Abstract
Halicephalobus gingivalis (previously Micronema deletrix) is a free-living nematode known to cause opportunistic infections, mainly in horses. Human infections are very rare, but all cases described to date involved fatal meningoencephalitis. Here we report the first case of H. gingivalis infection in an Australian human patient, confirmed by nematode morphology and sequencing of ribosomal DNA. The implications of this case are discussed, particularly, the need to evaluate real-time PCR as a diagnostic tool.
CASE REPORT
A 74-year-old lady from a regional town in the Eyre Peninsula of South Australia with a 4-day history of mental state deterioration, fever, and a loss of coordination was transferred to the Royal Adelaide Hospital. She was moderately immune suppressed by methotrexate and etanercept treatment for rheumatoid arthritis and had a history of diabetes. During the admission, her conscious state deteriorated rapidly, requiring mechanical ventilation and admission to the intensive care unit (ICU). Subsequently, she developed signs of brainstem involvement and exhibited a loss of corneal and gag reflexes. She was administered benzylpenicillin, ceftriaxone, and aciclovir for presumptive meningoencephalitis of bacterial or viral etiology. Cerebrospinal fluid (CSF) obtained by lumbar puncture demonstrated 280 ×106 polymorphonuclear leukocytes (PMN)/liter, 18 ×106 mononuclear lymphocytes/liter, elevated CSF protein of 1.59 g/liter, and CSF glucose of 3.3 mmol/liter; aerobic and anaerobic bacterial culture results were negative, as were PCR results for Streptococcus pneumoniae, Neisseria meningitidis, herpes simplex virus, and varicella-zoster virus. CSF India ink stain and cryptococcal antigen lateral flow assay (Immy, Inc., Norman, OK, USA) results were negative. Magnetic resonance imaging (MRI) of the brain exhibited a left-predominant, asymmetrical meningeal enhancement in the frontoparietal cortex, without any detectable brainstem changes. Two days later, repeated lumbar punctures showed a marked elevation of PMN to 2,500 ×106 cells/liter and 34 ×106 mononuclear cells/liter, with no bacteria detected upon Gram staining. CSF stained with Diff-Quick (Alere, Brisbane, Australia) showed 99% PMN with very few eosinophils. CSF protein was markedly elevated (5.46 g/liter), and CSF glucose was 1.3 mmol/liter. Microscopic examination of 100 μl of unstained CSF was performed after centrifugation at 700 × g for 10 min, but no amoebic trophozoites were detected. With a suspicion of parasitic infection, given the unexplained high PMN counts, the antimicrobial treatment strategy was changed to liposomal amphotericin B, sulfadiazine, pentamidine, and azithromycin to target protists such as amoebae and Toxoplasma gondii. CSF was subjected to PCR for Naegleria fowleri, Acanthamoeba sp., and Balamuthia mandrillaris, but all test results were negative. No anti-Strongyloides serum antibody (IgG) was detected in an enzyme-linked immunosorbent assay using somatic larval antigens from Strongyloides ratti (Bordier Affinity Products) (1, 2). At day 7 of admission, the patient died following a complete loss of brainstem functions.
An etiological diagnosis was made based on postmortem findings. Microscopy of CSF and brain tissue exhibited numerous motile nematodes containing oval, elongated, thin-shelled, colorless eggs of 40 to 55 μm by 20 to 25 μm in size (average of 10 eggs) (Fig. 1). The larvae in CSF were 250 to 300 μm long and 15 to 20 μm wide, with a rhabditoid esophagus (70 to 90 μm long). Larvae from CSF were cultured using a modified Strongyloides agar plate culture method by replacing fecal material with an Escherichia coli ATCC 25922 suspension together with 100 μl of CSF onto the middle of a Mueller-Hinton agar plate (Oxoid, Australia) (3, 4). Every 7 days, new plates were inoculated. E. coli grew in tracks created by motile nematodes as they moved out of the central inoculum, and microscopic examination revealed nematodes at different stages of development (Fig. 2). Only female adult worms were observed; they possessed didelphic reproductive tracts and reflexed ovaries at the posterior end, consistent with the description of Halicephalobus gingivalis (5). The live nematodes were fixed in ethanol. Subsequently, DNA was isolated from individual worms and subjected to PCR-based sequencing of nuclear large-subunit ribosomal DNA (LSU rDNA) (6). The sequences determined from four individual nematodes were all the same and had 99% homology (1,385/1,399 bases) to that of H. gingivalis SAN100, isolated from a horse in Guelph, Canada (GenBank accession no. AY293177.1 [7]).
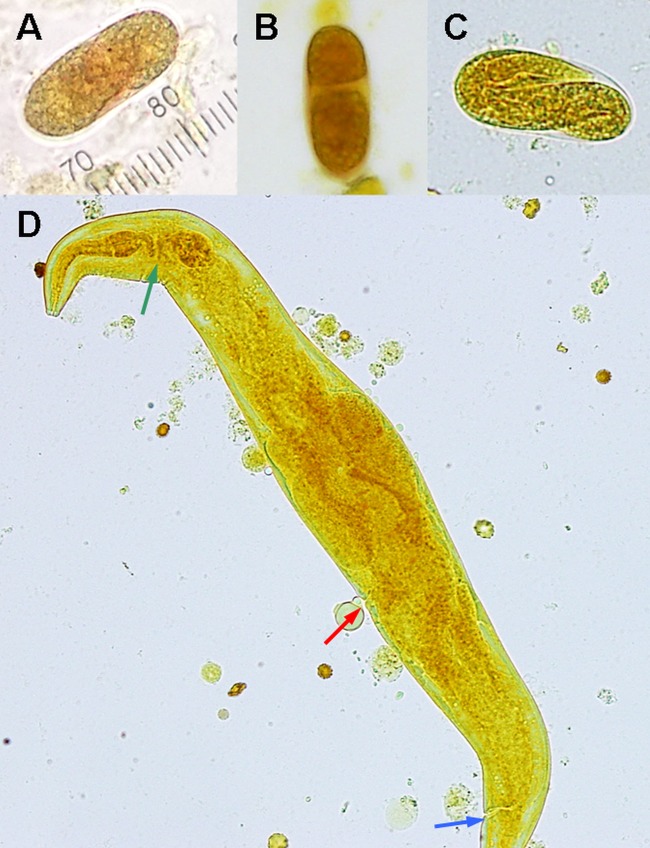
FIG 1.
(A to C) Iodine stain of CSF obtained postmortem shows different stages of H. gingivalis egg development. (A) Single-cell stage (1 scale unit = 2.5 μm). (B) Two-cell stage. (C) Larval stage. (D) Iodine stain of a fourth-stage larva (300 μm in length) demonstrates a short buccal cavity, nerve rings (green arrow) between two bulbs of the rhabditoid esophagus, reflexed ovary, presence of vulva (red arrow), and anal pore (blue arrow).
A complete postmortem examination was conducted, and a study of the brain revealed congested leptomeningeal blood vessels without significant opacity of the leptomeninges or CSF. The brain had a normal weight of 1,160 g, and there was no significant cerebral edema. There was extensive brain necrosis, primarily affecting the temporal lobes (bilaterally) and the right and left basal ganglia, anterior corpus callosum, right cerebral peduncle, and cerebellum. Histopathological examination of the brain showed meningoencephalitis, with mild to moderate perivascular inflammation comprising lymphocytes and macrophages and with no evidence of granulomatous inflammation (Fig. 2). The inflammation extended into the brain parenchyma, and there were multiple foci of necrosis and widespread cortical hypoxic-ischemic injury characterized by neuronal red cell change. Adult female nematodes, larvae, and eggs were observed in every section of the brain (bilateral hemispheres, cerebellum, brain stem, pituitary gland, and leptomeninges), primarily in the perivascular spaces, including areas within the brain parenchyma in which the presence of H. gingivalis was identified without any apparent associated inflammatory response. The spinal cord was not examined. The nematode was not observed in any other organs (including heart, lungs, liver, and kidneys).
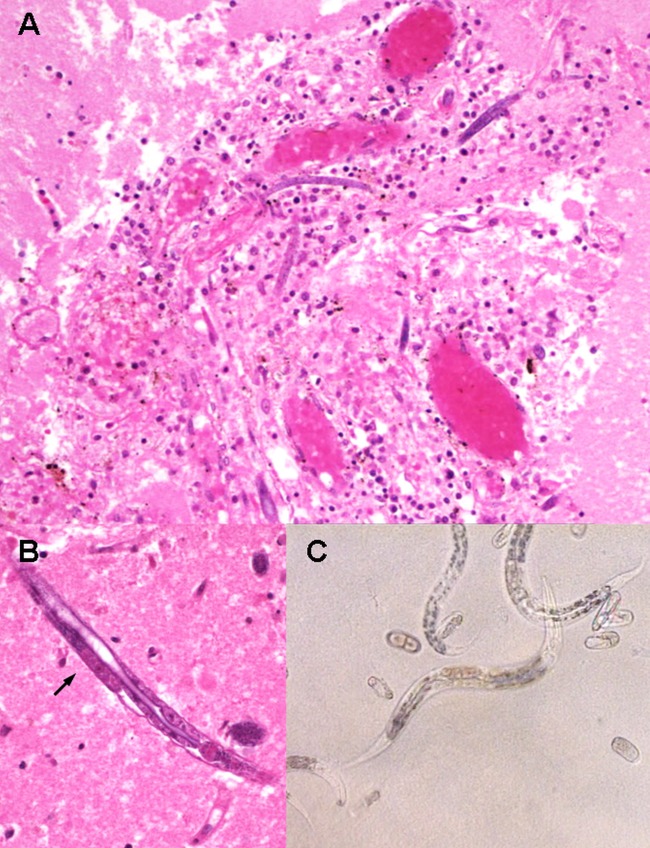
FIG 2.
(A) Hematoxylin and eosin (H&E) stain of brain tissue (under ×100 magnification) demonstrates perivascular inflammation with predominant macrophages and lymphocytes surrounding H. gingivalis larvae. (B) Third-stage larvae stained with H&E under ×400 magnification show presence of premature genital primordium (black arrow) and bulb of esophagus (at right end of nematode). (C) Microscopy examination of agar plate culture (×400 magnification) shows H. gingivalis larvae and eggs in various stages of development.
H. gingivalis belongs to the nematode family Paragrolaimidae. Currently, there are eight described species of Halicephalobus, and only H. gingivalis has been reported to infect humans and equines, predominantly horses (5) (Table 1 and Table 2). Only female worms have been isolated from parasitized hosts, confirming that H. gingivalis can reproduce parthenogenetically, although how H. gingivalis infects human and equine hosts is unknown (8–12). In the environment, H. gingivalis has been isolated from horse manure and compost (36). This organism has been reported from all inhabited continents except Australia (37), and isolates recovered from geographically distant localities appear to be genetically similar (6). In the present case, the affected woman had not traveled overseas or had contact with horses in the year prior to her presentation. Infection was likely acquired locally, but this cannot be confirmed as the epidemiology of H. gingivalis in Australia is unknown, and there is no published Australian case to date.
TABLE 1.
Reported cases of H. gingivalis infections in humans between 1975 and 2014a
| Yr | Demographics | Country | Clinical presentation | Premortem CSF findings | Identification of organism | Anthelmintic | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|
| 1975 | Human, 5-year-old male, immunocompetent | Canada | Meningoencephalitis 8 days after fall into manure spreader with facial & mandible injuries | CSF—lymphocytic pleocytosis; 300 cells, 50% lymphocytes, 50% macrophages | Autopsy; morphological diagnosis; spinal cord involvement | No | Death | 8 |
| 1979 | Human, 47-year-old male, immunocompetent | United States | Meningoencephalitis, brainstem signs | Not available | Autopsy; morphological diagnosis; brainstem involvement | No | Death | 9 |
| 1981 | Human, 54-year-old male, heavy alcohol use | United States | Decubitus ulcers over buttock, bilateral internuclear ophthalmoplegia, normal brain scan | CSF—lymphocytic pleocytosis | Autopsy; morphological diagnosis; brain, heart, liver, kidney involvement | No | Death | 10 |
| 2010 | Human, 39-year-old female, immunocompetent | United States | Meningoencephalitis; initial MRI & lumbar puncture normal, improved temporarily with cyclophosphamide + prednisolone; repeat MRI—bilateral ring enhancing lesions | CSF—lymphocytic pleocytosis | Autopsy; morphological diagnosis; brain involvement | No | Death | 11 |
| 2013 | Human, 65-year-old female, immunocompetent | United States | Blurring of vision, encephalopathy, fever, MRI unremarkable | CSF—PMN pleocytosis; 160 leukocytes, 35% macrophages, 27% eosinophils, 14% neutrophils, 20% lymphocytes | Autopsy; morphological diagnosis; brain involvement only | No | Death | 12 |
| 2014 | Human, 74-year-old female, immunosuppressed | Australia | Meningoencephalitis with brainstem signs, MRI frontoparietal meningitis | CSF—pleocytosis; 2,500 neutrophils, 34 mononuclear cells | Autopsy; morphological diagnosis confirmed with LSU rDNA PCR and sequencing | No | Death |
CSF, cerebrospinal fluid; MRI, magnetic resonance imaging; PMN, polymorphonuclear leukocytes; LSU rDNA, large subunit ribosomal DNA.
TABLE 2.
Reported cases of H. gingivalis infections in animals between 1987 and 2014
| Yr | Clinical detail | Country | Presentation | Laboratory diagnosis | Treatment | Outcome | Reference |
|---|---|---|---|---|---|---|---|
| 1987 | Horse | United Kingdom | Encephalitis | Autopsy; morphological diagnosis | No | Death | 13 |
| 1990 | Two horses | United States | Disseminated infection, lung infection, encephalitis, spinal cord lesions | Autopsy; morphological diagnosis | No | Death | 14 |
| 1992 | Horse | Scotland | Disseminated infection with encephalitis and renal abscess | Autopsy; morphological diagnosis | No | Death | 15 |
| 1993 | Horse | United States | Mandible osteomyelitis renal abscess encephalitis | Prospective; morphological diagnosis | Ivermectin followed with fenbendazole; deterioration on therapy | Death | 16 |
| 1993 | Horse | United States | Maxillary sinus abscess encephalitis | Prospective; morphological diagnosis | Fenbendazole for maxillary sinus infection; deterioration on therapy | Death | 17 |
| 1993 | Horse | United States | Posthitis | Prospective; morphological diagnosis | Ivermectin and diethylcarbamazine | Survival | 18 |
| 1995 | Horse | United States | Encephalitis | Autopsy; morphological diagnosis | Fenbendazole, dimethyl sulfoxide, dexamethasone, and butazolidine | Death | 19 |
| 1998 | Horse | Germany | Osteomyelitis gingivitis | Morphology | Unspecified | Unknown | 20 |
| 2000 | Two horses | United States | Encephalitis uveitis nephritis | Retrospective; morphology | Ivermectin | Death | 21 |
| 2000 | Zebra | United States | Ocular infection | Prospective; morphology | Ivermectin | Death | 22 |
| 2000 | Horse | Canada | Encephalitis | Autopsy; morphological diagnosis | No | Death | 7 |
| 2001 | Horse | United States | Encephalitis | Autopsy; morphological diagnosis | No | Death | 23 |
| 2001 | Horse | United States | Encephalitis | Autopsy; morphological diagnosis | No | Death | 24 |
| 2001 | Horse | Canada | Encephalitis | Prospective; morphological diagnosis | Ivermectin + surgical debulking of granulomas | Survival | 25 |
| 2004 | Donkey | United States | Renal abscess | Prospective; morphological diagnosis | Ivermectin | Survival | 26 |
| 2006 | Horse | United States | Encephalitis | Autopsy; morphological diagnosis | No | Death | 27 |
| 2007 | Horse | Japan | Encephalitis | LSU rDNAa PCR and sequencing | No | Death | 28 |
| 2007 | Horse | Switzerland | Posthitis | Prospective; morphological diagnosis | Prednisolone + topical moxidectin + oral moxidectin for 5 mo | Survival | 29 |
| 2008 | Horse | Canada | Mandibular abscess encephalitis | Prospective; morphological diagnosis | Progression on ivermectin, changed to thiabendazole | Death | 30 |
| 2011 | Horse | United Kingdom | Encephalitis | Autopsy; morphological diagnosis | No | Death | 31 |
| 2011 | Horse | Canada | Encephalitis | Autopsy; morphological diagnosis | No | Death | 32 |
| 2012 | Two horses | Iceland | Encephalitis | Autopsy; morphological diagnosis | No | Death | 33 |
| 2012 | Horse | Italy | Encephalitis | Autopsy; morphological diagnosis | No | Death | 34 |
| 2014 | Horse | South Korea | Encephalitis | LSU rDNAa PCR and sequencing | Unspecified anthelminthics | Death | 35 |
LSU rDNA, large subunit ribosomal DNA.
The present case is the sixth infection of a human by a Halicephalobus sp. described in the literature since 1975 (Table 1). Previously published human cases have all involved immunocompetent individuals in North America. All cases were fatal, with granulomatous encephalitis, suggestive of high neurotropism during infection. Diagnoses were made postmortem, and no anthelmintic treatment had been given. CSFs were obtained antemortem in three cases, white cells ranged from neutrophil to lymphocyte predominance, and pleocytosis with raised eosinophil levels was seen. Granulomatous inflammation was not seen in the current case, possibly due to the use of etanercept, a tumor necrosis factor-alpha (TNF-α) inhibitor, combined with methotrexate (38).
To date, 27 other cases of infection in animals have been described, mainly in horses, with 4 survivors (18, 25, 26, 29). Micronema deletrix was used as a synonym for this species in the 20th century. One horse with brain granulomata survived following aggressive debulking surgery complemented by ivermectin treatment (25). Transmission through a manure-contaminated wound had been proposed as the route of infection for one case but was not proven by autopsy (8). To date, human cases have not shed light on the route of transmission. In the present case, histopathological examinations of other organs did not indicate any dissemination of the nematode beyond the central nervous system (CNS). In animals, H. gingivalis had been linked to oromaxillary infections and posthitis, suggestive of initial mucosal exposure to the invasive larvae, followed by dissemination (17, 18, 20, 29, 30). Exposure through the oromaxillary route may explain the common neurological involvement.
All human cases of H. gingivalis infections reported to date were diagnosed at autopsy, despite antemortem suspicions of parasitic infection in some cases. Neurological nematodiasis is rare but can involve parasites such as Angiostrongylus cantonensis, Strongyloides stercoralis, Toxocara canis, Trichinella spiralis, and Gnathostoma spinigerum, typically associated with CSF and peripheral eosinophilia (39–46). During H. gingivalis infection, CSF may initially show only moderate pleocytosis and eosinophilia may be absent (10, 11) and larvae are usually not found in CSF obtained by lumbar puncture. To our knowledge, there is currently no immunoassay or PCR readily available for H. gingivalis to provide a timely diagnosis. The D2 and D3 domains of LSU rDNA might be suitable targets for development of a real-time diagnostic PCR (6). The preliminary diagnosis of H. gingivalis can be made from nematodes obtained at autopsy since (i) the eggs are distinctive being thin shelled, elongate, and oval at various stages of development, including mature larvae, and (ii) although the larvae have a rhabditiform esophagus and superficially resemble the rhabditiform larvae of S. stercoralis, H. gingivalis has two esophageal bulbs whereas S. stercoralis has one, and the esophageal neck and buccal capsule are longer in H. gingivalis. In addition, only filariform larvae of S. stercoralis, which have a cylindrical esophagus and a notched tail, have been found in the CNS in disseminated strongyloidiasis (47) and (iii) if adult nematodes are found, they are female only with distinctive morphology.
Treatment responses can be assessed only from previous cases in animals, as none of the human cases, including this case, received anthelmintics. Most affected animals deteriorated (16, 17, 19, 21, 22, 30) despite treatment, and the presence of live worms at autopsy suggests that the anthelmintic treatment was ineffective. In vitro susceptibility testing using microagar larval developmental tests (MALDTs) has been used to assess the effects of thiabendazole and ivermectin on the hatching rate and larval development of H. gingivalis (48). Thiabendazole at concentrations of 10 to 100 μg/ml showed a dose-dependent inhibition effect on the hatchability of eggs. However, no inhibition of larval development was observed. Thus, H. gingivalis appears to have some intrinsic tolerance of ivermectin, but larval development can be temporarily suppressed at 2 μg/ml. Reversal of inhibition can be seen, despite incubation with ivermectin for 72 h. Pharmacokinetic studies have showed that ivermectin rarely enters the CNS since it is actively removed by the P-glycoprotein, an abundant transporter protein in the brain. This can result in an undetectable CSF level, despite a parenteral dose of 200 μg/kg of body weight, which is often used for disseminated S. stercoralis infection (49). If high parenteral doses do allow ivermectin to penetrate the CNS, adverse CNS effects, including decreased consciousness, may occur (50). There is a paucity of pharmacokinetic data on cerebral penetration of thiabendazole. Using thiabendazole at 23 mg/kg every 12 h, one study has shown that the highest drug level detectable in CSF from an individual with cerebral strongyloidiasis was only 1.8 μg/ml (51), significantly below the concentration required for H. gingivalis egg inhibition. The benefit of adjunctive corticosteroid is also questionable due to paucity of evidence and lack of clinical improvement (52).
To date, all reported cases of human H. gingivalis infection have led to fatal meningoencephalitis, and diagnoses were made at autopsy. Brain biopsy should be considered for indeterminate cases of meningoencephalitis (53). Antemortem diagnosis remains a major challenge, as there is no laboratory test with a reasonable turnaround time, and routine CSF findings and radiologic features are nonspecific. Nonetheless, PCR-based sequencing of DNA from brain biopsy material and CSF could potentially assist in diagnosis. Treatment has not been described in human cases, but pharmacokinetic studies (48, 49) suggest that treatment with ivermectin or thiabendazole administered parenterally may not be effective because of poor killing effect per se and inability to achieve therapeutic levels in CNS.
Nucleotide sequence accession number.
The sequences determined in this work were deposited in GenBank under accession no. KP307928.
ACKNOWLEDGMENTS
We acknowledge the communications with Harsha Sheorey, St Vincent's Hospital, Melbourne; Norbert Ryan, Victorian Infectious Diseases Reference Laboratory, Melbourne; Andrew Butcher, School of Health Sciences, University of South Australia, Adelaide; Barbara Koszyca, SA Pathology, Adelaide; and staff members of the Department of Anatomical Pathology, SA Pathology, Adelaide.
REFERENCES
- 1.van Doorn HR, Koelewijn R, Hofwegen H, Gilis H, Wetsteyn JC, Wismans PJ, Sarfati C, Vervoort T, van Gool T. 2007. Use of enzyme-linked immunosorbent assay and dipstick assay for detection of Strongyloides stercoralis infection in humans. J Clin Microbiol 45:438–442. doi: 10.1128/JCM.01735-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loutfy MR, Wilson M, Keystone JS, Kain KC. 2002. Serology and eosinophil count in the diagnosis and management of strongyloidiasis in a non-endemic area. Am J Trop Med Hyg 66:749–752. [DOI] [PubMed] [Google Scholar]
- 3.Koga K, Kasuya S, Khamboonruang C, Sukhavat K, Ieda M, Takatsuka N, Kita K, Ohtomo H. 1991. A modified agar plate method for detection of Strongyloides stercoralis. Am J Trop Med Hyg 45:518–521. [DOI] [PubMed] [Google Scholar]
- 4.Intapan PM, Maleewong W, Wongsaroj T, Singthong S, Morakote N. 2005. Comparison of the quantitative formalin ethyl acetate concentration technique and agar plate culture for diagnosis of human strongyloidiasis. J Clin Microbiol 43:1932–1933. doi: 10.1128/JCM.43.4.1932-1933.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson RC, Linder KE, Peregrine AS. 1998. Halicephalobus gingivalis (Stefanski, 1954) from a fatal infection in a horse in Ontario, Canada with comments on the validity of H. deletrix and a review of the genus. Parasite 5:255–261. [DOI] [PubMed] [Google Scholar]
- 6.Nadler SA, Carreno RA, Adams BJ, Kinde H, Baldwin JG, Mundo-Ocampo M. 2003. Molecular phylogenetics and diagnosis of soil and clinical isolates of Halicephalobus gingivalis (Nematoda: Cephalobina: Panagrolaimoidea), an opportunistic pathogen of horses. Int J Parasitol 33:1115–1125. doi: 10.1016/S0020-7519(03)00134-6. [DOI] [PubMed] [Google Scholar]
- 7.Brojer JT, Parsons DA, Linder KE, Peregrine AS, Dobson H. 2000. Halicephalobus gingivalis encephalomyelitis in a horse. Can Vet J 41:559–561. [PMC free article] [PubMed] [Google Scholar]
- 8.Hoogstraten J, Young WG. 1975. Meningo-encephalomyelitis due to the saprophagous nematode, Micronema deletrix. Can J Neurol Sci 2:121–126. [DOI] [PubMed] [Google Scholar]
- 9.Shadduck JA, Ubelaker J, Telford VQ. 1979. Micronema deletrix meningoencephalitis in an adult man. Am J Clin Pathol 72:640–643. [DOI] [PubMed] [Google Scholar]
- 10.Gardiner CH, Koh DS, Cardella TA. 1981. Micronema in man: third fatal infection. Am J Trop Med Hyg 30:586–589. [DOI] [PubMed] [Google Scholar]
- 11.Ondrejka SL, Procop GW, Lai KK, Prayson RA. 2010. Fatal parasitic meningoencephalomyelitis caused by Halicephalobus deletrix: a case report and review of the literature. Arch Pathol Lab Med 134:625–629. doi: 10.1043/1543-2165-134.4.625. [DOI] [PubMed] [Google Scholar]
- 12.Papadi B, Boudreaux C, Tucker JA, Mathison B, Bishop H, Eberhard ME. 2013. Halicephalobus gingivalis: a rare cause of fatal meningoencephalomyelitis in humans. Am J Trop Med Hyg 88:1062–1064. doi: 10.4269/ajtmh.12-0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blunden AS, Khalil LF, Webbon PM. 1987. Halicephalobus deletrix infection in a horse. Equine Veterinary J 19:255–260. doi: 10.1111/j.2042-3306.1987.tb01399.x. [DOI] [PubMed] [Google Scholar]
- 14.Spalding MG, Greiner EC, Green SL. 1990. Halicephalobus (Micronema) deletrix infection in two half-sibling foals. J Am Vet Med Assoc 196:1127–1129. [PubMed] [Google Scholar]
- 15.Angus KW, Roberts L, Archibald DR, Fraser DG, Jackson F, Gibbons LM. 1992. Halicephalobus deletrix infection in a horse in Scotland. Vet Rec 131:495. [DOI] [PubMed] [Google Scholar]
- 16.Ruggles AJ, Beech J, Gillette DM, Midla LT, Reef VB, Freeman DE. 1993. Disseminated Halicephalobus deletrix infection in a horse. J Am Vet Med Assoc 203:550–552. [PubMed] [Google Scholar]
- 17.Trostle SS, Wilson DG, Steinberg H, Dzata G, Dubielzig RR. 1993. Antemortem diagnosis and attempted treatment of (Halicephalobus) Micronema deletrix infection in a horse. Can Vet J 34:117–118. [PMC free article] [PubMed] [Google Scholar]
- 18.Dunn DG, Gardiner CH, Dralle KR, Thilsted JP. 1993. Nodular granulomatous posthitis caused by Halicephalobus (syn. Micronema) sp. in a horse. Vet Pathol 30:207–208. doi: 10.1177/030098589303000215. [DOI] [PubMed] [Google Scholar]
- 19.Rames DS, Miller DK, Barthel R, Craig TM, Dziezyc J, Helman RG, Mealey R. 1995. Ocular Halicephalobus (syn. Micronema) deletrix in a horse. Vet Pathol 32:540–542. [DOI] [PubMed] [Google Scholar]
- 20.Teifke JP, Schmidt E, Traenckner CM, Bauer C. 1998. Halicephalobus (syn. Micronema) deletrix as a cause of granulomatous gingivitis and osteomyelitis in a horse. Tierarztl Prax Ausg G Grosstiere Nutztiere 26:157–161. (In German.) [PubMed] [Google Scholar]
- 21.Kinde H, Mathews M, Ash L, St Leger J. 2000. Halicephalobus gingivalis (H. deletrix) infection in two horses in southern California. J Vet Diagn Invest 12:162–165. [DOI] [PubMed] [Google Scholar]
- 22.Isaza R, Schiller CA, Stover J, Smith PJ, Greiner EC. 2000. Halicephalobus gingivalis (Nematoda) infection in a Grevy's zebra (Equus grevyi). J Zoo Wildl Med 31:77–81. doi: 10.1638/1042-7260(2000)031[0077:HGNIIA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 23.Wilkins PA, Wacholder S, Nolan TJ, Bolin DC, Hunt P, Bernard W, Acland H, Del Piero F. 2001. Evidence for transmission of Halicephalobus deletrix (H gingivalis) from dam to foal. J Vet Intern Med 15:412–417. doi: 10.1111/j.1939-1676.2001.tb02338.x. [DOI] [PubMed] [Google Scholar]
- 24.Johnson JS, Hibler CP, Tillotson KM, Mason GL. 2001. Radiculomeningomyelitis due to Halicephalobus gingivalis in a horse. Vet Pathol 38:559–561. doi: 10.1354/vp.38-5-559. [DOI] [PubMed] [Google Scholar]
- 25.Pearce SG, Boure LP, Taylor JA, Peregrine AS. 2001. Treatment of a granuloma caused by Halicephalobus gingivalis in a horse. J Am Vet Med Assoc 219:1735–1738, 1708. doi: 10.2460/javma.2001.219.1735. [DOI] [PubMed] [Google Scholar]
- 26.Schmitz DG, Chaffin MK. 2004. What is your diagnosis? Halicephalobus gingivalis. J Am Vet Med Assoc 225:1667–1668. doi: 10.2460/javma.2004.225.1667. [DOI] [PubMed] [Google Scholar]
- 27.Bryant UK, Lyons ET, Bain FT, Hong CB. 2006. Halicephalobus gingivalis-associated meningoencephalitis in a Thoroughbred foal. J Vet Diagn Invest 18:612–615. doi: 10.1177/104063870601800618. [DOI] [PubMed] [Google Scholar]
- 28.Akagami M, Shibahara T, Yoshiga T, Tanaka N, Yaguchi Y, Onuki T, Kondo T, Yamanaka T, Kubo M. 2007. Granulomatous nephritis and meningoencephalomyelitis caused by Halicephalobus gingivalis in a pony gelding. J Vet Med Sci 69:1187–1190. doi: 10.1292/jvms.69.1187. [DOI] [PubMed] [Google Scholar]
- 29.Muller S, Grzybowski M, Sager H, Bornand V, Brehm W. 2008. A nodular granulomatous posthitis caused by Halicephalobus sp. in a horse. Vet Dermatol 19:44–48. doi: 10.1111/j.1365-3164.2007.00643.x. [DOI] [PubMed] [Google Scholar]
- 30.Ferguson R, van Dreumel T, Keystone JS, Manning A, Malatestinic A, Caswell JL, Peregrine AS. 2008. Unsuccessful treatment of a horse with mandibular granulomatous osteomyelitis due to Halicephalobus gingivalis. Can Vet J 49:1099–1103. [PMC free article] [PubMed] [Google Scholar]
- 31.Hermosilla C, Coumbe KM, Habershon-Butcher J, Schöniger S. 2011. Fatal equine meningoencephalitis in the United Kingdom caused by the panagrolaimid nematode Halicephalobus gingivalis: case report and review of the literature. Equine Vet J 43:759–763. doi: 10.1111/j.2042-3306.2010.00332.x. [DOI] [PubMed] [Google Scholar]
- 32.Sponseller BT, Plattner BL, Hostetter JM. 2011. Pathology in practice. Halicephalobus gingivalis. J Am Vet Med Assoc 238:1265–1267. doi: 10.2460/javma.238.10.1265. [DOI] [PubMed] [Google Scholar]
- 33.Eydal M, Bambir SH, Sigurdarson S, Gunnarsson E, Svansson V, Fridriksson S, Benediktsson ET, Sigurdardóttir ÓG. 2012. Fatal infection in two Icelandic stallions caused by Halicephalobus gingivalis (Nematoda: Rhabditida). Vet Parasitol 186:523–527. doi: 10.1016/j.vetpar.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 34.Di Francesco G, Savini G, Maggi A, Cavaliere N, D'Angelo AR, Marruchella G. 2012. Equine meningo-encephalitis caused by Halicephalobus gingivalis: a case report observed during West Nile disease surveillance activities. Vet Ital 48:437–442, 431–436. (In English and Italian.) [PubMed] [Google Scholar]
- 35.Jung JY, Lee KH, Rhyoo MY, Byun JW, Bae YC, Choi E, Kim C, Jean YH, Lee MH, Yoon SS. 2014. Meningoencephalitis caused by Halicephalobus gingivalis in a thoroughbred gelding. J Vet Med Sci 76:281–284. doi: 10.1292/jvms.13-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steel H, de la Peña E, Fonderie P, Willekens K, Borgonie G, Bert W. 2010. Nematode succession during composting and the potential of the nematode community as an indicator of compost maturity. Pedobiologia 53:181–190. doi: 10.1016/j.pedobi.2009.09.003. [DOI] [Google Scholar]
- 37.Nishimura K, Hung T. 1997. Current views on geographic distribution and modes of infection of neurohelminthic diseases. J Neurol Sci 145:5–14. doi: 10.1016/S0022-510X(96)00293-6. [DOI] [PubMed] [Google Scholar]
- 38.Curtis JR, Patkar N, Xie A, Martin C, Allison JJ, Saag M, Shatin D, Saag KG. 2007. Risk of serious bacterial infections among rheumatoid arthritis patients exposed to tumor necrosis factor alpha antagonists. Arthritis Rheum 56:1125–1133. doi: 10.1002/art.22504. [DOI] [PubMed] [Google Scholar]
- 39.Cowie RH. 2013. Guest Editor's message: eosinophilic meningitis caused by Angiostrongylus cantonensis, the rat lungworm: biology, distribution, epidemiology, detection, diagnosis, treatment, and management. Hawaii J Med Public Health 72(Suppl 2):3–4. [PMC free article] [PubMed] [Google Scholar]
- 40.Morassutti AL, Thiengo SC, Fernandez M, Sawanyawisuth K, Graeff-Teixeira C. 2014. Eosinophilic meningitis caused by Angiostrongylus cantonensis: an emergent disease in Brazil. Mem Inst Oswaldo Cruz 109:399–407. doi: 10.1590/0074-0276140023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsai HC, Chen YS, Yen CM. 2013. Human parasitic meningitis caused by Angiostrongylus cantonensis infection in Taiwan. Hawaii J Med Public Health 72(Suppl 2):26–27. [PMC free article] [PubMed] [Google Scholar]
- 42.Espírito-Santo MC, Pinto PL, Mota DJ, Gryschek RC. 2013. The first case of Angiostrongylus cantonensis eosinophilic meningitis diagnosed in the city of Sao Paulo, Brazil. Rev Inst Med Trop Sao Paulo 55:129–132. doi: 10.1590/S0036-46652013000200012. [DOI] [PubMed] [Google Scholar]
- 43.Tsai HC, Liu YC, Kunin CM, Lee SS, Chen YS, Lin HH, Tsai TH, Lin WR, Huang CK, Yen MY, Yen CM. 2001. Eosinophilic meningitis caused by Angiostrongylus cantonensis: report of 17 cases. Am J Med 111:109–114. doi: 10.1016/S0002-9343(01)00766-5. [DOI] [PubMed] [Google Scholar]
- 44.Smallman LA, Young JA, Shortland-Webb WR, Carey MP, Michael J. 1986. Strongyloides stercoralis hyperinfestation syndrome with Escherichia coli meningitis: report of two cases. J Clin Pathol 39:366–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dutcher JP, Marcus SL, Tanowitz HB, Wittner M, Fuks JZ, Wiernik PH. 1990. Disseminated strongyloidiasis with central nervous system involvement diagnosed antemortem in a patient with acquired immunodeficiency syndrome and Burkitts lymphoma. Cancer 66:2417–2420. [DOI] [PubMed] [Google Scholar]
- 46.Rusnak JM, Lucey DR. 1993. Clinical gnathostomiasis: case report and review of the English-language literature. Clin Infect Dis 16:33–50. [DOI] [PubMed] [Google Scholar]
- 47.Cahill KM, Shevchuk M. 1996. Fulminant, systemic strongyloidiasis in AIDS. Ann Trop Med Parasitol 90:313–318. [DOI] [PubMed] [Google Scholar]
- 48.Fonderie P, Bert W, Hendrickx F, Houthoofd W, Moens T. 2012. Anthelmintic tolerance in free-living and facultative parasitic isolates of Halicephalobus (Panagrolaimidae). Parasitology 139:1301–1308. [DOI] [PubMed] [Google Scholar]
- 49.Nau R, Sorgel F, Eiffert H. 2010. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev 23:858–883. doi: 10.1128/CMR.00007-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rose CE, Paciullo CA, Kelly DR, Dougherty MJ, Fleckenstein LL. 23 March 2009, posting date Fatal outcome of disseminated strongyloidiasis despite detectable plasma and cerebrospinal levels of orally administered ivermectin. J Parasitol Res doi: 10.1155/2009/818296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arroyo JC, Brown A. 1987. Concentrations of thiabendazole and parasite-specific IgG antibodies in the cerebrospinal fluid of a patient with disseminated strongyloidiasis. J Infect Dis 156:520–523. [DOI] [PubMed] [Google Scholar]
- 52.Thanaviratananich S, Thanaviratananich S, Ngamjarus C. 2012. Corticosteroids for parasitic eosinophilic meningitis. Cochrane Database Syst Rev 10:CD009088. doi: 10.1002/14651858.CD009088.pub2. [DOI] [PubMed] [Google Scholar]
- 53.Huppatz C, Gawarikar Y, Levi C, Kelly PM, Williams D, Dalton C, Massey P, Givney R, Durrheim DN. 2010. Should there be a standardised approach to the diagnostic workup of suspected adult encephalitis? A case series from Australia. BMC Infect Dis 10:353. doi: 10.1186/1471-2334-10-353. [DOI] [PMC free article] [PubMed] [Google Scholar]