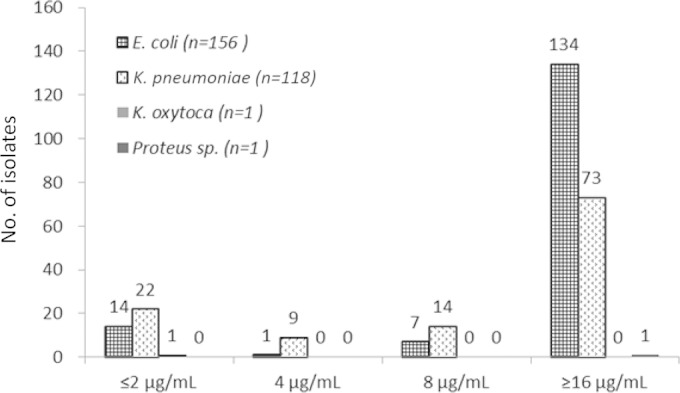
Cefepime MIC distribution for phenotypically confirmed ESBL-producing Enterobacteriaceae. The MICs are on the x axis.
The cefepime MIC distribution for P. aeruginosa is displayed in Fig. 1. When the new ≤2-μg/ml breakpoint was applied to P. aeruginosa, the overall %S was reduced from 77% to 43% (P < 0.01), as the S-DD limit of 4 μg/ml at 17% and the S-DD limit of 8 μg/ml at 17% comprised the remaining portion. The susceptibility of P. aeruginosa as defined by both sets of breakpoint values is shown in Fig. 2.
Cefepime is a commonly used broad-spectrum cephalosporin with potent activity against a wide variety of Gram-negative bacteria, including P. aeruginosa (5). The interpretive susceptibility criteria are determined by several different organizations, including the U.S. Food and Drug Administration (FDA), CLSI, and the European Committee on Antimicrobial Susceptibility Testing (EUCAST). Although these criteria are generally determined at the time of registration or shortly thereafter, periodic adjustments in these breakpoint values have been undertaken for a number of antimicrobials by these organizations in the postmarketing period. Recently, the FDA also changed the Enterobacteriaceae susceptibility interpretive criteria for cefepime to the same values as those of the CLSI (i.e., ≤2 μg/ml, 4 to 8 μg/ml, and ≥16) but with the 4- to 8-μg/ml category defined as intermediate, not S-DD. While the susceptibility range of 4 to 8 μg/ml is defined as intermediate for Enterobacteriaceae, the recommendation in the cefepime package insert is for the use of a 2-g dose every 8 h in a patient with normal renal function (6) when organisms with MICs of 4 to 8 μg/ml are identified. The criteria for P. aeruginosa remained unchanged at ≤8 (susceptible) and ≥16 μg/ml (resistant) in this most recently revised package insert.
Since the cefepime MIC distribution of these Enterobacteriaceae is overwhelmingly ≤2 or ≥16 μg/ml, the recently revised breakpoints did very little to change the overall %S for these organisms. However, since the MIC distribution of P. aeruginosa is heavily weighted to 4 and 8 μg/ml, the application of these revised breakpoints might have a profound effect on the reporting of the %S for this organism. Moreover, our group investigated cefepime exposures in patients infected with P. aeruginosa to identify the pharmacodynamic relationship that is predictive of a microbiological response (7). In that report, cefepime doses of 2 g every 8 h in patients with normal renal function were required to achieve adequate exposures and ultimately increase the probability of microbiological success against pathogens with cefepime MICs of 8 μg/ml.
The application of these CLSI susceptibility and S-DD breakpoints to P. aeruginosa appears to be important, because it will encourage clinicians to use higher cefepime doses for the substantial population of organisms having MICs of 4 or 8 μg/ml, which require higher cefepime exposures to optimize the pharmacodynamic profile of the compound in an attempt to maximize the clinical and microbiologic outcomes for the infected patient.
ACKNOWLEDGMENTS
We thank Mary Anne Banevicius, Henry Christensen, Jennifer Hull, Lucinda Lamb, Sara Robinson, Debra Santini, and Pamela Tessier for their collective efforts with MIC determinations.
This analysis was supported by internal funding from the Center for Anti-Infective Research and Development.
REFERENCES
- 1.Clinical and Laboratory Standards Institute. 2014. Performance standards for antimicrobial susceptibility testing; 24th informational supplement. CLSI document M100-S24 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 2.Clinical and Laboratory Standards Institute. 2010. Performance standards for antimicrobial susceptibility testing; 20th informational supplement. CLSI document M100-S20 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 3.Sutherland CA, Hamada Y, Nicolau DP. 2014. Impact of revised cefepime CLSI breakpoints on E. coli and K. pneumoniae susceptibility and potential impact if applied to P. aeruginosa, abstr C-099. Abstr 54th Intersci Conf Antimicrob Agents Chemother. American Society for Microbiology, 5 to 9 September 2014, Washington, DC. [Google Scholar]
- 4.Dortet L, Poirel L, Nordmann P. 2012. Rapid identification of carbapenemase types in Enterobacteriaceae and Pseudomonas spp. by using a biochemical test. Antimicrob Agents Chemother 56:6437–6440. doi: 10.1128/AAC.01395-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones RN, Kirby JT, Rhomberg PR. 2008. Comparative activity of meropenem in US medical centers (2007): initiating the 2nd decade of MYSTIC program surveillance. Diagn Microbiol Infect Dis 61:203–213. doi: 10.1016/j.diagmicrobio.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Hospira Inc. 2014. Maxipime. (Cefepime hydrochloride, USP) for injection. Hospira, Inc., Lake Forest, IL: http://www.hospira.com/Images/EN-3461_81-92609_1.pdf. [Google Scholar]
- 7.Crandon JL, Bulik CC, Kuti JL, Nicolau DP. 2010. Clinical pharmacodynamics of cefepime in patients infected with Pseudomonas aeruginosa. Antimicrob Agents Chemother 54:1111–1116. doi: 10.1128/AAC.01183-09. [DOI] [PMC free article] [PubMed] [Google Scholar]