Abstract
Candida inconspicua and Candida (Pichia) norvegensis are two emerging pathogenic species that exhibit reduced susceptibility to azole derivatives. Conventional (biochemical) approaches do not readily differentiate between the two species. The first aim of this work was to analyze the performance of biochemical, proteomic (matrix-assisted laser desorption ionization–time of flight [MALDI-TOF]), and molecular approaches in the precise identification of these species. These results then led us to sequence 3 genomic loci, i.e., the internal transcribed spacer (ITS) region of the ribosomal DNA (rDNA), the D1/D2 domain of the 28S rDNA, and the elongation factor 1α (EF-1α) gene, either directly or following cloning, of 13 clinical isolates and 9 reference strains belonging to the 5 species included in the Pichia cactophila clade, namely, Pichia cactophila, Pichia insulana, C. inconspicua, C. norvegensis, and P. pseudocactophila. Finally, isolates of C. inconspicua were challenged for sexual reproduction on the appropriate medium. Our results show that EF-1α sequencing and proteic profiling by MALDI-TOF are the two most efficient approaches to distinguish between C. norvegensis and C. inconspicua. As a characteristic of the P. cactophila clade, we found multiple alleles of the rDNA regions in certain strains belonging to the tested species, making ITS or D1/D2 sequencing not appropriate for identification. Whatever the method of identification, including MALDI-TOF and EF-1α sequencing, none could differentiate C. inconspicua from P. cactophila. The results of phylogenetic analysis and the generation of asci from pure cultures of all C. inconspicua strains both support the identification of P. cactophila as the teleomorph of C. inconspicua.
INTRODUCTION
Candida spp. remain the predominant cause of invasive fungal infections (1). The incidence of candidemia, the main clinical form of invasive candidiasis, significantly increased in the 1980s (2). Concomitantly, non-C. albicans species have emerged among the causative agents of candidiasis (3). The reasons for this emergence remain unclear, but selective pressure due to a larger use of azole derivatives has been suggested (4). Indeed, in addition to C. glabrata and C. krusei, the two main fluconazole-resistant species isolated from patients with candidemia, a number of fluconazole-resistant species are now regularly isolated from deep-seated infections. Together, these “rare” species may account for as much as 10% of the etiologic agents in some medical centers (5).
Candida inconspicua and Candida norvegensis (teleomorph [tel.]: Pichia norvegensis) are among those fluconazole-resistant emerging species that are more frequently isolated from invasive infections (6). In addition to this increasing concern, these two species are known to be difficult to differentiate from each other using routine techniques such as biochemical panels. Indeed, auxanograms such as ID32C differentiate the two species only according to the hydrolysis of esculin, a phenotypic trait whose variability is well known (7). These quite similar biochemical profiles may reflect the close phylogenetic relationship between these 2 species. Indeed, both species belong to the Pichia cactophila clade, which, in addition to C. inconspicua and C. norvegensis, encompasses three teleomorphic stages, namely, P. cactophila, Pichia insulana, and Pichia pseudocactophila (8, 9).
For such “difficult-to-identify” yeast species, the molecular approach, mainly using sequence analysis of the internal transcribed spacer (ITS) or D1/D2 domain of the large subunit of the ribosomal DNA (rDNA), is considered the reference method for a definitive identification. However, direct sequencing of rDNA fragments amplified from C. norvegensis and C. inconspicua is frequently unsuccessful (personal data).
Here, we report on the performance of biochemical, proteomic (matrix-assisted laser desorption ionization–time of flight [MALDI-TOF] mass spectrometry), and molecular approaches, including sequencing of a fragment of the transcription elongation factor 1α (EF-1α) gene, for the specific identification of clinical isolates of C. inconspicua and C. norvegensis. Sequence-based identification allowed us to better characterize the rDNA content of these species. This, along with a study of the sexual reproduction of these yeasts, led us to suggest that P. cactophila is the sexual morph of Candida inconspicua.
MATERIALS AND METHODS
Strains.
Thirteen clinical isolates identified using a biochemical panel (ID32C; bioMérieux, Marcy l'Etoile, France) as either C. inconspicua (n = 9) or C. norvegensis (n = 4) were used in this study (Table 1). In addition, 9 reference strains of P. cactophila (n = 5), C. inconspicua (n = 1), P. norvegensis (n = 1), P. insulana (n = 1), and P. pseudocactophila (n = 1) were included. Detailed references of the strains used in this study are presented in Table S1 in the supplemental material.
TABLE 1.
Biological characteristics of 13 clinical isolates and 8 CBS reference strains belonging to species of the P. cactophila cladea
| Strain | Identification result(s) |
Molecular analysis |
||||||
|---|---|---|---|---|---|---|---|---|
| ID32C (esculin hydrolysis) identification | MALDI-TOF (no. of replicates with LS > 2) | ITS | Best hit | D1/D2 | Best hit(s) | EF-1 | Best hit(s) | |
| SA952 | C. norvegensis (Pos) | P. cactophila (4) | PSC | NI | USC | NI | HPM | C. inconspicua |
| SA1635 | C. inconspicua (Neg) | P. cactophila (4) | PSC | NI | USC | NI | HPM | C. inconspicua |
| SA1729 | C. inconspicua (Neg) | C. inconspicua (2), P. cactophila (2) | PSC | NI | PSC | NI | HPM | C. inconspicua |
| SA1730 | C. norvegensis (Pos) | C. inconspicua (2), P. cactophila (2) | PSC | NI | HPM | C. inconspicua, P. cactophila | HPM | C. inconspicua |
| SA1457 | C. inconspicua (Neg) | C. inconspicua (3), P. cactophila (1) | PSC | NI | USC | NI | HPM | C. inconspicua |
| SA1458 | C. inconspicua (Neg) | P. cactophila (4) | PSC | NI | USC | NI | HPM | C. inconspicua |
| SA1460 | C. norvegensis (Pos) | P. norvegensis (3) | PSC | P. norvegensis | USC | NI | CC | C. norvegensis |
| SA1459 | C. inconspicua (Neg) | P. cactophila (4) | PSC | NI | USC | NI | HPM | C. inconspicua |
| SA1461 | C. inconspicua (Neg) | P. cactophila (3) | CC | C. inconspicua | USC | NI | HPM | C. inconspicua |
| SA1462 | C. inconspicua (Neg) | C. inconspicua (2), P. cactophila (2) | PSC | NI | USC | NI | HPM | C. inconspicua |
| SA1463 | C. inconspicua (Neg) | C. inconspicua (2), P. cactophila (2) | CC | C. inconspicua | USC | NI | HPM | C. inconspicua |
| SA2546 | C. norvegensis (Pos) | P. norvegensis (4) | USC | NI | HPM | P. norvegensis | CC | C. norvegensis |
| SA2185 | C. inconspicua (Neg) | P. cactophila (4) | PSC | NI | PSC | NI | HPM | C. inconspicua, P. cactophila |
| CBS6926 | C. inconspicua (Neg) | P. cactophila (4) | USC | NI | HPM | C. inconspicua, P. cactophila | HPM | C. inconspicua |
| CBS7059 | C. inconspicua (Neg) | P. cactophila (4) | PSC | NI | PSC | NI | HPM | C. inconspicua |
| CBS7103 | C. inconspicua (Neg) | P. cactophila (4) | PSC | NI | PSC | NI | HPM | C. inconspicua |
| CBS2155 | NI (Neg) | P. cactophila (4) | CC | C. inconspicua | PSC | NI | HPM | C. inconspicua |
| CBS6928 | C. inconspicua (Neg) | P. cactophila (4) | CC | C. inconspicua | HPM | C. inconspicua, P. cactophila | HPM | C. inconspicua |
| CBS180 | C. inconspicua (Neg) | P. cactophila (4) | CC | C. inconspicua | HPM | C. inconspicua, P. cactophila | HPM | C. inconspicua |
| CBS6564 | C. norvegensis (Pos) | P. norvegensis (4) | CC | P. norvegensis | HPM | P. norvegensis | CC | P. norvegensis |
| CBS6929 | NI (Neg) | NI | PSC | NI | HPM | P. pseudocactophila | CC | P. pseudocactophila |
| CBS11169 | C. inconspicua (Neg) | NI | PSC | C. inconspicua | HPM | P. insulana | CC | P. cactophila, C. inconspicua, P. pseudocactophila |
LS, log score; Pos, positive; Neg, negative; NI, nonidentified; PSC, partially superimposed chromatograms; USC, uninterpretable superimposed electropherograms; HPM, heterozygous point mutations; CC, clear chromatograms.
Biochemical-phenotypic identification.
All strains were identified with the use of an ID32C auxanogram panel (bioMérieux), according to the manufacturer's recommendations. Particular attention was paid to the esculin hydrolysis, the unique trait thought to be able to distinguish between C. inconspicua (lack of hydrolysis) and C. norvegensis (presence of hydrolysis). The percentage of identification based on the relative similarity between the biochemical profile of the tested strain and the analyzed profiles was recorded. According to the manufacturer, the identification is considered very good above 99%, good between 90% and 99%, and acceptable between 80% and 90%. It is worth noting that P. cactophila, P. insulana, and P. pseudocactophila are not included in the ID32C database.
MALDI-TOF mass spectrometry.
Strains were analyzed by MALDI-TOF mass spectrometry using a Bruker Microflex mass spectrometer (Bruker, Wissembourg, France). Briefly, strains were cultured on CHROMagar Candida plates (BD, Pont-de-Claix, France) during 48 h at 37°C. Proteins from two yeast colonies were extracted using an ethanol-acetonitrile-formic acid treatment following the manufacturer's recommendations. Extracts were then spotted in quadruplicate onto a MALDI-TOF anchor chip and submitted for analysis. Bruker Biotyper version 2.0 was used for identification. A log score of >2 is considered to represent confidence for species identification according to the manufacturer's recommendations. Based on previous experiments (personal data), we also considered a species identification to be correct when at least 3 of the 4 spectra reached a log score of >1.7 for the same species. Bruker database version 2.0 contains representatives of C. inconspicua (one strain), P. norvegensis (five strains), and P. cactophila (three strains). Neither P. pseudocactophila nor P. insulana is included in the database.
Molecular analysis.
All strains were subjected to direct sequencing of the D1/D2 domain and the ITS region of the ribosomal DNA and a fragment of the EF-1α gene. Briefly, DNA from isolated colonies obtained after 48 h was extracted using a heat shock-Chelex resin protocol (10) on CHROMagar Candida medium incubated at 37°C. Amplification was performed in a 50-μl reaction mixture containing a 0.5 μM concentration of each primer (Eurogentec, Angers, France), a 0.25 mM concentration of each deoxynucleoside triphosphate (equimolar concentrations of dATP, dCTP, dGTP, and dTTP) (New England BioLabs, Evry, France), 0.5 U of DreamTaq polymerase (Fermentas, Saint Rémy-lès-Chevreuse, France), and 5 μl of DNA. The ITS1-ITS4 and NL1-NL4 primer pairs used for the amplification of the ITS region and the D1/D2 domain, respectively, have been published previously (11, 12). A specific primer pair (EF1_F [GTGTCGGTGAATTCGAAGCTGGTA] and EF1_R [GGTGGGTATTCAGTGAAAGC]) was designed to amplify a ca. 780-bp fragment of the EF-1α gene of the considered species.
Direct sequencing was performed using a BigDye Terminator V3.1 kit (Life Technologies, Saint Aubin, France) and run in a 3500xL Dx genetic analyzer (Life Technologies). Chromatograms were then edited and manually corrected using BioEdit software version 7.0.9.0.
Cloning of the rDNA intergenic spacer region.
Because the chromatograms of the ITS region obtained through direct sequencing frequently resulted in superimposed traces, we decided to clone those amplicons before sequencing. The PCR products were purified using a MinElute PCR purification kit (Qiagen, Courtaboeuf, France) and then cloned into the plasmid vector PCR II Topo (Invitrogen). The plasmid was used to transform chemocompetent NeB 5-alpha Escherichia coli cells (New England BioLabs), according to the manufacturer's instructions. For each amplicon (i.e., strain), 7 to 20 bacterial clones were further sequenced with the primers used for amplification. After plasmid DNA extraction using a PureLink Quick Plasmid Miniprep kit (Life Technologies), recombinant plasmids were sequenced in both directions using a BigDye Terminator v3.1 kit (Sanger ABI 3730xl; GATC Biotech, Mulhouse, France).
Phylogenetic analysis.
Sequences were aligned using Clustal X software (version 2.0.10) (13) and were used to draw trees using the maximum parsimony and neighbor-joining methods implemented in the MEGA5.2.2 software (14).
Sexual reproduction.
In order to investigate the potential sexual reproduction of C. inconspicua, strains were grown on Sabouraud chloramphenicol gentamicin agar (Bio-Rad, Marnes la Coquette, France) and were then spotted onto potassium acetate agar plates (yeast extract [0.25%], glucose [0.1%], potassium acetate [10%], agar [20%]) and incubated at 30°C for 1 week. Strains were spotted individually on the potassium acetate plates but also in combination with every other strain used in this study. As it readily produced asci, P. cactophila CBS6926 was used alone as positive control. Each day, a thin smear of the culture was stained using the Wirtz stain that distinguishes the blastoconidia (stained in pink) from the ascospores (in green) (15).
Nucleotide sequence accession numbers.
Accession numbers of the new sequences described in this study are registered in GenBank under accession numbers KM252801 to KM252842.
RESULTS
Molecular analysis.
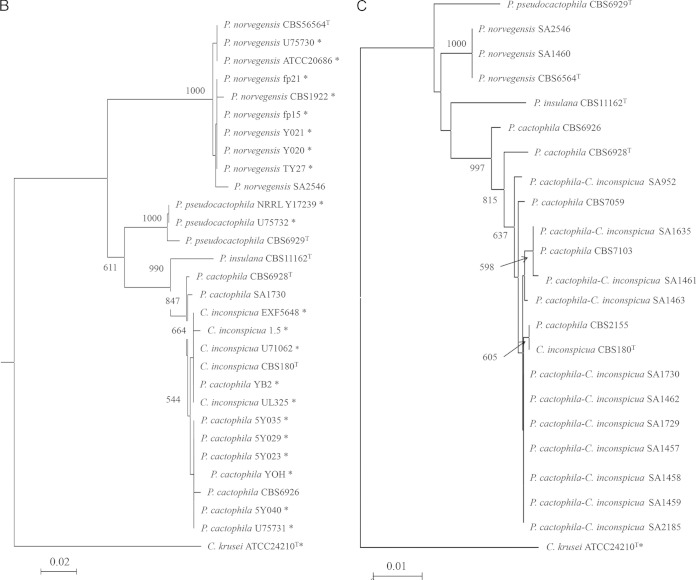
Analysis of the sequences of the ITS region is shown in Table 1 and in Table S3 in the supplemental material. Traces for one clinical isolate (SA2546) and one P. cactophila reference strain (CBS6926) were repeatedly unreadable throughout their length. In contrast, for six strains, a complete chromatogram was obtained. BLASTN comparison of the sequences obtained from C. inconspicua (CBS180) and P. cactophila (CBS2155 and CBS6928) reference strains and two clinical isolates retrieved C. inconspicua as the best hit with 99% identity (or 100%, omitting heterozygous point mutations). The sequence obtained from the P. norvegensis reference strain (CBS6564) matched with a P. norvegensis sequence in GenBank (99% identity). Five reference strains and 11 clinical isolates presented superimposed chromatograms that were partially interpretable. Sharp analysis of these chromatograms suggested the superimposition of multiple staggered traces (see Fig. S1 in the supplemental material). Thus, ITS amplicons from 5 P. cactophila reference strains were cloned into the PCR II Topo plasmid vector and 7 to 20 clones were sequenced for each amplicon. Unambiguous chromatograms were obtained in all cases. Six different alleles, ITSa to ITSf, were identified (Table 2). Alignment of these sequences showed insertions or deletions at positions 310 and/or 350. One to three different alleles were identified per strain, in accordance with the superimposed traces obtained from direct sequencing (Fig. 1).
TABLE 2.
Alignment of 6 different alleles of the ITS locus found in P. cactophila reference strains after cloning and sequencing
| ITS sequencea | No. of clones |
||||||
|---|---|---|---|---|---|---|---|
| CBS2155 (n = 10) | CBS6926 (n = 10) | CBS6928 (n = 20) | CBS7059 (n = 7) | CBS7103 (n = 15) | CBS2155 (n = 10) | ||
| ITSa | CCGTCTTTCGGTGGCTCCCCCGAAATGGAACGATTGCGGGCTTAG | 3 | 3 | 8 | |||
| ITSb | CC---TTTGGGTGGCTCCCCCGAAATGGAACGATTGCGGGCT-AG | 2 | |||||
| ITSc | CC---TTTGGGTGGCTCCCCCGAAATGGAACGATTGCGGGCTTAG | 10 | 6 | 5 | 10 | ||
| ITSd | CCAC--TTCGGTGGCTCCCCCGAAATGGAACGATTGCGGGCT-AG | 1 | |||||
| ITSe | CCGCC--TCGGTGGCTCCCCCGAAATGGAACGATTGCGGGCT-AG | 20 | 1 | ||||
| ITSf | CCGCC--TCGGTGGCTCCCCCGAAATGGAACGATTGCGGGCTTAG | 3 | |||||
| ··***·***·································*·· | |||||||
Data represent nucleotide sequences starting at position 308 of the ITS sequence of the CBS180 strain (GenBank accession number AB179767). Asterisks represent nucleotide divergences between alleles.
FIG 1.
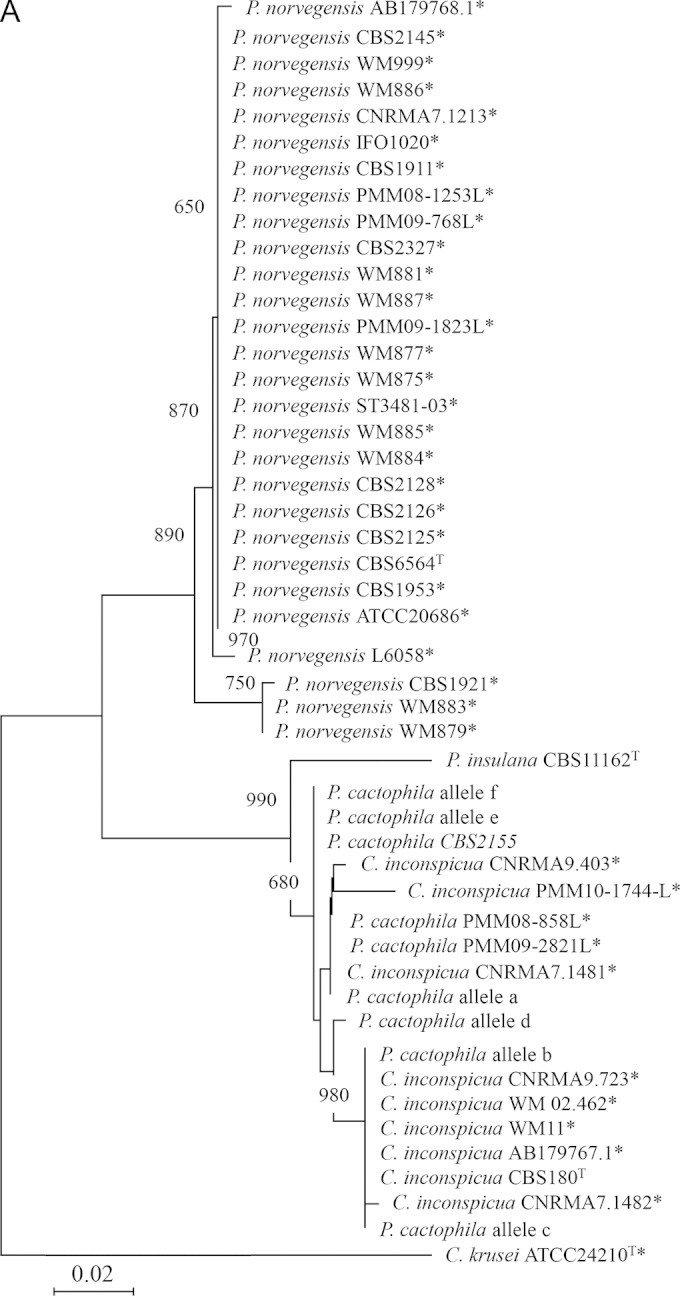
Phylogenetic trees built with the neighbor-joining method using sequences of clinical and reference strains belonging to the P. cactophila clade. Candida krusei was used as an outgroup. Bootstrap values are from 1,000 replicates and are given at branch nodes. Only bootstrap values over 750 are indicated. The tree is drawn to scale with branch lengths in the units of the number of nucleotide changes over the whole sequence. For the ITS locus, P. cactophila alleles a to f correspond to the different alleles characterized after cloning and sequencing the ITS region from different P. cactophila strains (see Table 2).
The analysis of the D1/D2 domain revealed somewhat similar results (Table 1; see also Table S4 in the supplemental material). Eight strains among 22 had chromatograms that could be read on both strands, on a length of 571 to 604 bp. BLASTN comparison of the sequences obtained from 2 P. cactophila (CBS6926 and CBS6928) and the C. inconspicua (CBS180) reference strains, as well as a clinical isolate (SA1730), returned C. inconspicua and P. cactophila as best hits with similar E values. One clinical isolate (SA2546) showed 99% nucleotide identity with P. norvegensis. BLASTN comparison of the sequences from the P. norvegensis, P. insulana, and P. pseudocactophila reference strains returned adequate identification results in all cases. In contrast, 9 clinical isolates presented direct traces that were fully uninterpretable. In addition, 3 reference strains and 2 clinical isolates had a clear chromatogram over only a limited sequence portion (51 to 108 bp). Sharp analysis of these chromatograms suggested, again, a combination of multiple staggered traces.
In contrast, clear chromatograms of EF-1α sequence were obtained for all the strains tested (Table 1; see also Table S2 in the supplemental material). For the 6 C. inconspicua and P. cactophila reference strains, and for 11 clinical isolates, BLASTN comparisons to GenBank data returned 2 best hits of either P. cactophila or C. inconspicua with similar E values. In these cases, 3 to 13 coincident peaks were found in the chromatograms of the forward and reverse sequences, suggestive of heterozygous point mutations in diploid cells. P. norvegensis was the best hit for the P. norvegensis reference strain and for 2 clinical isolates.
Phylogenetic analysis of the P. cactophila clade.
For each locus, we used our own sequences and sequences retrieved from GenBank to build phylogenetic trees using the neighbor-joining method (Fig. 1). In each case, Candida krusei (tel. Issatchenkia orientalis) ATCC 24210 served as the outgroup.
Overall, the topologies of the trees were similar whatever the locus analyzed. The P. norvegensis strains formed a cluster strongly supported by a bootstrap value of between 89% and 100%. Similarly, the P. insulana, C. inconspicua, and P. cactophila strains grouped into a cluster with a bootstrap value at 99.7% to 99.9%. The differentiation of a cluster gathering P. cactophila and C. inconspicua was variably supported according to the locus: 84.7% for the D1/D2 domain and 68% for the ITS region. Finally, P. cactophila strains can always be differentiated from C. inconspicua strains.
Interspecies and intraspecies variability.
The same sequences were used to estimate interspecies and intraspecies sequence divergence according to the locus (see Table S5 in the supplemental material). The interspecies variability between P. pseudocactophila, P. norvegensis, and P. insulana and an “artificial” group comprising the P. cactophila and C. inconspicua strains ranged between 0.015 and 0.093 according to the locus. In contrast, the interspecies variability between C. inconspicua and P. cactophila ranged between 0.001 and 0.011, values equivalent to those found for intraspecies variability, estimated for the other species tested in this study (see Table S5).
Sexual reproduction of C. inconspicua and P. cactophila.
Since both molecular and spectrometric approaches failed to clearly delineate C. inconspicua and P. cactophila, we hypothesized that these 2 species may represent the anamorph and the teleomorph of the same species, respectively. Subcultured on potassium acetate agar, in a pure culture or a culture mixed with any other strain, all the clinical isolates identified as either C. inconspicua or P. cactophila and the C. inconspicua CBS180 reference strain were able to form asci containing 1 to 4 ascospores within 1 week (Fig. 2). Similarly, we found sexual reproduction for P. pseudocactophila and C. (Pichia) norvegensis strains.
FIG 2.
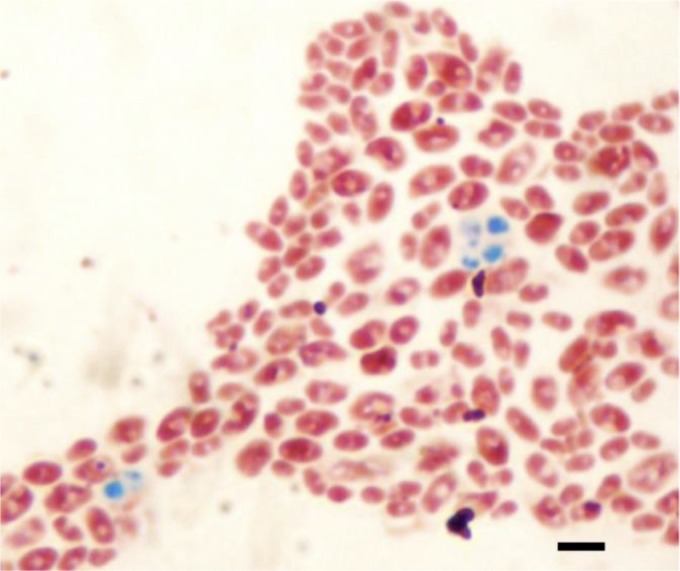
Example of sexual reproduction obtained with the C. inconspicua CBS180 strain. Strains were spotted on potassium acetate agar, and Wirtz staining was used to reveal the presence of ascospores (in green) and blastospores (in pink). The black bar represents 5 μm.
Performance of routine identification methods.
The performances were evaluated by comparison to the identification obtained through the EF-1α locus sequencing, considered the reference in this study (Table 1).
ID32C returned an identification of C. inconspicua for 14 strains molecularly identified as C. inconspicua or P. cactophila. Three C. norvegensis strains (one reference strain and two clinical isolates) were correctly identified. One P. cactophila reference strain (CBS2155) was not identified, and two clinical isolates of C. inconspicua, molecularly confirmed, were identified as C. norvegensis.
Reference strains of C. inconspicua (n = 1) and P. cactophila (n = 5) were identified as P. cactophila using MALDI-TOF analysis. For the 11 clinical strains molecularly identified as either C. inconspicua or P. cactophila, MALDI-TOF analysis returned an identification as either P. cactophila (n = 6; 55%) or P. cactophila-C. inconspicua (n = 5; 45%). All the C. (Pichia) norvegensis strains were correctly identified using MALDI-TOF analysis. Similar results were obtained using Sabouraud chloramphenicol gentamicin as the primary medium for isolation (data not shown).
DISCUSSION
Candida inconspicua and C. norvegensis are two emerging species whose specific identification is frequently incorrect (7). The first aim of this study was to compare the performances of biochemical, proteomic (MALDI-TOF), and molecular (direct sequencing) approaches for definitive identification.
From our results, it appears that sequencing the EF-1α locus is the most suitable approach for the identification of these species. Indeed, chromatograms are consistently easy to read, and the sequences are divergent enough to distinguish between all the different species of the clade, with the notable exception of P. cactophila and C. inconspicua (see below). Taking these results as the reference, we confirmed that identification based on ID32C may be not the most reliable approach since a misidentification between C. inconspicua and C. norvegensis occurred in two cases and one C. inconspicua strain was not identified. In contrast, these data confirmed the usefulness of MALDI-TOF analysis as a powerful tool to differentiate closely related species such as C. norvegensis and C. inconspicua. The lack of discrimination between P. cactophila and C. inconspicua seen using MALDI-TOF analysis, which has been shown to be able to discriminate between some cryptic species (16), was in agreement with the results of molecular methods and the hypothesis that C. inconspicua is the anamorph of P. cactophila (see below).
Analysis of ribosomal DNA sequences, notably, the D1/D2 domain of the large subunit and the ITS loci, is considered a method of choice for the identification of ascomycetous yeast species (17, 18). The rDNA of eukaryotes consists of a tandem repeat of a region that includes 4 genes, the 18S, 5.8S, 28S, and 5S units. In fungal genomes, there are generally a few dozen to a few thousand repeats that are arranged either in a single large cluster or in multiple tandem arrays found on one or a few chromosomes (19). It is considered that polymorphism between rDNA repeat units is rare, supporting the idea of concerted evolution for these regions. However, after cloning was performed, we found, in this study, the existence of multiple (as many as 3) alleles of the ITS region for some strains, including in some of the reference strains. This feature appeared to be a distinctive trait of the clade, since strains from all five species tested exhibit such a profile, while C. krusei (tel. I. orientalis), one of the most closely related species, differs on this point. Since rDNA alleles are usually similar in diploid yeast cells, this supports the idea of the existence of multiple nonhomologous loci of rDNA and/or a diversity of sequence among the tandem repeats of rDNA and/or the presence of hybrids in the clade. The latter hypothesis seems less plausible, as hybrids often lose one of the parental rDNA copies (20). The former has been demonstrated for members of the subphylum Pezizomycotina, which contained multiple dispersed copies of the 5S regions with multiple different types (21). This is also the case for other eukaryotes such as those belonging to the Apicomplexa phylum. For example, Plasmodium, Eimeria, and Toxoplasma spp. (22–24) are known to contain dispersed and highly divergent 18S rDNAs in their genome. Diversity of sequence among the repeats of rDNA has been shown for flatworms that may have a few distinct rRNA types maintained within the genome (25). Among the hemiascomycete yeasts, it is worth noting that C. glabrata possesses two clusters of rDNA but with identical sequences (19). On the other hand, Yarrowia (Candida) lipolytica harbors divergent rDNA sequences with extended microheterogeneity among the repeats and differences in the number of repeats (26). Additional studies are required to specify the molecular mechanisms involved in this phenomenon in the P. cactophila clade.
Several arguments led us to postulate that C. inconspicua and P. cactophila are the anamorph and teleomorph of the same species. First, it is noticeable that while MALDI-TOF analysis is well known for its ability in differentiating closely related species, even within species complexes, this method failed to distinguish between C. inconspicua and P. cactophila. Also, we found very limited nucleotide divergence between these 2 species in the loci tested, with values of variability at a level more in accordance with intraspecies variability. Finally, phylogenetic analyses using any of the three loci tested in this study failed to distinguish these 2 species but rather support the idea of a single cluster gathering C. inconspicua and P. cactophila strains. This is in agreement with a previous study by Kurtzman et al., who concluded that C. inconspicua and P. cactophila are conspecific (9). Indeed, in their study, the type strains of C. inconspicua and of P. cactophila, subjected to sequencing at 4 loci (D1/D2 domain, small subunit of the rDNA, EF-1α, and mitochondrial rDNA small subunit), were found to differ by only a single nucleotide insertion/deletion plus a nucleotide substitution in the D1/D2 domain.
The hypothesis that P. cactophila is the teleomorph of C. inconspicua was further supported by the evidence indicating that C. inconspicua does undergo sexual reproduction. Indeed, we showed that all our clinical strains of C. inconspicua-P. cactophila as well as the C. inconspicua reference strain were able to form asci. Within these structures, ascospores were very similar to those described for other Pichia species: evanescent asci containing 1 to 4 ascospores (27). Analysis of sequence chromatograms of the EF-1α locus that contained features suggestive of heterozygous point mutations supports the idea of the diploid nature of P. cactophila that has been previously reported (28). Those cells may convert to ascospores, but the heterothallic or homothallic pattern of sexuality has not been yet determined.
In conclusion, our report adds evidence that mass spectrometry MALDI-TOF analysis is a powerful tool for the identification of yeast species, including closely related ones that are difficult to distinguish using biochemical profiles. Our study also demonstrated that, in contrast to the vast majority of fungal species, the use of ITS or D1/D2 loci should be avoided for C. inconspicua-C. norvegensis identification due to the existence of multiple divergent alleles containing an insertion or deletion within a given strain. Finally, on the basis of molecular and spectrometric analyses and a study of the sexual reproduction, we strongly hypothesize that C. inconspicua is the anamorph stage of P. cactophila.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge S. Vellaissamy and members of the Laboratoire Commun de Biologie et Génétique Moléculaire for excellent technical assistance, V. Letscher, C. L'Ollivier, M. Cornet, and F. Dalle for kindly sending us clinical isolates, and Gianluigi Cardinali for kindly providing us the P. insulana strain.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.02913-14.
REFERENCES
- 1.Pfaller MA, Diekema DJ. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, Moss M. 2003. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 3.Trick WE, Fridkin SK, Edwards JR, Hajjeh RA, Gaynes RP. 2002. Secular trend of hospital-acquired candidemia among intensive care unit patients in the United States during 1989–1999. Clin Infect Dis 35:627–630. doi: 10.1086/342300. [DOI] [PubMed] [Google Scholar]
- 4.Pfaller MA, Diekema DJ, Rinaldi MG, Barnes R, Hu B, Veselov AV, Tiraboschi N, Nagy E, Gibbs DL. 2005. Results from the ARTEMIS DISK Global Antifungal Surveillance Study: a 6.5-year analysis of susceptibilities of Candida and other yeast species to fluconazole and voriconazole by standardized disk diffusion testing. J Clin Microbiol 43:5848–5859. doi: 10.1128/JCM.43.12.5848-5859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tortorano AM, Peman J, Bernhardt H, Klingspor L, Kibbler CC, Faure O, Biraghi E, Canton E, Zimmermann K, Seaton S, Grillot R. 2004. Epidemiology of candidaemia in Europe: results of 28-month European Confederation of Medical Mycology (ECMM) hospital-based surveillance study. Eur J Clin Microbiol Infect Dis 23:317–322. doi: 10.1007/s10096-004-1103-y. [DOI] [PubMed] [Google Scholar]
- 6.Guitard J, Angoulvant A, Letscher-Bru V, L'Ollivier C, Cornet M, Dalle F, Grenouillet F, Lacroix C, Vekhoff A, Maury E, Caillot D, Charles PE, Pili-Floury S, Herbrecht R, Raffoux E, Brethon B, Hennequin C. 2013. Invasive infections due to Candida norvegensis and Candida inconspicua: report of 12 cases and review of the literature. Med Mycol 51:795–799. doi: 10.3109/13693786.2013.807444. [DOI] [PubMed] [Google Scholar]
- 7.Majoros L, Kardos G, Belak A, Maraz A, Asztalos L, Csanky E, Barta Z, Szabo B. 2003. Restriction enzyme analysis of ribosomal DNA shows that Candida inconspicua clinical isolates can be misidentified as Candida norvegensis with traditional diagnostic procedures. J Clin Microbiol 41:5250–5253. doi: 10.1128/JCM.41.11.5250-5253.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganter PF, Cardinali G, Boundy-Mills K. 2010. Pichia insulana sp. nov., a novel cactophilic yeast from the Caribbean. Int J Syst Evol Microbiol 60:1001–1007. doi: 10.1099/ijs.0.014258-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurtzman CP, Robnett CJ, Basehoar-Powers E. 2008. Phylogenetic relationships among species of Pichia, Issatchenkia and Williopsis determined from multigene sequence analysis, and the proposal of Barnettozyma gen. nov., Lindnera gen. nov and Wickerhamomyces gen nov. FEMS Yeast Res 8:939–954. doi: 10.1111/j.1567-1364.2008.00419.x. [DOI] [PubMed] [Google Scholar]
- 10.Hennequin C, Abachin E, Symoens F, Lavarde V, Reboux G, Nolard N, Berche P. 1999. Identification of Fusarium species involved in human infections by 28S rRNA gene sequencing. J Clin Microbiol 37:3586–3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurtzman CP, Robnett CJ. 1997. Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5′ end of the large-subunit (26S) ribosomal DNA gene. J Clin Microbiol 35:1216–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.James SA, Collins MD, Roberts IN. 1996. Use of an rRNA internal transcribed spacer region to distinguish phylogenetically closely related species of the genera Zygosaccharomyces and Torulaspora. Int J Syst Bacteriol 46:189–194. doi: 10.1099/00207713-46-1-189. [DOI] [PubMed] [Google Scholar]
- 13.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yarrow D. 1998. Methods for the isolation, maintenance and identification of yeasts, p 77–100. In Kurtzman CP, Fell JW (ed), The yeasts, a taxonomic study, 4th ed Elsevier, Amsterdam, Netherlands. [Google Scholar]
- 16.Alanio A, Beretti JL, Dauphin B, Mellado E, Quesne G, Lacroix C, Amara A, Berche P, Nassif X, Bougnoux ME. 2011. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry for fast and accurate identification of clinically relevant Aspergillus species. Clin Microbiol Infect 17:750–755. doi: 10.1111/j.1469-0691.2010.03323.x. [DOI] [PubMed] [Google Scholar]
- 17.Iwen PC, Hinrichs SH, Rupp ME. 2002. Utilization of the internal transcribed spacer regions as molecular targets to detect and identify human fungal pathogens. Med Mycol 40:87–109. doi: 10.1080/mmy.40.1.87.109. [DOI] [PubMed] [Google Scholar]
- 18.Mannarelli BM, Kurtzman CP. 1998. Rapid identification of Candida albicans and other human pathogenic yeasts by using short oligonucleotides in a PCR. J Clin Microbiol 36:1634–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dujon B, Sherman D, Fischer G, Durrens P, Casaregola S, Lafontaine I, De Montigny J, Marck C, Neuveglise C, Talla E, Goffard N, Frangeul L, Aigle M, Anthouard V, Babour A, Barbe V, Barnay S, Blanchin S, Beckerich JM, Beyne E, Bleykasten C, Boisrame A, Boyer J, Cattolico L, Confanioleri F, De Daruvar A, Despons L, Fabre E, Fairhead C, Ferry-Dumazet H, Groppi A, Hantraye F, Hennequin C, Jauniaux N, Joyet P, Kachouri R, Kerrest A, Koszul R, Lemaire M, Lesur I, Ma L, Muller H, Nicaud JM, Nikolski M, Oztas S, Ozier-Kalogeropoulos O, Pellenz S, Potier S, Richard GF, Straub ML, Suleau A, Swennen D, Tekaia F, Wesolowski-Louvel M, Westhof E, Wirth B, Zeniou-Meyer M, Zivanovic I, Bolotin-Fukuhara M, Thierry A, Bouchier C, Caudron B, Scarpelli C, Gaillardin C, Weissenbach J, Wincker P, Souciet JL. 2004. Genome evolution in yeasts. Nature 430:35–44. doi: 10.1038/nature02579. [DOI] [PubMed] [Google Scholar]
- 20.Gabaldón T. 2008. Large-scale assignment of orthology: back to phylogenetics? Genome Biol 9:235. doi: 10.1186/gb-2008-9-10-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rooney AP, Ward TJ. 2005. Evolution of a large ribosomal RNA multigene family in filamentous fungi: birth and death of a concerted evolution paradigm. Proc Natl Acad Sci U S A 102:5084–5089. doi: 10.1073/pnas.0409689102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Sherry S, Ogedengbe ME, Hafeez MA, Barta JR. 2013. Divergent nuclear 18S rDNA paralogs in a turkey coccidium, Eimeria meleagrimitis, complicate molecular systematics and identification. Int J Parasitol 43:679–685. doi: 10.1016/j.ijpara.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Nishimoto Y, Arisue N, Kawai S, Escalante AA, Horii T, Tanabe K, Hashimoto T. 2008. Evolution and phylogeny of the heterogeneous cytosolic SSU rRNA genes in the genus Plasmodium. Mol Phylogenet Evol 47:45–53. doi: 10.1016/j.ympev.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 24.Rooney AP. 2004. Mechanisms underlying the evolution and maintenance of functionally heterogeneous 18S rRNA genes in Apicomplexans. Mol Biol Evol 21:1704–1711. doi: 10.1093/molbev/msh178. [DOI] [PubMed] [Google Scholar]
- 25.Wicht B, Ruggeri-Bernardi N, Yanagida T, Nakao M, Peduzzi R, Ito A. 2010. Inter- and intra-specific characterization of tapeworms of the genus Diphyllobothrium (Cestoda: Diphyllobothriidea) from Switzerland, using nuclear and mitochondrial DNA targets. Parasitol Int 59:35–39. doi: 10.1016/j.parint.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 26.van Heerikhuizen H, Ykema A, Klootwijk J, Gaillardin C, Ballas C, Fournier P. 1985. Heterogeneity in the ribosomal RNA genes of the yeast Yarrowia lipolytica; cloning and analysis of two size classes of repeats. Gene 39:213–222. doi: 10.1016/0378-1119(85)90315-4. [DOI] [PubMed] [Google Scholar]
- 27.Barnett JA, Payne R, Yarrow D. 1990. Yeasts: characteristics and identification, 2nd ed Cambridge University Press, Cambridge, United Kingdom. [Google Scholar]
- 28.Kurtzman CP. 2011. Pichia E.C. Hansen 1904, p 2354 In Kurtzman CP, Fell JW, Boekhout T (ed), The yeasts, a taxonomic study, 5th ed, vol 1 Elsevier, Amsterdam, Netherlands. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.