Abstract
Sonication improved the diagnosis of orthopedic implant-associated infections (OIAI). We investigated the diagnostic performance of sonication fluid inoculated into blood culture bottles in comparison with that of intraoperative tissue and sonication fluid cultures. Consecutive patients with removed orthopedic hardware were prospectively included and classified as having OIAI or aseptic failure (AF) according to standardized criteria. The diagnostic procedure included the collection of five intraoperative tissue cultures and sonication of the removed device, followed by conventional culture of the sonication fluid. Cultures were incubated for 7 days (aerobic) or 14 days (anaerobic). In addition, 10 ml of sonication fluid was inoculated into each aerobic and anaerobic BacT/Alert FAN blood culture bottle and incubated in the automated blood culture system for 5 days. Of 75 included patients, 39 had OIAI and 36 AF. The sensitivity of sonication fluid inoculated into blood culture bottles (100%) was higher than that of conventional sonication fluid (87%; P = 0.05) or intraoperative tissue cultures (59%; P < 0.01). Previous antibiotic therapy reduced the culture sensitivity of conventional sonication fluid to 77% and that of intraoperative tissue to 55%, while it remained 100% for blood culture-inoculated sonication fluid. The time to positivity was shorter in blood culture-inoculated sonication fluid, with detection of 72% of microorganisms after 1 day of incubation, than for intraoperative tissue and conventional sonication fluid cultures, with detection of 18% and 28% of microorganisms, respectively. In conclusion, compared to conventional sonication fluid and intraoperative tissue cultures, sonication fluid inoculated into blood culture bottles improved the diagnosis of OIAI and considerably reduced the time to culture positivity.
INTRODUCTION
The pathogenesis of orthopedic implant-associated infections (OIAI) is related to biofilms, rendering these infections difficult to diagnose. Microorganisms in biofilms are embedded in a hydrated extracellular matrix, adhered to the surface, and transformed in a less metabolically active state than microorganisms in biofilms (1). An accurate diagnosis of OIAI is crucial for treatment success. A combination of various preoperative and intraoperative methods is usually needed for microbiological diagnosis of OIAI (2, 3). However, current diagnostic methods have limited sensitivity, with 10 to 30% false-negative results, and/or lack specificity (2).
Research and development of new diagnostic methods aim to improve the diagnostic accuracy and speed of microbial detection in foreign-body infections. Sonication of explanted implants, designed to remove attached biofilms, significantly improved the culture sensitivity compared to that of conventional microbiological methods using synovial fluid or intraoperative tissue samples. Sonication was evaluated in infections involving prosthetic joints, fracture fixation devices, vascular grafts, neurosurgical shunts, breast implants, and cardiac devices (4–7). Despite the use of sonication, however, cultures remain negative for several reasons, such as inappropriate culture media, short incubation time, loss of microbial viability during specimen transport, or prior antimicrobial therapy (8).
Various methods have been investigated to further improve the diagnosis of OIAI. For example, the culture sensitivity was improved by the inoculation of synovial fluid immediately after aspiration into aerobic and anaerobic blood culture bottles (9, 10). Similarly, authors have suggested the inoculation of homogenized tissue suspension in blood culture bottles (11, 12). In a recent study of prosthetic joint infection (13), sonication fluid inoculated into blood culture bottles showed higher sensitivity than inoculated synovial fluid (88% versus 64%; P = 0.009), especially in cases with previous antibiotic treatment (81% versus 52%; P = 0.031). This observation is not surprising, as similar results have been demonstrated with conventional synovial and sonication fluid cultures on blood agar plates and enrichment growth media. Other investigators routinely inoculated sonication fluid in blood culture bottles (14). However, it remains unclear whether inoculation into blood culture bottles increases the sensitivity of sonication fluid culture (especially in patients receiving antibiotics prior to sampling) and potentially reduces the time to culture positivity. This question has a high clinical relevance, which needs to be resolved before general use of automated blood culture systems for sonication fluid can be routinely recommended.
In this study, we evaluated the diagnostic performance of sonication fluid inoculated into BacT/Alert FAN blood culture bottles and compared it with that of intraoperative tissue and conventional sonication fluid cultures. Consecutive patients with removed orthopedic hardware were prospectively included. In all patients, independent of whether infection or an aseptic reason was suspected, standardized comprehensive preoperative and intraoperative diagnostic procedures were performed to minimize the probability of misclassification of OIAI as aseptic failure (AF) or vice versa by interpreting contaminating microorganisms as pathogens. To our knowledge, this is the first study in which a direct diagnostic performance comparison between conventional sonication fluid cultures and blood culture-inoculated sonication fluid was performed.
(Part of these results were presented at the European Congress of Clinical Microbiology and Infectious Diseases [ECCMID] in Barcelona, Spain, 10 to 13 May 2014, and at the European Bone and Joint Infection Society [EBJIS] in Utrecht, Netherlands, 11 to 13 September 2014.)
MATERIALS AND METHODS
Study design.
A cohort study was conducted in two tertiary medical care centers, Hospital del Mar (≈400 beds) and Hospital de l'Esperança (≈200 beds), in Barcelona, Spain. The study protocol was reviewed and approved by the institutional review board before patient inclusion. A standardized comprehensive diagnostic algorithm was applied to all patients to accurately determine the cause of prosthesis failure. This algorithm included standardized sampling of five intraoperative tissue specimens, sonication of removed orthopedic prosthetic components, prolonged incubation of synovial, intraoperative tissue, and sonication fluid cultures, and inoculation of sonication fluid into aerobic and anaerobic BacT/Alert FAN blood culture bottles with antimicrobial removal systems (bioMérieux, Marcy l'Etoile, France).
Study population.
In the participating hospitals, we prospectively included all consecutive patients ≥18 years old hospitalized from June 2013 through December 2013 in whom a joint prosthesis or fracture fixation device was removed for any reason. If obvious contaminations of the explanted components occurred during surgery, transport, or processing of the prosthesis in the laboratory, subjects were excluded. The following information was recorded: demographic, clinical, radiological, laboratory, and microbiological data and information on the type of surgical management and antimicrobial therapy.
Study definitions.
OIAI was defined as when at least one of the following criteria was present (15): (i) visible purulence of the site aspirate or at the surgical site (as determined by the surgeon), (ii) the presence of a sinus tract communicating with the implant, and/or (iii) clinical signs of infection, such as warmth, redness, or wound drainage (as determined by the surgeon). Additionally, in prosthetic joint infections, the following criteria were also considered: (iv) acute inflammation in histopathology sections of intraoperative tissue (as determined by the pathologist) and (v) acute inflammation in preoperative joint aspiration (leukocyte count of >1.7 g/liter or >65% granulocytes in a knee prosthesis [16] or leukocyte count of >4.2 g/liter or >80% granulocytes in a hip prosthesis [17]). AF was defined as when the implant was removed in the absence of these criteria for OIAI. Previous antimicrobial therapy was defined as any antibiotic received for ≥24 h within the 14 days prior to surgery.
Synovial fluid.
Synovial fluid was aspirated preoperatively and transferred into two vials. One of the vials contained EDTA for the determination of the leukocyte count and the percentage of granulocytes. The other was a native vial for culture. In the microbiology laboratory, 0.1 ml was inoculated on each PoliViteX (bioMérieux, Marcy l'Etoile, France) agar plate (incubated 7 days aerobically at 37°C with 5% CO2) and Schaedler agar plate enriched with 5% sheep blood (bioMérieux, Marcy l'Etoile, France) (incubated 14 days anaerobically at 37°C). Additionally, 0.5 ml of synovial fluid was inoculated in thioglycolate broth (BBL enriched thioglycolate medium with vitamin K and hemin; Becton Dickinson and Company, USA), and residual volumes were inoculated into a BacT/Alert (bioMérieux, Marcy l'Etoile, France) anaerobic bottle and incubated for 5 days.
Intraoperative tissue samples.
Tissue specimens were collected in native vials and were individually homogenized in 0.5 ml thioglycolate broth for 1 min using a mortar and pestle. Aliquots of 0.5 ml of tissue homogenate samples were inoculated on PoliViteX agar plates, on Schaedler agar plates enriched with 5% sheep blood, and in thioglycolate broth. The aerobic cultures were incubated at 37°C for 7 days, and the anaerobic ones were incubated for 14 days. Each distinctive colony morphology was identified using standard microbiological techniques. Low-virulence microorganisms, such as coagulase-negative staphylococci, Corynebacterium spp., Bacillus spp., or Propionibacterium spp., were considered pathogens when the same organism was isolated in at least two samples.
Sonication of removed implants.
The removed orthopedic hardware was explanted aseptically in the operating room and transported to the microbiology laboratory in solid polyethylene containers, with screw tops and airtight inner seals. The containers were previously autoclaved at 121°C for 15 min and double packed. Sonication of the hardware was performed in the microbiological laboratory, as previously described (18). The container with the hardware was vortexed for 30 s, followed by sonication for 1 min (at a frequency of 40 ± 5 kHz), and then vortexed again for 30 s. For sonication, a Bransonic ultrasound bath (model SM25E-MT; Branson Ultrasonics Corporation, Geneva, Switzerland) was used. Aliquots (0.5 ml) of sonication fluid were plated onto PoliViteX chocolate agar plates and Schaedler agar plates enriched with 5% sheep blood or inoculated into thioglycolate broth. The cultures were incubated at 37°C for 7 days (aerobically) or 14 days (anaerobically). Sonication fluid cultures were considered positive when ≥50 CFU of the same organism morphology grew per milliliter of sonication fluid, as previously defined (15). If the patient had previously received antibiotics, any growth in the sonication fluid culture was considered positive (19).
Blood culture BacT/Alert method.
Ten milliliters of sonication fluid was inoculated into aerobic and anaerobic BacT/Alert FAN blood culture bottles with antimicrobial removal systems. These bottles were incubated into the automated BacT/Alert system (bioMérieux, Marcy l'Etoile, France) for 5 days. For bottles that flagged positive, a Gram stain was performed and an inoculum was plated onto PoliViteX chocolate agar plates, blood agar plates (bioMérieux, Marcy l'Etoile, France), and Schaedler agar plates enriched with 5% sheep blood. If no organisms were seen by Gram staining, the BacT/Alert bottle was returned to the BacT/Alert instrument for further monitoring. The aerobic agar plates were incubated for 2 more days, and the anaerobic ones were incubated at 37°C to complete 14 days.
Statistical analysis.
Comparisons between categorical variables were performed using McNemar's χ2 or Fisher's exact test, as appropriate. Continuous variables were compared using the Mann-Whitney U test. P values of less than 0.05 were considered statistically significant. Calculations and graphics were performed using Prism software (version 6.05; GraphPad, La Jolla, CA).
RESULTS
Study population.
We included 75 patients in whom an orthopedic hardware was removed and submitted to the microbiology laboratory for sonication. No patients were excluded because of obvious contaminations of the implant during handling or in the microbiology laboratory. AF was diagnosed in 36 cases (48%) and OIAI in 39 cases (52%). Further characteristics of the 75 patients are summarized in Table 1. A joint prosthesis was explanted in 45 (60%) cases, and a fracture fixation device was explanted in 30 (40%) cases. Patients with OIAI most commonly had visible pus surrounding the implant (72%) and acute inflammation in tissue histopathology (82%). About half of the patients with OIAI (56%) received antibiotics within 14 days prior to sampling.
TABLE 1.
Characteristics of 75 study patients with aseptic failure and implant-associated infection
| Characteristic | Value |
P valueb | |
|---|---|---|---|
| Aseptic failure (n = 36 patients) | Orthopedic implant-associated infection (n = 39 patients) | ||
| Median patient age, yrs (range) | 73 (27–89) | 73 (48–87) | 0.968 |
| No. (%) of males | 12 (33) | 15 (43) | 0.554 |
| No. (%) of patients by type of prosthesis | |||
| Joint prosthesis (n = 45) | 27 (75) | 18 (46) | 0.091 |
| Fracture fixation device (n = 30) | 9 (25) | 21 (54) | 0.004 |
| No. (%) of patients with clinical signs of infection | |||
| Sinus tract | 0 | 8 (21) | <0.001 |
| Other local signs of infectiona | 0 | 19 (49) | <0.001 |
| Visible pus surrounding the implant | 0 | 28 (72) | <0.001 |
| No. (%) of patients with acute inflammation in tissue histopathology | 0 | 32 (82) | <0.001 |
| Mean synovial fluid cell count (range) | |||
| Leukocyte count (109/liter) | 0.4 (0.05–0.5) | 52 (1.2–78) | <0.001 |
| % granulocytes | 17 (2–40) | 95 (55–98) | <0.001 |
| No. (%) of patients who received antibiotics prior to sampling | 0 | 22 (56) | <0.001 |
Local signs of infection include warmth, redness, and wound drainage.
Bolding represents statistical significance.
Performance of diagnostic methods.
Table 2 summarizes the culture accuracy of intraoperative tissue, conventional sonication fluid, and sonication fluid inoculated into blood culture bottles from patients with OIAI and AF. The sensitivity of sonication fluid culture was significantly higher than that for intraoperative tissue culture (87% versus 59%; P < 0.01). The sensitivity was improved to 100% by inoculating the sonication fluid into blood culture bottles (P = 0.05).
TABLE 2.
Diagnostic performance of three diagnostic methods in 75 patients with removed orthopedic hardwarea
| Diagnostic method | % sensitivity (95% CI) | % specificity (95% CI) | % PPV (95% CI) | % NPV (95% CI) |
|---|---|---|---|---|
| Intraoperative tissue samples | 59 (42–74) | 100 (90–100) | 100 (85–100) | 69 (55–81) |
| Conventional sonication fluid | 87 (73–96) | 100 (90–100) | 100 (90–100) | 88 (74–96) |
| Inoculation of sonication fluid in blood culture bottles | 100 (91–100) | 100 (90–100) | 100 (91–100) | 100 (90–100) |
Out of the 75 patients, 39 had orthopedic implant-associated infections and 36 had aseptic failure. 95% CI, 95% confidence interval; PPV, positive predictive value; NPV, negative predictive value.
Effect of previous antibiotic treatment on culture sensitivity.
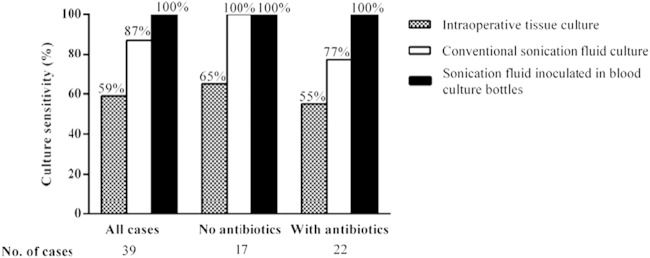
Figure 1 shows the culture sensitivities of intraoperative tissue cultures, conventional sonication fluid cultures, and blood culture-inoculated sonication fluid. Previous antibiotic therapy reduced the culture sensitivity of intraoperative tissue from 65% to 55% and of conventional sonication fluid from 100% to 77%, while it remained 100% in blood culture-inoculated sonication fluid. Among the 36 cases with AF, none received antimicrobial treatment previous to surgery, and all cases were negative by all three diagnostic methods.
FIG 1.
Culture sensitivities of intraoperative tissue, conventional sonication fluid, and sonication fluid inoculated into BacT/Alert bottles in 39 orthopedic implant-associated infection cases (stratified to whether or not receiving antibiotics prior to sampling).
Microbiological findings in patients with OIAI.
Table 3 summarizes the microbiological findings of individual diagnostic methods in 39 patients with OIAI. When conventional and blood culture-inoculated sonication fluid cultures were used, more pathogens were detected (45 and 50 organisms, respectively) than by intraoperative tissue cultures (30 pathogens). In addition, mixed infections (i.e., isolation of ≥2 microorganisms) were detected more frequently in sonication fluid than in intraoperative tissue cultures (21% versus 13%). Negative cultures were observed less frequently in conventional sonication fluid than in intraoperative tissue (13% versus 41%; P < 0.01). All patients with negative cultures received antibiotics prior to surgery. By inoculating sonication fluid into blood culture bottles, no false-negative cultures were observed. All 5 infections, which were detected only by blood culture-inoculated sonication fluid, were acute infections caused by coagulase-negative Staphylococcus (n = 1), Staphylococcus aureus (n = 1), viridans group Streptococcus (n = 1), and Gram-negative bacilli (n = 1).
TABLE 3.
Microbiological findings in 39 orthopedic implant-associated infection cases according to type of diagnostic method
| Characteristic | Value |
||
|---|---|---|---|
| Intraoperative tissue culture | Conventional sonication fluid culture | Sonication fluid inoculated in blood culture bottles | |
| No. (%) of cases by no. of microorganisms detected | |||
| 1 | 18 (46) | 26 (67) | 31 (79) |
| ≥2 | 5 (13)a | 8 (21)b | 8 (21)b |
| 0 | 16 (41) | 5 (13) | 0 (0) |
| Total no. of microorganisms isolated | 30 | 45 | 50 |
| No. (%) of microorganisms with Gram-positive cocci | 16 (53) | 27 (60) | 30 (60) |
| Coagulase-negative staphylococci | 8 | 16 | 17 |
| S. aureus | 3 | 4 | 5 |
| Viridans group streptococci | 1 | 3 | 4 |
| Enterococcus spp. | 4 | 4 | 4 |
| No. (%) of microorganisms with Gram-negative bacilli | 10 (33) | 10 (22) | 12 (24) |
| E. coli | 2 | 2 | 2 |
| Enterobacter spp. | 2 | 2 | 3 |
| Klebsiella spp. | 1 | 1 | 2 |
| Proteus spp. | 4 | 4 | 4 |
| P. aeruginosa | 1 | 1 | 1 |
| No. (%) of microorganisms that were anaerobes | 2 (7) | 5 (11) | 5 (10) |
| P. acnes | 2 | 5 | 5 |
| No. (%) of other microorganisms | 2 (7) | 3 (7) | 3 (6) |
| Candida spp. | 1 | 1 | 1 |
| Corynebacterium spp. | 1 | 2 | 2 |
Klebsiella spp. and Proteus spp.; Enterococcus spp., Proteus spp., and Pseudomonas spp.; Staphylococcus epidermidis and Staphylococcus warneri; Corynebacterium spp., Enterococcus spp., and Proteus spp.; S. epidermidis and Staphylococcus simulans.
Klebsiella spp. and Proteus spp.; Enterococcus spp., Proteus spp., and Pseudomonas spp.; S. epidermidis and S. warneri; Corynebacterium spp., Enterococcus spp., and Proteus spp.; S. epidermidis and S. simulans; S. epidermidis and Streptococcus spp.; Corynebacterium spp., S. epidermidis, and Enterococcus spp.; S. epidermidis and P. acnes.
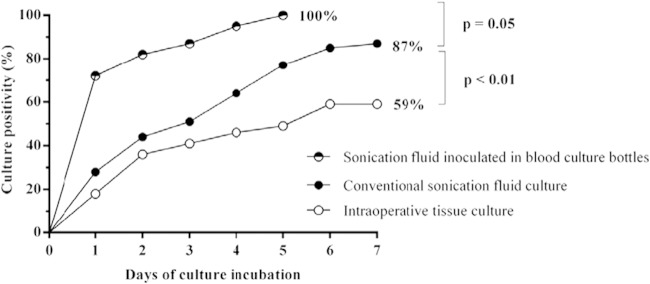
Time to culture positivity in patients with OIAI.
Figure 2 illustrates the time to culture positivity in 39 OIAI cases. After 1 day of incubation, 18% of intraoperative tissue and 28% of conventional sonication fluid cultures were positive, whereas blood culture-inoculated sonication fluid cultures were positive in 72% (P < 0.01). All OIAI cases (100%) were detected by sonication fluid inoculated in blood culture bottles on day 5 of incubation. In contrast, sonication fluid culture detected 87% and intraoperative tissue culture detected 59% of OIAI cases on day 7 of incubation. In this series, prolonged anaerobic incubation of intraoperative tissue and conventional sonication cultures up to 14 days did not yield additional microorganisms.
FIG 2.
Time to culture positivity of 39 orthopedic implant-associated infection cases.
DISCUSSION
The inoculation of synovial fluid and intraoperative tissue homogenate into aerobic and anaerobic blood culture bottles was shown to improve the culture sensitivity in prosthetic joint infection (9–11). Later, several researchers demonstrated improved culture sensitivity of sonication fluid cultures compared to that of intraoperative tissue cultures in the diagnosis of OIAI (18, 20–22). In a recent publication, the inoculation of sonication fluid into blood culture bottles was compared with the inoculation of synovial fluid (13). However, the same sample (in this case sonication fluid) was not compared in this study. Therefore, it remained unclear whether the inoculation of sonication fluid into blood culture bottles increases the culture sensitivity compared to that of conventional sonication fluid cultures. Especially, it remained unclear whether inoculation may reduce the time to culture positivity or whether patients receiving antibiotics prior to sampling may benefit from this method.
In this study, we compared the performance of sonication fluid inoculated into blood culture bottles with that of intraoperative tissue and sonication fluid cultures. When sonication fluid was inoculated into blood culture bottles, the culture sensitivity was higher (100%) than that in conventional sonication fluid (87%) and intraoperative tissue samples (59%). Despite the fact that sonication may be harmful to microorganisms, especially to Gram-negative bacilli and anaerobic bacteria (23), three additional OIAI cases, one caused by Propionibacterium acnes and 2 caused by Gram-negative bacilli, were detected by sonication, corroborating the efficacy of the sonication procedure.
The inoculation of sonication fluid in blood culture bottles has several advantages over conventional solid medium cultures. First, previous studies suggested that microorganisms are present in the sonication fluid in culture-negative cases of prosthetic joint infection (or at least their DNA), as detected by broad-range PCR (24) or multiplex PCR (15, 25). However, it remains unclear whether culture negativity is caused by nonviability of the microorganisms or just low microbial quantity, below the detection limit of conventional sonication fluid cultures. Second, by use of a blood culture system, a 20-fold-increased volume of sonication fluid compared to the volume of agar plate cultures (10 ml versus 0.5 ml) is investigated. This study also suggests that a larger sample volume does not compromise the culture specificity, since no false-positive cultures in AF cases were observed. Third, growth media in blood culture bottles contain antimicrobial removal systems and allow growth of microorganisms immediately after inoculation. The main disadvantage of the inoculation of sonication fluid in blood culture bottles is a potential decrease in specificity by losing the ability of set colony count thresholds to define a positive culture. Therefore, a combination of independent microbiologic diagnostic tests should be performed, including periprosthetic tissue cultures and conventional sonication fluid cultures.
In the study by Shen et al. (13), researchers detected six additional infections by inoculated sonication fluid than by synovial fluid in patients who had previously received antibiotics. In our study, previous antibiotic therapy reduced the culture sensitivities of conventional sonication fluid culture (from 100% to 73%) and intraoperative tissue culture (from 65% to 55%). However, the sensitivity of inoculated sonication fluid into blood culture bottles was not affected (100%). Therefore, the inoculation of sonication fluid culture into blood culture bottles with antimicrobial removal systems may be particularly useful in patients who previously received antimicrobials.
Interestingly, five additional cases of OIAI were detected in our study by the inoculation of sonication fluid into blood culture bottles (and not by conventional sonication fluid culture). All were acute infections in patients receiving previous antibiotics. This can be explained by better antibiotic activity on early biofilms in acute infections than on mature biofilms in chronic infections (25). This explanation is in line with a previous observation that sonication increased the culture sensitivity in chronic but not in acute prosthetic joint infections (19). We hypothesize that biofilms in chronic infections involve more layers and are more firmly attached to the surface; therefore, sonication can fully exhibit its detachment effect and improved detection characteristics.
Despite the fact that the use of sonication improves the diagnosis of OIAI, a considerable number of these infections are culture negative (8, 19, 25, 26). Culture-negative OIAI may be due to the use of prior antimicrobial therapy, inadequate diagnostic methods, or the inability of a pathogen to grow with the current available culture methods (18, 27, 28). In our study, sonication succeeded in significantly reducing the rate of culture-negative OIAI from 41% (by intraoperative tissue culture) to 13%. These results are in agreement with the prior study, which demonstrated that sonication fluid provided faster microbial detection than intraoperative tissue samples (8). In our study, we also observed that the time to microbial detection was further reduced by inoculating the sonication fluid culture into blood culture bottles, detecting 72% of infections already after 1 day of culture incubation.
Blood culture bottles are widely used in microbiology laboratories for the diagnosis of sepsis or different types of infections, such as arthritis, when inoculated with sterile fluids (10). However, the contamination risk may be increased. Careful manipulation should be applied, especially when the inoculation of sonication fluid into blood culture bottles is performed. In our study, we applied an enhanced diagnostic algorithm to all included patients independent of microbiological findings and did not observe false-positive culture in AF cases. By inoculating sonication fluid into blood culture bottles, no false-positive results in this method were observed, therefore increasing the sensitivity without compromising specificity. By counting single-positive tissue samples, the specificity was compromised, as shown by false-positive tissue cultures in aseptic failure cases (29). In contrast, in the study by Shen et al. (13), coagulase-negative staphylococci were isolated from some blood culture bottles inoculated with sonication fluid from patients with AF, which may reflect contamination or the misclassification of patients.
In conclusion, sonication fluid inoculated into blood culture bottles with antimicrobial removal systems, compared to conventional sonication fluid and intraoperative tissue cultures, improved the diagnosis of OIAI and considerably reduced the time to culture positivity. This method demonstrated high sensitivity and specificity, especially in patients receiving antibiotics previous to surgery. By the inoculation of sonication fluid into blood culture bottles, 72% of microorganisms were detected after 1 day of incubation, whereas intraoperative tissue and conventional sonication fluid cultures detected only 18% and 28% of OIAI after 1 day. This simple and readily available diagnostic method may significantly improve the diagnosis of implant-associated infections in the future.
REFERENCES
- 1.Donlan RM. 2002. Biofilms: microbial life on surfaces. Emerg Infect Dis 8:881–890. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corvec S, Portillo ME, Pasticci BM, Borens O, Trampuz A. 2012. Epidemiology and new developments in the diagnosis of prosthetic joint infections. Int J Artif Organs 35:923–934. doi: 10.5301/ijao.5000168. [DOI] [PubMed] [Google Scholar]
- 3.Zimmerli W, Trampuz A, Ochsner PE. 2004. Prosthetic-joint infections. N Engl J Med 351:1645–1654. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]
- 4.Rohacek M, Weisser M, Kobza R, Schoenenberger AW, Pfyffer GE, Frei R, Erne P, Trampuz A. 2010. Bacterial colonization and infection of electrophysiological cardiac devices detected with sonication and swab culture. Circulation 121:1691–1697. doi: 10.1161/CIRCULATIONAHA.109.906461. [DOI] [PubMed] [Google Scholar]
- 5.Rieger UM, Pierer G, Luscher NJ, Trampuz A. 2009. Sonication of removed breast implants for improved detection of subclinical infection. Aesthetic Plast Surg 33:404–408. doi: 10.1007/s00266-009-9333-0. [DOI] [PubMed] [Google Scholar]
- 6.Trampuz A, Piper KE, Hanssen AD, Osmon DR, Cockerill FR, Steckelberg JM, Patel R. 2006. Sonication of explanted prosthetic components in bags for diagnosis of prosthetic joint infection is associated with risk of contamination. J Clin Microbiol 44:628–631. doi: 10.1128/JCM.44.2.628-631.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Portillo ME, Corvec S, Borens O, Trampuz A. 2013. Propionibacterium acnes: an underestimated pathogen in implant-associated infections. Biomed Res Int 2013:804391. doi: 10.1155/2013/804391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Portillo ME, Salvado M, Alier A, Martinez S, Sorli L, Horcajada JP, Puig L. 2014. Advantages of sonication fluid culture for the diagnosis of prosthetic joint infection. J Infect 69:35–41. doi: 10.1016/j.jinf.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Barrack RL, Harris WH. 1993. The value of aspiration of the hip joint before revision total hip arthroplasty. J Bone Joint Surg Am 75:66–76. [DOI] [PubMed] [Google Scholar]
- 10.Hughes JG, Vetter EA, Patel R, Schleck CD, Harmsen S, Turgeant LT, Cockerill FR III. 2001. Culture with BACTEC Peds Plus/F bottle compared with conventional methods for detection of bacteria in synovial fluid. J Clin Microbiol 39:4468–4471. doi: 10.1128/JCM.39.12.4468-4471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Velay A, Schramm F, Gaudias J, Jaulhac B, Riegel P. 2010. Culture with BACTEC Peds Plus bottle compared with conventional media for the detection of bacteria in tissue samples from orthopedic surgery. Diagn Microbiol Infect Dis 68:83–85. doi: 10.1016/j.diagmicrobio.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Minassian AM, Newnham R, Kalimeris E, Bejon P, Atkins BL, Bowler IC. 2014. Use of an automated blood culture system (BD BACTEC) for diagnosis of prosthetic joint infections: easy and fast. BMC Infect Dis 14:233. doi: 10.1186/1471-2334-14-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen H, Tang J, Wang Q, Jiang Y, Zhang X. 2014. Sonication of explanted prosthesis combined with incubation into BD Bactec bottles for pathogen diagnosis of prosthetic joint infection. J Clin Microbiol 53:777–781. doi: 10.1128/JCM.02863-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janz V, Wassilew GI, Hasart O, Matziolis G, Tohtz S, Perka C. 2013. Evaluation of sonicate fluid cultures in comparison to histological analysis of the periprosthetic membrane for the detection of periprosthetic joint infection. Int Orthop 37:931–936. doi: 10.1007/s00264-013-1853-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Achermann Y, Vogt M, Leunig M, Wust J, Trampuz A. 2010. Improved diagnosis of periprosthetic joint infection by multiplex PCR of sonication fluid from removed implants. J Clin Microbiol 48:1208–1214. doi: 10.1128/JCM.00006-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trampuz A, Hanssen AD, Osmon DR, Mandrekar J, Steckelberg JM, Patel R. 2004. Synovial fluid leukocyte count and differential for the diagnosis of prosthetic knee infection. Am J Med 117:556–562. doi: 10.1016/j.amjmed.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 17.Schinsky MF, Della Valle CJ, Sporer SM, Paprosky WG. 2008. Perioperative testing for joint infection in patients undergoing revision total hip arthroplasty. J Bone Joint Surg Am 90:1869–1875. doi: 10.2106/JBJS.G.01255. [DOI] [PubMed] [Google Scholar]
- 18.Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Osmon DR, Mandrekar JN, Cockerill FR, Steckelberg JM, Greenleaf JF, Patel R. 2007. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med 357:654–663. doi: 10.1056/NEJMoa061588. [DOI] [PubMed] [Google Scholar]
- 19.Portillo ME, Salvado M, Trampuz A, Plasencia V, Rodriguez-Villasante M, Sorli L, Puig L, Horcajada JP. 2013. Sonication versus vortexing of implants for diagnosis of prosthetic joint infection. J Clin Microbiol 51:591–594. doi: 10.1128/JCM.02482-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holinka J, Bauer L, Hirschl AM, Graninger W, Windhager R, Presterl E. 2011. Sonication cultures of explanted components as an add-on test to routinely conducted microbiological diagnostics improve pathogen detection. J Orthop Res 29:617–622. doi: 10.1002/jor.21286. [DOI] [PubMed] [Google Scholar]
- 21.Piper KE, Jacobson MJ, Cofield RH, Sperling JW, Sanchez-Sotelo J, Osmon DR, McDowell A, Patrick S, Steckelberg JM, Mandrekar JN, Fernandez Sampedro M, Patel R. 2009. Microbiologic diagnosis of prosthetic shoulder infection by use of implant sonication. J Clin Microbiol 47:1878–1884. doi: 10.1128/JCM.01686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tande AJ, Patel R. 2014. Prosthetic joint infection. Clin Microbiol Rev 27:302–345. doi: 10.1128/CMR.00111-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monsen T, Lovgren E, Widerstrom M, Wallinder L. 2009. In vitro effect of ultrasound on bacteria and suggested protocol for sonication and diagnosis of prosthetic infections. J Clin Microbiol 47:2496–2501. doi: 10.1128/JCM.02316-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomez E, Cazanave C, Cunningham SA, Greenwood-Quaintance KE, Steckelberg JM, Uhl JR, Hanssen AD, Karau MJ, Schmidt SM, Osmon DR, Berbari EF, Mandrekar J, Patel R. 2012. Prosthetic joint infection diagnosis using broad-range PCR of biofilms dislodged from knee and hip arthroplasty surfaces using sonication. J Clin Microbiol 50:3501–3508. doi: 10.1128/JCM.00834-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Portillo ME, Salvado M, Sorli L, Alier A, Martinez S, Trampuz A, Gomez J, Puig L, Horcajada JP. 2012. Multiplex PCR of sonication fluid accurately differentiates between prosthetic joint infection and aseptic failure. J Infect 65:541–548. doi: 10.1016/j.jinf.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 26.Berbari EF, Marculescu C, Sia I, Lahr BD, Hanssen AD, Steckelberg JM, Gullerud R, Osmon DR. 2007. Culture-negative prosthetic joint infection. Clin Infect Dis 45:1113–1119. doi: 10.1086/522184. [DOI] [PubMed] [Google Scholar]
- 27.Cazanave C, Greenwood-Quaintance KE, Hanssen AD, Karau MJ, Schmidt SM, Gomez Urena EO, Mandrekar JN, Osmon DR, Lough LE, Pritt BS, Steckelberg JM, Patel R. 2013. Rapid molecular microbiologic diagnosis of prosthetic joint infection. J Clin Microbiol 51:2280–2287. doi: 10.1128/JCM.00335-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tande AJ, Cunningham SA, Raoult D, Sim FH, Berbari EF, Patel R. 2013. A case of Q fever prosthetic joint infection and description of an assay for detection of Coxiella burnetii. J Clin Microbiol 51:66–69. doi: 10.1128/JCM.02352-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Portillo ME, Salvado M, Alier A, Sorli L, Martinez S, Horcajada JP, Puig L. 2013. Prosthesis failure within 2 years of implantation is highly predictive of infection. Clin Orthop Relat Res 471:3672–3678. doi: 10.1007/s11999-013-3200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]