Abstract
A global reemergence of human enterovirus 68 (EV-D68) associated with severe respiratory illness occurred in 2014. We developed and validated an EV-D68-specific real-time reverse transcription-PCR (RT-PCR) for the detection of EV-D68 in respiratory samples. The rapid diagnosis of EV-D68 may contribute to better management of EV-D68 infections.
TEXT
Human enterovirus 68 (EV-D68) infections are associated with severe respiratory illness in pediatric patients (1). In the last decade, increased circulation of this virus has been reported worldwide (2–5). From mid-August 2014 to 13 October 2014, an outbreak with 691 laboratory-confirmed EV-D68 infections was observed in the United States (6, 7).
At present, positive EV-D68 samples are identified using a generic human enterovirus (HEV)/human rhinovirus (HRV) real-time reverse transcription-PCR (RT-PCR), followed by sequencing of amplicons. The aim of the present study was to develop and validate an EV-D68-specific real-time RT-PCR for the detection and quantification of EV-D68 in respiratory samples.
The primers and the probe (Table 1) were designed with Primer Express software, version 3.0 (Applied Biosystems, Foster City, CA) using all available EV-D68 sequences (n = 133) (see Table S1 in the supplemental material) of the 5′ untranslated region retrieved from the GenBank database. The primer and probe sequences were evaluated in silico by querying the NCBI nucleotide database for related sequences (n = 45) (see Table S1). No matches with enteroviruses (HEVs) or rhinoviruses (HRVs) were found for the forward primer and probe, while the reverse primer showed 62% to 87% identity with some HEVs, such as EV-A90 and coxsackievirus A24.
TABLE 1.
Primers and probes for EV-D68-specific real-time RT-PCR
| Primer or probe name | Sequence (5′ to 3′) | Locationa |
|---|---|---|
| EV-D68Pavia-For | GGCGGCCTACTCATGG | 367–382 |
| EV-D68Pavia-Rev | AGACTCTTCACACCTTGTTCATGT | 401–426 |
| EV-D68Pavia-Probe | FAM-AAAACCATGAGACGCT-MGBb | 385–400 |
Nucleotide position according to reference sequence of strain Fermon (GenBank accession number AY426531).
FAM, 6-carboxyfluorescein; MGB, minor groove binder.
A DNA plasmid used as the standard calibrator was produced by cloning a 610-bp fragment of EV-D68 into a plasmid vector from a positive respiratory sample amplified using primers bracketing the real-time RT-PCR target region (5′-TTAAAACAGCYYTGGGGTTGTTCCCA-3′ [forward] and 5′-GATTGTCACCATAAGCAGCC-3′ [reverse]). The RT-PCR product was cloned into the plasmid vector pCR2.1 (Invitrogen, Carlsbad, CA) and sequenced for verification. The DNA plasmid was purified using the QIAprep Maxiprep kit (Qiagen) and quantified with a TBS-380 minifluorometer (Turner BioSystems, Sunnyvale, CA) and the Quant-iT PicoGreen double-stranded DNA reagent (Invitrogen, Carlsbad, CA) to generate standard curves for quantification determinations. The analytical evaluation of the EV-D68-specific real-time RT-PCR was performed according to previously published recommendations (8).
The one-step real-time RT-PCR was developed using the QuantiFast pathogen RT-PCR +IC kit (Qiagen, Hilden, Germany) and was carried out in an ABI 7300 real-time PCR system (Applied Biosystems, Foster City, CA, USA). The real-time RT-PCR conditions were as follows: 5 μl of extracted RNA, 5 μl of 5× QuantiFast pathogen RT-PCR master mix, 0.25 μl of QuantiFast pathogen RT mix, 100 nM primers and probe (0.03 μl of stock 80 μM), 2.5 μl of 10× internal control RNA, 2.5 μl of 10× internal control assay, 0.50 μl of High-ROX dye solution, and RNase-free water to adjust the volume to 25 μl. The cycling conditions for the amplification were as follows: reverse transcription at 50°C for 20 min, an initial PCR activation step at 95°C for 5 min, and 45 cycles of two-step cycling for 10 s at 95°C and for 30 s at 60°C.
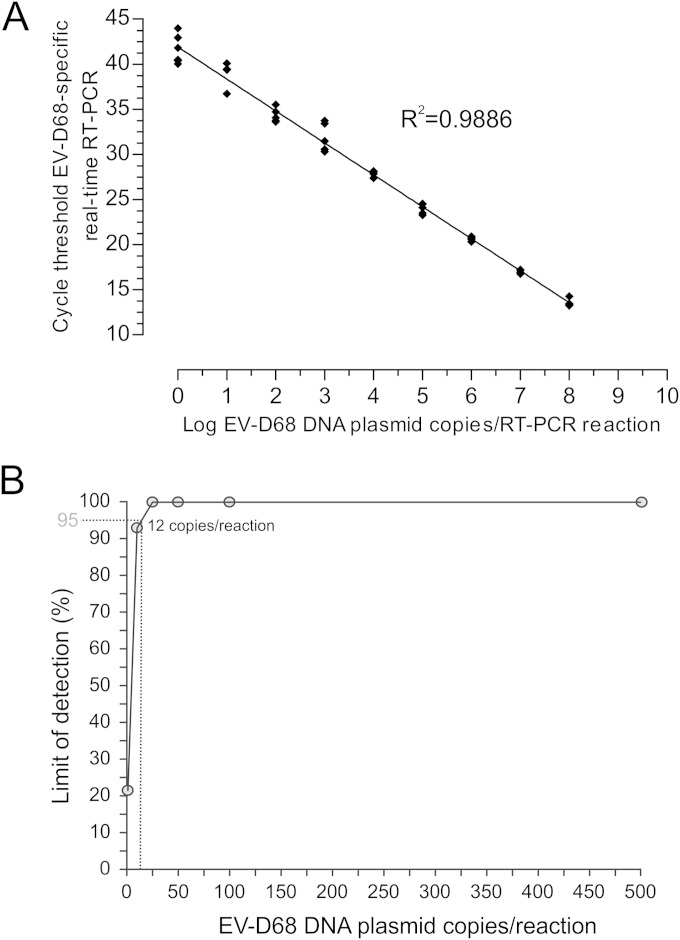
A linearity study was performed on serial 10-fold dilutions of quantified plasmid DNA in RT-PCR-grade water (Ambion, Austin, TX). A linear correlation between the log starting copy number and cycle threshold (CT) was achieved with 8.0 to 0.1 log10 DNA plasmid copies/μl (Fig. 1A). At each concentration, four replicates were tested in a single run. Liner regression analysis of the CT values against the log10 EV-D68 plasmid concentration resulted in an R2 value of 0.9886 (Fig. 1A). The precision of the assay, defined as the coefficient of variation (CV), was measured using the linearity test results and the average intra-assay CV value, 2.7% ± 1.4% (Fig. 1A), while the average interassay variability was 2.9% ± 0.9% (data not shown).
FIG 1.
(A) Linear range for the EV-D68 real-time RT-PCR assay. The standard curve was obtained by plotting the CT values against the starting copy number. The coefficient R2 of linear regression is shown. (B) Probit regression analysis to determine the limit of detection (LOD) for the EV-D68-specific real-time RT-PCR. TheLOD with a 95% probability is represented by a gray dotted line.
The analytical sensitivity of the assay was assessed by determining the lower limit of detection (LOD) using a dilution series of standard DNA plasmids at the following concentrations: 1, 10, 25, 50, 100, and 500 copies/reaction. From each dilution, 10 replicates were tested in 3 different assays on different days. The proportion of the positive results obtained from each input concentration was analyzed using probit regression analysis. The probit analysis showed a 95% LOD of 13 copies/reaction corresponding to 715 copies/ml in bronchoalveolar lavage fluid and 286 copies/ml in nasal swab (Fig. 1B) samples for our current extraction protocol (EZ1 system; Qiagen, Hilden, Germany).
To assess the effects of nucleic acid extraction and the sample matrix, three aliquots of DNA plasmids containing 6.0 (mean CT value, 20.6), 4.0 (27.9), and 2.0 (34.5) log10 DNA plasmid copies/μl were added to nasal swabs negative for EV-D68 before the extraction. The samples were extracted using the EZ1 system (Qiagen, Hilden, Germany) with the EZ1 virus minikit v2.0 (Qiagen, Hilden, Germany) and subsequently amplified with the EV-D68 real-time RT-PCR. The average CT difference between the obtained results and the DNA plasmid quantity was 1.0 ± 0.4.
The specificity of the EV-D68 real-time RT-PCR was evaluated by testing a set of reference strains (25 HEV, 2 parechovirus, and 4 HRV), 10 HEV isolates, and 25 positive respiratory samples containing 17 different HEV genotypes. Finally, 56 HRV-positive samples containing HRV strains belonging to species A, B, or C (as previously determined by VP4/VP2 gene sequencing) were also tested. None of the reference strains, HEV isolates, or HEV-positive or HRV-positive samples produced a detectable signal. Finally, the inclusivity of the EV-D68 real-time RT-PCR was assessed by testing a series of 21 sequencing-confirmed EV-D68-positive samples (4). All positive EV-D68 samples were correctly detected with a median CT value of 25.5 (range, 18.4 to 31.4).
This new real-time RT-PCR is a useful tool for detecting EV-D68 in respiratory samples. The rapid diagnosis of EV-D68 may contribute to better management of EV-D68 infections.
Supplementary Material
ACKNOWLEDGMENTS
We thank Daniela Sartori for manuscript editing and Laurene Kelly for revision of the English.
This work was supported by the Ministero della Salute, Fondazione IRCCS Policlinico San Matteo, Ricerca Corrente (grant 80622) and Progetto Cariplo 2011-0517, Milan, Italy.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.03691-14.
REFERENCES
- 1.Oberste MS, Maher K, Schnurr D, Flemister MR, Lovchik JC, Peters H, Sessions W, Kirk C, Chatterjee N, Fuller S, Hanauer JM, Pallansch MA. 2004. Enterovirus 68 is associated with respiratory illness and shares biological features with both the enteroviruses and the rhinoviruses. J Gen Virol 85:2577–2584. doi: 10.1099/vir.0.79925-0. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2011. Clusters of acute respiratory illness associated with human enterovirus 68—Asia, Europe, and United States, 2008–2010. MMWR Morb Mortal Wkly Rep 60:1301–1304. [PubMed] [Google Scholar]
- 3.Meijer A, Benschop K, Donker G, van der Avoort H. 2014. Continued seasonal circulation of enterovirus D68 in the Netherlands, 2011–2014. Euro Surveill 19:20935 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20935. [DOI] [PubMed] [Google Scholar]
- 4.Piralla A, Girello A, Grignani M, Gozalo-Margüello M, Marchi A, Marseglia G, Baldanti F. 2014. Phylogenetic characterization of enterovirus 68 strains in patients with respiratory syndromes in Italy. J Med Virol 86:1590–1593. doi: 10.1002/jmv.23821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piralla A, Lilleri D, Sarasini A, Marchi A, Zecca M, Stronati M, Baldanti F, Gerna G. 2012. Human rhinovirus and human respiratory enterovirus (EV68 and EV104) infections in hospitalized patients in Italy, 2008–2009. Diagn Microbiol Infect Dis 73:162–167. doi: 10.1016/j.diagmicrobio.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 2014. Enterovirus D68 in the United States, 2014. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/non-polio-enterovirus/outbreaks/EV-D68-outbreaks.html. [Google Scholar]
- 7.Midgley CM, Jackson MA, Selvarangan R, Turabelidze G, Obringer E, Johnson D, Giles BL, Patel A, Echols F, Oberste MS, Nix WA, Watson JT, Gerber SI. 2014. Severe respiratory illness associated with enterovirus D68—Missouri and Illinois, 2014. MMWR Morb Mortal Wkly Rep 63:798–799. doi: 10.1111/ajt.13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burd EM. 2010. Validation of laboratory-developed molecular assays for infectious diseases. Clin Microbiol Rev 23:550–576. doi: 10.1128/CMR.00074-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.