Abstract
We report a case of babesiosis in a traveler from India who was diagnosed with malaria on the basis of blood smears. Pan-Plasmodium PCR was positive, though species-specific assays were negative. Reexamination of blood smears and Babesia-specific PCR confirmed babesiosis. We highlight the overlapping clinical and diagnostic features of malaria and babesiosis and the potential cross-reactivity of Plasmodium primers in cases of babesiosis.
CASE REPORT
A 77-year-old male citizen of India traveled to Canada for the purpose of “visiting friends and relatives” (VFR), arriving 2 weeks prior to his presentation to the emergency room. He presented with a 16-day history of fever, anorexia, headache, myalgia, and fatigue. Comorbidities included type II diabetes and hypertension.
On examination, the patient appeared well. Temperature was 39.2°C, heart rate was 78 beats per minute (bpm), and blood pressure was 120/60 mm Hg. There was no rash or lymphadenopathy. Laboratory investigations revealed anemia and thrombocytopenia, with a hemoglobin of 97 g/liter (normal, 140 to 160 g/liter), a white blood cell (WBC) count of 7.3 × 109/liter (normal, 4 × 109 to 11 × 109/liter), and a platelet count of 81 × 109/liter (normal, 150 × 109 to 400 × 109/liter). Hepatic transaminases were 71 U/liter (aspartate transaminase [AST]) and 73 U/liter (alanine aminotransferase [ALT]) (normal, <40 U/liter). Creatinine (71 μmol/liter) revealed normal renal function.
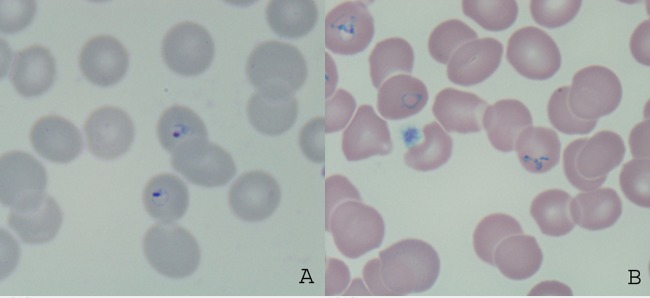
Thick and thin blood films for malaria were positive for ring stages of Plasmodium spp. at a parasitemia of 6% (Fig. 1A), most suspicious for Plasmodium falciparum. However, a rapid diagnostic test (RDT) for malaria was negative (BinaxNOW Malaria; Alere, ME). Whole blood and thin smears were then sent from the hospital laboratory to the parasitology section of the provincial reference laboratory, Public Health Ontario Laboratory (PHOL), for confirmation of Plasmodium species and quantitation of parasitemia.
FIG 1.
(A) Ring-stage trophozoites of Babesia spp. visualized by Giemsa staining and thin-film microscopy. Note the absence of Schuffner's stippling and unenlarged erythrocytes. (B) Pyriform and triad appearance of Babesia spp. visualized by Giemsa staining and thin-film microscopy. Note the pleiomorphic ring stages.
At PHOL, Giemsa-stained thin films were prepared, the RDT (BinaxNOW) was repeated, and genus- and species-specific real-time PCR assays were initiated. DNA extraction and quantitative real-time PCR (qPCR) were conducted to confirm species as previously described (1, 2). In brief, human β2-microglobulin (B2MG) extraction control, Plasmodium genus-specific, P. falciparum/Plasmodium vivax species-specific duplex, and Plasmodium malariae/Plasmodium ovale species-specific duplex qPCRs were performed as previously described (1, 2, 3). All qPCR assays were run using an ABI 7900HT real-time PCR system and under the following conditions: 50°C for 2 min, 95°C for 10 min, and 45 cycles of 95°C for 15 s and 60°C for 1 min. TaqMan universal PCR master mix (12.5 μl; Life Technologies), 5 μl of DNA, and primers and probes at concentrations reported previously were used for a final volume of 25 μl per reaction. All qPCR amplification curves were analyzed using a manual CT threshold of 0.02 and an automatic baseline. A result was called positive if the CT value was <40 for B2MG, Plasmodium genus-specific, and P. falciparum/P. vivax species-specific PCRs and <38 for P. malariae/P. ovale species-specific PCR in the presence of a logarithmic amplification curve. A true negative would be characterized by the absence of a smooth, logarithmic amplification curve at a CT of <40 or <38, respectively.
Examination of Giemsa-stained thin smears revealed small ring-stage trophozoites and the presence of pyriform triads resembling Maltese crosses (Fig. 1B), with a parasitemia of 6.9%. There was no evidence of Schuffner's stippling and no enlargement of parasitized erythrocytes (Fig. 1B). The RDT was negative. The pan-Plasmodium qPCR was positive, with a CT value of 35, which would correspond to a parasitemia of <0.1%. Species-specific duplex qPCR was negative for P. falciparum, P. vivax, P. ovale, and P. malariae. Given the highly characteristic appearance of parasite morphology on thin-film examination, a report of presumptive Babesia spp. was issued to the hospital. In addition, two qPCR assays were performed for Babesia microti, a presumptive assay for the η subunit of the chaperonin-containing t-complex polypeptide l (CCTη) and a confirmatory assay for the 18S rRNA gene (4, 5), which are used routinely at the National Microbiology Laboratory in Winnipeg, Canada, and demonstrate no cross-reactivity with P. falciparum, P. vivax, or other tick-borne pathogens such as Borrelia burgdorferi or Anaplasma phagocytophilum. CCTη PCR was carried out using 400 nM B. microti CCTη forward primer 5′ CAAGTTGGAGGCAATTCATAGC 3′, reverse primer 5′ CACAGCTTCCCAAACAAGAGTC 3′, and a 125 nM concentration of the probe 5′ 6FAM-ACGAGTCCTCCTGTTGCTTTGGCC-MGB 3′. 18S rRNA PCR was performed with a 200 nM concentration of the B. microti 18S forward primer 5′AGCCATGCATGTCTTAGTATAAGCTTT 3′, reverse primer 5′ CACGGTTATCCATGTAAAACGAACA 3′, and a 100 nM concentration of the probe 5′ 6FAM-AATGGCTCATTAAAACAGTTATAG-MGB 3′. Running conditions were the same as those for the malaria qPCR described above. The result was positive, with a CT value of 20 and a logarithmic amplification curve.
Upon further investigation of the clinical history, the patient acknowledged a 6-week stopover in Massachusetts near the New Hampshire border before his arrival from India in Canada. There, he stayed with other family residing in a wooded area, and the patient acknowledged finding ticks on his person prior to and after his arrival in Canada. He was treated with a full course of atovaquone and clindamycin and recovered uneventfully. Lyme serologic testing was reactive, and he was also given a 3-week course of doxycycline.
Given the false-positive pan-Plasmodium qPCR assay, we BLAST searched the Babesia 18S region in silico to elucidate sequence homology with our Plasmodium primers and probe. Sequence homologies of our Plasmodium genus primers and probe to the 18S rRNA Babesia microti region were as follows: forward primer, 54%; reverse primer, 85%; and probe, 94%.
Malaria remains the top specific cause of fever in the returned traveler (6, 7), and India is the top single source country for malaria cases evaluated at Canadian GeoSentinel Surveillance Network sites (8). Every year, travelers returning from the tropics die of P. falciparum malaria (9); thus, fever in the returned traveler should be construed as a medical emergency warranting immediate exclusion of malaria as the underlying cause.
Travelers traveling for the purpose of VFR are overrepresented among cases of malaria imported to North America (7, 10), and this may certainly bias the evaluation of febrile travelers returning from areas of particularly high risk for malaria, including India. This case underscores the need for a detailed travel history, as outlined in the initial assessment guidelines for fever in the returning international traveler (11).
As with the similar clinical features of malaria and babesiosis, the laboratory diagnostic features of each infection may overlap (Table 1). The appearance of ring-stage trophozoites of Babesia is very similar to that of P. falciparum (Fig. 1), and the two may be easily confused in routine diagnostic laboratories. Like P. falciparum, the ring stages of Babesia are small, and parasitized erythrocytes remain unenlarged. In addition, babesiosis tends to cause parasitemia of >1%, which is also consistent with falciparum malaria, and the classic Maltese cross morphology may be inapparent to the untrained observer and sufficiently rare as to go unnoticed by the trained observer. Differences such as the more pleiomorphic ring stages of Babesia (Fig. 1B) compared to P. falciparum and the higher proportion of extracellular forms may be subtle. The performance of rapid diagnostic tests for nonfalciparum malaria is often poor, with sensitivities ranging from 30 to 70% for P. ovale and P. vivax, respectively (2, 12). Thus, a positive thin smear by microscopy with a negative RDT may be erroneously attributed to nonfalciparum malaria, rather than babesiosis, especially when clinical and travel details are absent from the malaria requisition or patient's chart. In a situation where parasitemia is high and the RDT is negative, additional history should be gathered and reference-level diagnostics obtained.
TABLE 1.
Comparison of expected malaria diagnostic test results in malaria versus babesiosis
| Test | Expected result(s) for: |
|
|---|---|---|
| Babesia spp. | Plasmodium spp. | |
| Thin-film microscopy | Rings; tetrads (Maltese cross; rarely seen); parasitemia of >5% (common) | Rings; amoeboid trophozoites; schizonts; gametocytes; Schuffner's dots for P. vivax or P. ovale; parasitemia of >5% (less common) |
| Malaria rapid diagnostic test | Negative | Positive for P. falciparum with sensitivity of ≥95% at parasite density of 200 per μl blood; less sensitive for nonfalciparum species |
| Malaria qPCR | May be cross-reactive with standard 18S genus level assays; high CT value out of proportion to parasitemia observed on thin film | Logarithmic amplification curve with a low (congruent) CT value at a parasitemia of >1% |
Real-time PCR is often used in reference laboratories to resolve discrepancies between malaria diagnostics, such as smear and RDT. In our laboratory, our pan-Plasmodium assay targets the 18S rRNA region of the parasite, which happens to share a high degree of sequence homology with the other human hemosporidian, Babesia; hence the false positivity of malaria PCR in this case. As observed in this case, a high CT value on qPCR in the context of high parasitemia by thin-film microscopy should raise the suspicion of a competing diagnosis and false positivity of the assay. Indeed, when Babesia-specific qPCR was performed, the low CT value was more congruent with what would be expected from a specimen with 6.9% parasitemia.
In summary, the experience of our patient highlights the need for a thorough travel history, consideration of all possible travel-related diagnoses, and the limitations of reference laboratory tests, including PCR, that are used for malaria confirmation. Discrepancies in malaria diagnostics should be investigated and resolved by arbiter assays at the reference level.
REFERENCES
- 1.Khairnar K, Martin D, Lau R, Ralevski F, Pillai DR. 2009. Multiplex real-time quantitative PCR, microscopy and rapid diagnostic immuno-chromatographic tests for the detection of Plasmodium spp: performance, limit of detection analysis and quality assurance. Malar J 8:284. doi: 10.1186/1475-2875-8-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phuong M, Lau R, Ralevski F, Boggild AK. 2014. Sequence-based optimization of a quantitative real-time PCR assay for the detection of Plasmodium ovale and P. malariae. J Clin Microbiol 52:1068–1073. doi: 10.1128/JCM.03477-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shokoples SE, Ndao M, Kowalewska-Grochowska K, Yanow SK. 2009. Multiplexed real-time PCR assay for discrimination of Plasmodium species with improved sensitivity for mixed infections. J Clin Microbiol 47:975–980. doi: 10.1128/JCM.01858-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakajima R, Masayoshi T, Kazunori O, Zamoto-Niikura A, Wei Q, Kawabuchi-Kurata T, Nishida A, Ishihara C. 2009. Babesia microti-group parasites compared phylogenetically by complete sequencing of the CCTη gene in 36 isolates. J Vet Med Sci 71:55–68. doi: 10.1292/jvms.71.55. [DOI] [PubMed] [Google Scholar]
- 5.Bullard JMP, Ahsanuddin AN, Perry AM, Lindsay R, Iranpour M, Dibernardo A, Van Caeseele PG. 2014. The first case of locally acquired tick-borne Babesia microti infection in Canada. Can J Infect Dis Med Microbiol 25:e87–e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson ME, Weld LH, Boggild A, Keystone JS, Kain KC, von Sonnenberg F, Schwartz E. 2007. Fever in returned travelers: results from the GeoSentinel Surveillance Network. Clin Infect Dis 44:1560–1568. doi: 10.1086/518173. [DOI] [PubMed] [Google Scholar]
- 7.Leder K, Torresi J, Libman MD, Cramer JP, Castelli F, Schlagenhauf P, Wilder-Smith A, Wilson ME, Keystone JS, Schwartz E, Barnett ED, von Sonnenburg F, Brownstein JS, Cheng AC, Sotir MJ, Esposito DH, Freedman DO, GeoSentinel Surveillance Network. 2013. GeoSentinel surveillance of illness in returned travelers, 2007-2011. Ann Int Med 158:456–468. doi: 10.7326/0003-4819-158-6-201303190-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boggild AK, Geduld J, Libman M, Ward BJ, McCarthy AE, Hajek J, Ghesquiere W, Vincelette J, Kuhn S, Freedman DO, Kain KC. 2014. Travel-acquired infections in Canada: CanTravNet 2011–2012. Can Commun Dis Rep 40:313–325. http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/14vol40/dr-rm40-16/dr-rm40-16-surv-eng.php. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensenius M, Han PV, Schlagenhauf P, Schwartz E, Parola P, Castelli F, von Sonnenburg F, Loutan L, Leder K, Freedman DO, GeoSentinel Surveillance Network. 2013. Acute and potentially life-threatening tropical diseases in western travelers—a GeoSentinel multicenter study, 1996-2011. Am J Trop Med Hyg 88:397–404. doi: 10.4269/ajtmh.12-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freedman DO. 2008. Malaria prevention in short-term travelers. N Engl J Med 359:603–612. doi: 10.1056/NEJMcp0803572. [DOI] [PubMed] [Google Scholar]
- 11.Boggild A, Ghesquiere W, McCarthy A, Committee to Advise on Tropical Medicine and Travel (CATMAT). 2011. Fever in the returning international traveller: initial assessment guidelines. Can Commun Dis Rep 37:ASC-3 http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/11vol37/acs-3/index-eng.php Accessed 21 January 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maltha J, Gillet P, Jacobs J. 2013. Malaria rapid diagnostic tests in travel medicine. Clin Microbiol Infect 19:408–415. doi: 10.1111/1469-0691.12152. [DOI] [PubMed] [Google Scholar]