Abstract
We report a fatal case of acute lower respiratory tract disease with human rhinovirus C (HRV-C) as the unique cause in a 19-month-old girl with a history of repeated episodes of bronchiolitis. HRV-C type 8 nucleic acids were observed in respiratory, stool, and cerebrospinal fluid samples, and infectious virions were isolated from patient serum after inoculation onto reconstituted airway epithelia.
CASE REPORT
In September 2012, a 19-month-old girl with severe acute respiratory distress was admitted to the pediatric intensive care unit (ICU). The child was born at term and healthy but had a history of repeated episodes of bronchiolitis that required hospitalization when she was 5, 6, and 8 months old. Viral diagnosis of these episodes had never been performed. No allergic sensitization was known, and she had received no treatment for asthma nor any inhaled corticosteroids. Her parents had no known history of asthma or atopy. The child had no known underlying condition. Thirty-six hours before hospitalization, the child suffered from rhinitis, respiratory symptoms, and wheezing, suggesting bronchiolitis. Upon admission, she was unconscious and cyanotic with a pulse at 155 beats/min, oxygen saturation at 48%, blood pressure at 125/74 mm Hg, and a temperature of 37.2°C. Plastic bronchitis was suspected, and the patient was treated with high-output oxygenotherapy, intravenous glucocorticoid (1 mg/kg methylprednisolone), and inhaled adrenalin and terbutalin, but her condition did not improve significantly. Laboratory tests revealed a leukocyte count of 31 × 109 cells/liter (75% segmented neutrophils, 17% lymphocytes, and 7% monocytes) and a serum C-reactive protein level of 19.2 mg/liter. A chest X-ray showed a compressive pneumothorax that was immediately treated. Despite orotracheal intubation and institution of mechanical ventilation, profound bradychardia occurred, followed by cardiac arrest. Resuscitation attempts failed and the child died a few hours after hospitalization.
An autopsy was conducted with the parents' consent, and microbiological investigations were performed on specimens collected just after the patient's death. None of the microbiological cultures and investigations yielded any pathogenic bacteria in the patient's blood, cerebrospinal fluid (CSF), urine, stool, throat, tracheal aspiration, or lung biopsy specimens (Table 1). Culture from lung biopsy specimens was negative for mycobacteria. Nucleic acids were extracted with the NucliSENS easyMAG automated system according to the manufacturer's instructions (bioMérieux, Lyon, France). The different specimens were tested for human enterovirus (HEV), human herpesviruses (HHV) 1 to 6, Pneumocystis jirovecii, and a panel of 22 respiratory pathogens, including human rhinovirus (HRV), using PCR (Table 1). Cepheid HEV-specific PCR (Cepheid, Sunnyvale, CA, USA) was positive for blood and stool specimens and at the lower limit of detection for the CSF sample, while multiplex PCR (RespiFinder SMART 22 assay; Pathofinder, Maastricht, Netherlands) was positive for HEV and HRV in nasopharyngeal aspirate specimens (Table 1). For genotyping, RNA extracts from serum, stool, and nasopharyngeal aspirate specimens were reverse transcribed. A generic nested PCR assay, which amplifies a partial sequence of the HEV VP1 coding region (1), was first attempted but gave negative results on all samples tested, even those with low cycle threshold (CT) values. Therefore, a second nested PCR assay specific for the HRV VP4/VP2 genomic region (2) was performed and allowed virus genotyping in serum, stool, and nasopharyngeal aspirate samples (Table 1). The VP4/VP2 sequences showed a 92% nucleotide homology with the corresponding region of the HRV-C type 8 (HRV-C8) prototype strain (Table 1) (GenBank accession number GQ223227; http://www.picornaviridae.com/enterovirus/rv-c/rv-c_seqs.htm). Phylogenetic analysis confirmed the genotype assignment (data not shown). Globally, these PCR typing results excluded an HEV infection and were in favor of a cross-detection of HRV-C8 by the Cepheid HEV-specific PCR.
TABLE 1.
Virological findings from patient specimens
| Specimen type | Result obtained usinga: |
|||
|---|---|---|---|---|
| HHV PCR | HEV PCR (CT value) | Respiratory virus multiplex PCRe | Viral culture | |
| Nasopharyngeal aspirate | Neg | ND | Pos for HEV/HRV | Posd |
| Blood (serum) | Negb | Pos (28)d | ND | Posd |
| Stool | ND | Pos (29)d | ND | ND |
| Cerebrospinal fluid | Neg | Pos (45) | ND | ND |
| Frozen lung tissue from autopsyc | ND | Pos (24)d | ND | ND |
Neg, negative; Pos, positive; ND, not done; HHV PCR, human herpesvirus PCR; HEV PCR, human enterovirus PCR (Cepheid); CT, cycle threshold. Tests for HHV 1 to 3 were conducted using LightMix kit HSV-1/2 (herpes simplex virus)/VZV (varicella-zoster virus) (TIB Molbiol). Tests for HHV 4 to 6 were conducted using Argene kits (bioMérieux). PCRs for HHV 1 to 6 were performed on a LightCycler 480 (Roche, Meylan, France).
Only HHV 4 to 6 were tested in serum.
Tested negative for Pneumocystis jirovecii.
Typed as HRV-C8 by sequencing.
The respiratory virus multiplex PCR assay used was the Respifinder SMART 22, which allows the detection of 18 viruses (rhinovirus/enterovirus, influenza A/B/A H1N1, adenovirus, metapneumovirus, respiratory syncytial viruses A and B, parainfluenza viruses 1 to 4, coronavirus NL63/OC43/229E/HKU1, and bocavirus) and 4 bacteria (Chlamydia pneumoniae, Mycoplasma pneumoniae, Legionella pneumophila, and Bordetella pertussis). This assay was performed on a LightCycler 480.
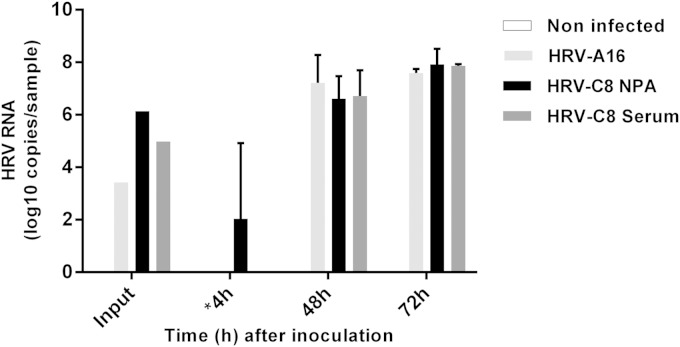
The autopsy revealed significant abnormalities only in the lungs and trachea, with acute diffuse alveolitis and tracheobronchitis. Real-time reverse transcription (RT)-PCR and VP4-VP2 sequencing performed on nucleic acids extracted from a frozen lung autopsy fragment confirmed the presence of HRV-C8. To assess whether blood positivity was associated with the presence of HRV-C8 replication-competent virions, we inoculated the patient's serum, as well as the nasopharyngeal aspirates (both stored in sterile phosphate-buffered saline at −80°C), into reconstituted human airway epithelia (MucilAir; Epithélix Sàrl, Plan-les-Ouates, Geneva, Switzerland) as previously described (3). Efficient viral replication was observed for the two specimens (Fig. 1). The presence of infectious HRV-C8 was confirmed by VP4/VP2 sequencing of the viruses collected from the apical face of the tissue 48 h postinfection (Table 1).
FIG 1.
HRV RNA loads measured by real-time RT-PCR at the apical surface of three-dimensional human airway epithelium reconstituted in vitro and inoculated with HRV-A16, the patient's nasopharyngeal aspirate, or the patient's serum or not infected (3). Absolute copy numbers present in the input (100 μl) or apical washes (200 μl) collected at different time points are indicated. Error bars show standard deviations calculated from two independent biological replicates. *, RNA load measured 4 h postinoculation represents the leftover inoculum. NPA, nasopharyngeal aspirate.
HRV is the most frequent cause of the common cold and other self-limited upper respiratory tract illnesses, but it can be associated with lower respiratory tract complications, including recurrent wheezing, bronchitis, asthma exacerbation, and pneumonia (4). Fatal cases caused by HRV infections are infrequent and have been described mostly in patients with severe underlying conditions (5–8). As HRV is also commonly detected in asymptomatic individuals, particularly in children, it may be difficult to establish a causal link between viral detection and symptoms. We report a systemic HRV-C type 8 infection as the cause of fatal acute respiratory distress in a 19-month-old girl with a history of repeated episodes of bronchiolitis. Both host and viral factors may have contributed to the disease severity and death. Some hosts, e.g., asthmatic subjects, present a deficient innate immune response to HRV and are predisposed to severe lower respiratory illnesses upon infection (9–11). In our case, we cannot rule out that the patient suffered from an unknown underlying disease, including infantile asthma, that could have predisposed her to a severe and fatal rhinovirus infection. In the absence of ethical authorization, genetic investigations were not carried out to explore a primary immunodeficiency or asthma susceptibility.
Viral factors may also have an impact on disease severity. HRV-Cs could present a higher virulence and be associated with asthma exacerbations and pediatric hospitalizations (12–14). In this study, HRV RNA was detected at a low level in the CSF and at higher concentrations in the lung and fecal samples. The latter observation suggests replication in the gastrointestinal tract, although isolation in tissue culture was not attempted due to the limited amount of clinical specimen available. An open question remains whether this HRV-C strain resisted the acidic environment of the gastrointestinal tract or if infants can present a less acidic gastrointestinal environment under some circumstances (15–17). In addition to this potential unusual tropism for the gastrointestinal tract, HRV RNA was identified in blood samples. The presence of HRV nucleic acids in blood has been reported previously. It was shown to be more frequent for HRV-C than for the other two HRV species (17–19) and more common in children with asthma exacerbation (19). However, the presence of HRV nucleic acids is not necessarily associated with infectious virions. To our knowledge, HRV culture from blood samples has only been described once, in 1970 (20). In that study, Urquhart and Stott isolated two HRV-A clinical strains (HRV-A15 and -A22) in cell culture after inoculation with the serum of two children who died of acute respiratory illness. In our case, replication-competent HRV-C virions were successfully isolated from the patient's serum in human airway epithelia.
In conclusion, HRV-Cs are known to bind to an as-yet-uncharacterized receptor that differs from the HRV-A and -B receptors (4). Thus, the nature of infected cells and the exact tissue tropism of HRV-C types are not yet totally elucidated. Recovery of infectious virus from blood, either as free virions or cell-associated virus, suggests that authentic viremia with HRV-C may occur in given situations and lead to dissemination beyond the respiratory tract. The presence of HRV-C in the bloodstream could affect the clinical outcome of viral infection and cause more severe disease, particularly in immunocompromised hosts or individuals with asthma and other comorbidities.
ACKNOWLEDGMENTS
We thank Isabelle Piuz for technical assistance and Rosemary Sudan for editorial assistance.
This work was supported by the Swiss National Science Foundation (grant 310030_146151 to C.T.) and the Sandoz Foundation (grant 9975 to C.T.).
All authors declare no potential conflicts of interest.
REFERENCES
- 1.Nix WA, Oberste MS, Pallansch MA. 2006. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J Clin Microbiol 44:2698–2704. doi: 10.1128/JCM.00542-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Linsuwanon P, Payungporn S, Samransamruajkit R, Posuwan N, Makkoch J, Theanboonlers A, Poovorawan Y. 2009. High prevalence of human rhinovirus C infection in Thai children with acute lower respiratory tract disease. J Infect 59:115–121. doi: 10.1016/j.jinf.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tapparel C, Sobo K, Constant S, Huang S, Van Belle S, Kaiser L. 2013. Growth and characterization of different human rhinovirus C types in three-dimensional human airway epithelia reconstituted in vitro. Virology 446:1–8. doi: 10.1016/j.virol.2013.06.031. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs SE, Lamson DM, St George K, Walsh TJ. 2013. Human rhinoviruses. Clin Microbiol Rev 26:135–162. doi: 10.1128/CMR.00077-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asner SA, Petrich A, Hamid JS, Mertz D, Richardson SE, Smieja M. 2014. Clinical severity of rhinovirus/enterovirus compared to other respiratory viruses in children. Influenza Other Respir Viruses 8:436–442. doi: 10.1111/irv.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gutman JA, Peck AJ, Kuypers J, Boeckh M. 2007. Rhinovirus as a cause of fatal lower respiratory tract infection in adult stem cell transplantation patients: a report of two cases. Bone Marrow Transplant 40:809–811. doi: 10.1038/sj.bmt.1705827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hai le T, Bich VT, Ngai le K, Diep NT, Phuc PH, Hung VP, Taylor WR, Horby P, Liem NT, Wertheim HF. 2012. Fatal respiratory infections associated with rhinovirus outbreak, Vietnam. Emerg Infect Dis 18:1886–1888. doi: 10.3201/eid1811.120607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louie JK, Yagi S, Nelson FA, Kiang D, Glaser CA, Rosenberg J, Cahill CK, Schnurr DP. 2005. Rhinovirus outbreak in a long term care facility for elderly persons associated with unusually high mortality. Clin Infect Dis 41:262–265. doi: 10.1086/430915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caliskan M, Bochkov YA, Kreiner-Moller E, Bonnelykke K, Stein MM, Du G, Bisgaard H, Jackson DJ, Gern JE, Lemanske RF Jr, Nicolae DL, Ober C. 2013. Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. N Engl J Med 368:1398–1407. doi: 10.1056/NEJMoa1211592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, Bartlett NW, Kebadze T, Mallia P, Stanciu LA, Parker HL, Slater L, Lewis-Antes A, Kon OM, Holgate ST, Davies DE, Kotenko SV, Papi A, Johnston SL. 2006. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med 12:1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 11.Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, Holgate ST, Davies DE. 2005. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med 201:937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox DW, Bizzintino J, Ferrari G, Khoo SK, Zhang G, Whelan S, Lee WM, Bochkov YA, Geelhoed GC, Goldblatt J, Gern JE, Laing IA, Le Souef PN. 2013. Human rhinovirus species C infection in young children with acute wheeze is associated with increased acute respiratory hospital admissions. Am J Respir Crit Care Med 188:1358–1364. doi: 10.1164/rccm.201303-0498OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee WM, Lemanske RF Jr, Evans MD, Vang F, Pappas T, Gangnon R, Jackson DJ, Gern JE. 2012. Human rhinovirus species and season of infection determine illness severity. Am J Respir Crit Care Med 186:886–891. doi: 10.1164/rccm.201202-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller EK, Edwards KM, Weinberg GA, Iwane MK, Griffin MR, Hall CB, Zhu Y, Szilagyi PG, Morin LL, Heil LH, Lu X, Williams JV, New Vaccine Surveillance Network . 2009. A novel group of rhinoviruses is associated with asthma hospitalizations. J Allergy Clin Immunol 123:98–104.e1. doi: 10.1016/j.jaci.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harvala H, McIntyre CL, McLeish NJ, Kondracka J, Palmer J, Molyneaux P, Gunson R, Bennett S, Templeton K, Simmonds P. 2012. High detection frequency and viral loads of human rhinovirus species A to C in fecal samples; diagnostic and clinical implications. J Med Virol 84:536–542. doi: 10.1002/jmv.23203. [DOI] [PubMed] [Google Scholar]
- 16.Lau SK, Yip CC, Lung DC, Lee P, Que TL, Lau YL, Chan KH, Woo PC, Yuen KY. 2012. Detection of human rhinovirus C in fecal samples of children with gastroenteritis. J Clin Virol 53:290–296. doi: 10.1016/j.jcv.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tapparel C, L'Huillier AG, Rougemont AL, Beghetti M, Barazzone-Argiroffo C, Kaiser L. 2009. Pneumonia and pericarditis in a child with HRV-C infection: a case report. J Clin Virol 45:157–160. doi: 10.1016/j.jcv.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuji N, Suzuki A, Lupisan S, Sombrero L, Galang H, Kamigaki T, Tamaki R, Saito M, Aniceto R, Olveda R, Oshitani H. 2011. Detection of human rhinovirus C viral genome in blood among children with severe respiratory infections in the Philippines. PLoS One 6:e27247. doi: 10.1371/journal.pone.0027247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xatzipsalti M, Kyrana S, Tsolia M, Psarras S, Bossios A, Laza-Stanca V, Johnston SL, Papadopoulos NG. 2005. Rhinovirus viremia in children with respiratory infections. Am J Respir Crit Care Med 172:1037–1040. doi: 10.1164/rccm.200502-315OC. [DOI] [PubMed] [Google Scholar]
- 20.Urquhart GE, Stott EJ. 1970. Rhinoviraemia. Br Med J 4:28–30. doi: 10.1136/bmj.4.5726.28. [DOI] [PMC free article] [PubMed] [Google Scholar]