Abstract
The involvement of protein kinase C (PKC) in isometric tension development of rat mesenteric arteries was investigated. Non-selective inhibition of PKC and selective inhibition of the epsilon isoform were performed using the PKC inhibitor, chelerythrine, and non-viral genetransfer of a kinase inactive mutant of PKCepsilon (PKCepsilon-KN), respectively. Chelerythrine (2.5 or 5.0 microM) significantly and equally attenuated phenylephrine-induced but not potassium-induced contractions. Higher concentrations of chelerythrine (10 microM) caused the vessels to lose responsiveness to both phenylephrine and potassium chloride. Transfection of blood vessels with epsilon-KN also resulted in significant attenuation of contractile responses to phenylephrine. Potassium chloride-induced responses were not altered in transfected arteries. In a separate group of vessels, the relationship between [Ca2+]i and isometric tension was evaluated. These studies suggested that calcium sensitivity of the contractile apparatus was decreased in vessels when PKC-epsilon activity was compromised. The results of the study suggest that PKC-epsilon can modulate phenylephrine-induced contraction in mesenteric arteries via calcium-independent pathways.
Keywords: Vascular smooth muscle cells, Gene transfection, Electroporation, Chelerythrine Chloride, Dual recording, Calcium, Tension
2. INTRODUCTION
The role of protein kinase C epsilon (PKCepsilon) in Ca2+-independent smooth muscle contraction has been disputed. Support for the role of PKCpsilon came from findings that phorbol esters that activate PKC induce smooth muscle contraction without enhancing phosphorylation of the myosin light chain (1, 2, 3, 4, 5) and that the ferret portal vein which lacks PKC-epsilon fails to develop Ca2+-independent contraction (1). Subsequently, the role of PKC-epsilon was challenged by Somlyo et al. (6) on the basis that inhibition of myosin phosphatase by microcystin induced Ca2+-independent contraction of rabbit portal vein even after PKC-epsilon was downregulated by chronic phorbol ester treatment. In spite of the potential role of PKC-epsilon, neither study was able to selectively alter the PKC-epsilon isoform (7, 8).
Although some pharmacological agents have been developed, pharmacological targeting of specific subtypes of PKC is still problematic because of similarities in the target sites for the inhibitors (9). Pharmacological studies suggest that even non-specific inhibition of PKC by agents such as chelerythrine (one of the most commonly used protein kinase C inhibitors) produces undesirable side effects by inhibiting phospholipase C and phospholipase D at 100 microM (10) and phosphodiesterases at 10 microM (11). These are the drug concentrations widely used for inhibiting PKC.
However, considering also that PKCepsilon has a unique motif for actin-binding (12) and the ability to phosphorylate a thin filament-associated protein, calponin (13, 14), the physiological roles of the kinase in cytoskeletal dynamics, requires further investigation. Recently, we have developed a method to transfer genes to the mesenteric arteries resulting in short term gene expression (=7 days) (15). In the present study, we have transfected arteries with a kinase inactive mutant of the PKCepsilon gene. This approach allows for specific attenuation of PKCepsilon activity without affecting the other PKC isoforms. We also examined the effects of the PKC inhibitor chelerythrine.
3. MATERIALS AND METHODS
3.1. Materials
Male Sprague-Dawley rats (200 – 400 g, n = 61) were purchased from Charles River Laboratories, housed in an environmentally controlled vivarium and fed a standard pellet diet and water ad libitum. Chelerythrine chloride was purchased from Sigma Chemical (St. Louis, MO).
3.2. Measurement of isometric contractile tension in mesenteric arteries
Rats were anesthetized with isoflurane and euthanized by thoracotomy and removal of the heart. A segment of small intestine and adjacent mesentery was rapidly excised and placed in cold (4 °C) physiological salt solution (PSS) containing (in mM) 119.0 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgSO4, 25.0 NaHCO3, 1.2 KH2PO4, 0.027 EDTA, and 5.5 glucose. With the aid of a stereomicroscope, a 2 mm-length segment of a first or second-order branch of the superior mesenteric artery was excised, then mounted on a small vessel wire myograph (JP Trading, Aarhus, Denmark). The buffer in the chamber (5 ml) was maintained at 37.0 °C and continuously bubbled with a mixture of 95 % O2 and 5 % CO2. The pH of the bathing was adjusted at 7.4. The normalization procedure of Mulvany and Halpern was used to optimize vessel internal circumference for isometric tension development which was set at 0.9 x internal circumference when internal pressure is 100 mmHg (i.e., 0.9 x IC100) (18). The arteries were allowed to equilibrate for 20 min and were exposed to at least two consecutive priming doses of phenylephrine (10−5 M) to ensure that maximal responses were reproducible. Experiments were then terminated if reproducible responses could not be obtained. After thorough rinsing with normal PSS and waiting for 20 min, a cumulative concentration-effect curve to phenylephrine (10−9 to 10−3.5 M) was generated for each vessel segment. After thorough rinsing with normal PSS and incubation with chelerythrine chloride (2.5, 5.0, or 10 microM. n = 5, 4, or 4, respectively) or 0.1 %DMSO as vehicle (n = 5) for 20 min, the second concentration-effect curve was generated Vessel segments were then challenged by incremental concentrations of KCl (30, 60, and 120 mM) added to the buffer by substituting KCl for NaCl.
3.3. Non-viral gene-transfer of DNA to the vessels
3.3.1. Preparation of plasmids
A plasmid encoding a kinase inactive mutant of PKCepsilon PKCepsilon pSRDHis-PKCepsilon was used. The plasmid was a gift from Dr. Ohno and described previously (16, 17). Plasmids were grown in Escherichia coli and purified using Qiagen Giga-prep kits, as described by the manufacturer (Qiagen, Chatsworth, CA). The concentration of plasmid DNA was adjusted to 2 mg/ml in 10 mM Tris, pH 8.0, 1 mM EDTA, and 140 mM NaCl. This procedure produced purified DNA with > 80 % in a supercoiled form, free from RNA.
3.3.2. Animal surgery and electroporation
A group of animals (n = 25) were prepared for electroporation as described by Martin et al (15). Briefly, the rat was anesthetized with isoflurane and had a 5-cm midline incision made to expose the small intestine with adjacent mesentery. A 7 – 10 cm segment of distal small intestine beginning at ileocecal junction was fanned out over sterile gauze pads moistened with lactate Ringer’s solution. The mesentery was cut away from a segment of neurovascular bundle including the first or second order branch of the mesenteric artery, which was then placed into an electroporation probe specially designed for this purpose (15). Electroporation was carried out using a square wave electroporator (BTX ECM2001, Genetronics, Inc., San Diego, CA) at a field strength of 200 V/cm with eight 10-ms duration square wave pulses. One minute later, the solution with or without DNA (sham-operated group) was removed. Incisions were closed in layers with sutures and metal wound clips. Anesthetic was discontinued, the animals were allowed to recover and returned to the vivarium. Forty-eight hours later, the rats were anesthetized, the abdomen reopened and the electroporated vessels harvested. The animals were euthanatized by ventricular transection. All animal experiments were conducted in accordance with institutional guidelines in compliance with the recommendations of the Guide for Care and Use of Laboratory Animals.
3.3.3. Experimental protocol 1: Phenylephrine-induced isometric contraction
Vessels from control, transfected, or sham-operated animals were mounted on the myograph for further study. Cumulative concentration-effect responses to phenylephrine (10−9 to 10−3.5 M) were evaluated. After thorough rinsing with PSS, vessels were allowed to return to baseline conditions, then were challenged with 30, 60, and/or 120 mM KCl, as stated previously.
3.3.4. Experimental protocol 2: Simultaneous measurements of calcium and tension
Arteries were prepared on the myograph as previously described. The vessels (n = 14) were then incubated with the acetoxymethyl ester form of the fluorescent Ca2+-indicator indo-1 (indo-1 AM) for 120 min as previously described (15). The arteries were washed with PSS and left undisturbed for 20 min before the myograph was mounted on the stage of an inverted, photometer-equipped microscope (Nikon DIAPHOT, Japan). Vessels were illuminated with monochromatic light (350 nm), and emitted light fractions were detected at wavelengths of 405 and 485 nm using photomultiplier tubes (Photon Technology International, New Jersey). The signals from the photomultipliers and force transducer were digitized and stored on a computer to yield simultaneous recordings of Ca2+-dependent fluorescence and force generation.
3.4. Data analysis
Force values were divided by wall length (2x segment length) of the vessels and expressed as tension (mN/mm). Nonlinear regression curves were fit to individual sets of concentration-effect data, from which EC50 and maximal tension values were calculated and expressed as mean ± SE. The EC50 and maximal tension were evaluated by either Student’s t-test between two groups or ANOVA. Dunnet’s post-hoc test was applied when necessary. A value of P < 0.05 was considered statistically significant for comparisons of individual data sets. For simultaneous [Ca2+]i and tension measurements, the fluorescence ratio of F405/F485 is used as an index of [Ca2+]i.
4. RESULTS
4.1. Effects of chelerythrine on phenylephrine concentration-effect curves
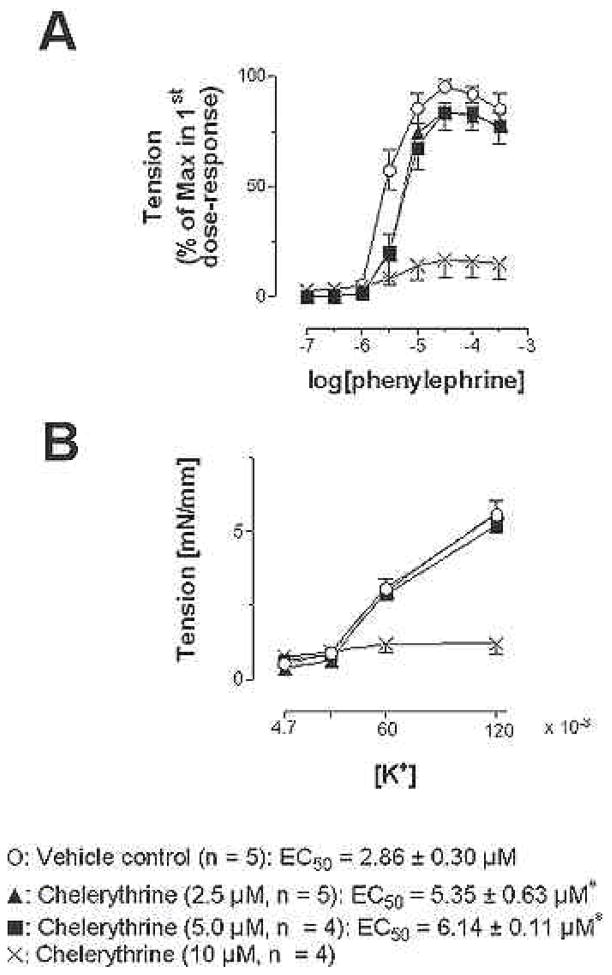
Figure 1 shows the effects of chelerythrine on responsiveness of mesenteric arteries to phenylephrine. A twenty-minute incubation of chelerythrine at concentrations of 2.5 and 5.0 microM equally attenuated phenylephrine-induced contraction of rat mesenteric arteries. The EC50 values of 5.35 ± 0.63 and 6.14 ± 0.11 microM for 2.5 and 5.0 microM chelerythrine, respectively, were not statistically different. The maximal tension development, 4.85 ± 0.53 mN/mm and 4.21 ± 0.52 mN/mm, were not different. However, 10 microM chelerythrine abolished the concentration-effect responses to phenylephrine (Figure 1A). While 2.5 and 5.0 microM chelerythrine chloride had no significant effects on potassium-induced contraction (Figure 1B), the 10 microM chelerythrine caused the vessels to lose responsiveness even to the high potassium exposure. There was no significant effect in the phenylephrine-induced responses before and after vehicle (0.1 % DMSO) treatment. The EC50 values are 2.25 ± 0.26 and 2.86 ± 0.30 microM, respectively.
Figure 1.
Effects of chelerythrine treatments on constrictor responses to phenylephrine (A) and potassium chloride (B) in rat mesenteric artery segments. Vessels were challenged with vasocontrictor, exposed to either vehicle (0.1 % DMSO) or chelerythrine at either 2.5, 5.0, or 10 microM for 20 min and then challenged again with vasoconstrictor. Data were normalized to maximal response and the chelerythrine treated responses were compared to the vehicle control.. An asterisk indicates a significant difference from vehicle control (P < 0.05).
4.2. Effects of non-viral gene-transfer of a PKCepsilon kinase inactive mutant (PKCepsilon-KN) on phenylephrine concentration-effect curves
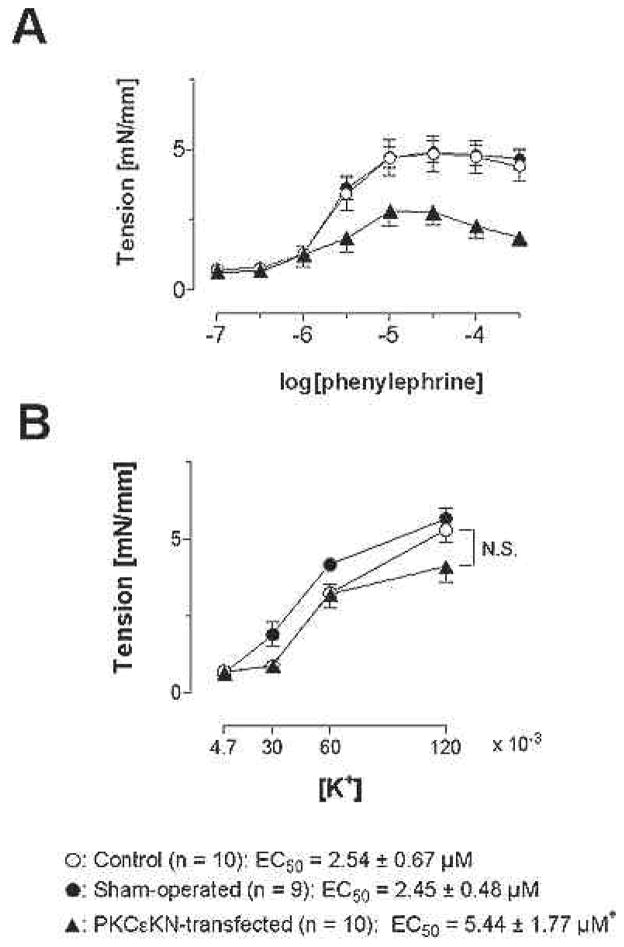
Transfection of PKCepsilon-KN significantly attenuated the phenylephrine-induced concentration effect curves (EC50: 2.45 ± 0.48 microM for sham-operated, 5.44 ± 1.77 microM for epsilon-KN) and suppressed the maximal force development (5.10 ± 0.40 mN/mm for sham-operated, 2.64 ± 0.45 mN/mm for PKCepsilon-KN, Figure 2A). On the other hand, potassium-induced contraction was not affected (Figure 2B). Electroporation alone did not affect the responsiveness of the vessels to phenylephrine or potassium.
Figure 2.
Effects of PKCepsilon-KN transfection on constrictor responses to phenylephrine (A) and potassium (B) in rat mesenteric artery segments. Phenylephrine responses were attenuated by transfection; the potassium chloride responses were not altered by transfection.. This data suggest that receptor but not depolarization induced activation of mesenteric artery smooth muscle is dependent upon PKCepsilon. An asterisk indicates a significant difference from sham-operated group (P < 0.05).
4.3. Effects of PKCepsilon-KN transfection on [Ca2+]i and tension
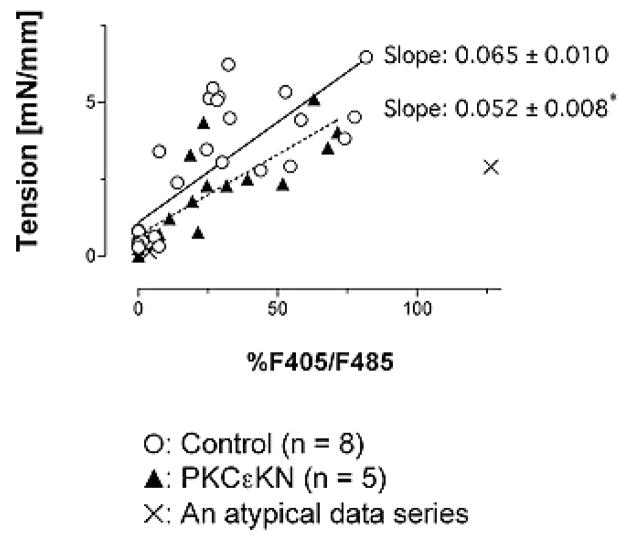
Simultaneous recordings of [Ca2+]i and tension were performed on control and PKCepsilon-KN-transfected preparations. Vessel segments were challenged with phenylephrine at concentrations corresponding to the EC15 (1.6 microM), EC50 (2.4 microM), and EC85 (7.8 microM), values obtained in the aforementioned experiments. Maximal [Ca2+]i was defined as the each vessel’s response to 120 mM KCl. Tension was plotted as a function of maximal calcium mobilization. The slope of this relationship was used as index of calcium sensitivity. The correlation coefficient values for control and the PKCepsilon-KN-transfected vessels were 0.77 and 0.83, respectively. The slope of PKCepsilon-KN-transfected vessels was significantly lower than that of the control group (Figure 3). A data set in the PKCepsilon-KN-transfected vessels, where 120 mM KCl did not produce maximal contraction, was excluded from calculation as a statistical outlier. If the oulier was included included, the slope would be 0.021 with higher correlation coefficient (0.99).
Figure 3.
The relationship between tension generation and cytosolic calcium in control and transfected vessels. The slope of the relationship was significantly decreased in vessels transfected with a kinase inactive mutant of PKCepsilon. This data suggests that calcium sensitivity may be mediated partially by PKCepsilon. An asterisk indicates a significant difference from vehicle control (P < 0.05).
5. DISCUSSION
Among the eleven known isoforms of protein kinase C, the alpha, beta and epsilon isoforms have been the major candidates suggested to be involved in smooth muscle excitation-contraction coupling. Roles for these isoforms have been implicated in spontaneous calcium spikes in A7r5 cells (7) and in acetylcholine-induced contraction of canine colon smooth muscle cells, where acetic acid/ethanol-induced inflammation suppressed contractility as well as their translocation from cytosol to membrane fraction (19). Other studies suggested that PKC regulates cytosolic calcium by either directly inhibiting LCa channels or by indirectly attenuating KCa channels, which helps membrane potential depolarization and thereby contraction of vascular smooth muscle cells. (20). In addition, PKC is one of the kinases that activates CPI-17 (i.e., 17 kDa protein kinase C-potentiated inhibitor protein), which inhibits myosin light chain phosphatase (MLCP) (21).
Consensus on the involvement of PKCepsilon in vascular smooth muscle contraction, however, has not yet been reached. Most studies investigating the role of PKCepsilon have focused on correlating the contractile responses to the presence of a certain isoform and/or non-specific downregulation of the kinase isoforms. The role of PKCepsilon therein has been controversial (6, 13). One study suggests that prolonged incubation with phorbol esters downregulates the expression of most PKC isoforms but not PKCepsilon in a cultured rat aortic smooth muscle cell line, A7r5 (7, 8). Thus, selective alteration of the function of PKCepsilon was attempted in the present study by using non-viral gene-transfer as a means for short-term in vivo expression of the kinase inactive mutant (PKCepsilon-KN) in blood vessels. Phenylephrine-induced contraction was significantly inhibited (Figure 2) and the contractile sensitivity to [Ca2+]i. also significantly decreased in the vessels transfected with PKCepsilon-KN (Figure 3). These results suggest that PKCepsilon is involved in tension development of rat mesenteric arteries, at least in part, through enhancing the calcium sensitivity.
Another important finding of the present study is that pharmacological inhibition of PKC with its commonly used inhibitor, chelerythrine, drastically abolished vessel responsiveness to both phenylephrine and potassium at 10 microM, a commonly used concentration for chelerythrine (Figure 1). In the present study, isometric tension development of the vessel to a cumulative application of phenylephrine was significantly suppressed by 2.5 microM chelerythrine. Doubling the inhibitor concentration to 5.0 microM did not result in further suppression. Therefore, the concentration of 2.5 microM, which is approximately 3.8 fold above its IC50 (22), was regarded as sufficient to maximally inhibit the PKC activities in the present study. However, a higher concentration of chelerythrine (10 microM), which is widely used for inhibiting PKC, made the vessels almost unresponsive to both phenylephrine and potassium stimuli. These effects suggest that chelerythrine at high concentrations can impair viability of the vessels, which may explain why basal tension tended to slightly increase in the group exposed to 10 microM chelerythrine. To this end we suggest that higher concentrations of chelerythrine may produce confounding results.
In summary, the present study revealed that non-selective inhibition of PKC by chelerythrine can suppress phenylephrine-induced but not potassium-induced contractions in rat mesenteric arteries. Further, the phenylephrine-induced contractions can also be attenuated in vivo by gene transfer of a dominant-negative mutant of PKCepsilon which also significantly suppresses contractile sensitivity to [Ca2+]i. Thus, PKC can modulate phenylephrine-induced contraction, possibly through changing calcium sensitivity in a PKC-epsilon dependent manner. This is one of the first papers to demonstrate the physiological modulation of blood vessel behavior in an intact animal using gene-transfer. Electroporation-mediated gene-transfer to the mesenteric vessel, as has been reported by Martin et al. (15), was successfully applied here without toxic side effects such as altered basal tone or loss of responses. Thus, this methodology should be appropriate for rapid screening and evaluation of certain gene products in the future.
Acknowledgments
This research was supported by grants from the National Institution of Health (NIDDK-51430 and HL59956). Travis J. Rutland was supported by the American Heart Association (AHA) Health Sciences Fellowship from the Southeastern Affiliate of the AHA. Jennifer Young was supported by the AHA, Midwestern Affiliate. The authors are grateful for the excellent technical support of Hong Yu.
References
- 1.Jiang MJ, Morgan KG. Agonist-specific myosin phosphorylation and intracellular calcium during isometric contractions of arterial smooth muscle. Pflügers Arch. 1989;413:637–643. doi: 10.1007/BF00581814. [DOI] [PubMed] [Google Scholar]
- 2.Singer HA, Baker KM. Calcium dependence of phorbol 12,13-dibutyrate-induced force and myosin light chain phosphorylation in arterial smooth muscle. J Pharmacol Exp Ther. 1987;243:814–821. [PubMed] [Google Scholar]
- 3.Sato K, Hori M, Ozaki H, Takano-Ohmuro H, Tsuchiya T, Sugi H, Karaki HH. Myosin phosphorylation-independent contraction induced by phorbol ester in vascular smooth muscle. J Pharmacol Exp Ther. 1992;261:497–505. [PubMed] [Google Scholar]
- 4.Fulginiti J, Singer HA, Moreland RS. Phorbol ester-induced contractions of swine carotid artery are supported by slowly cycling crossbridges which are not dependent on calcium or myosin light chain phosphorylation. J Vasc Res. 1993;30:315–322. doi: 10.1159/000159012. [DOI] [PubMed] [Google Scholar]
- 5.Takuwa Y. Regulation of vascular smooth muscle contraction. The roles of Ca2+, protein kinase C and myosin light chain phosphatase. Jpn Heart J. 1996;37:793–813. doi: 10.1536/ihj.37.793. [DOI] [PubMed] [Google Scholar]
- 6.Walker LA, Gailly P, Jensen PE, Somlyo AV, Somlyo AP. The unimportance of being (protein kinase C) epsilon. FASEB J. 1998;12:813–821. doi: 10.1096/fasebj.12.10.813. [DOI] [PubMed] [Google Scholar]
- 7.Fan J, Byron KL. Ca2+ signaling in rat vascular smooth muscle cells: a role for protein kinase C at physiological vasoconstrictor concentrations of vasopressin. J Physiol. 2000;524:821–831. doi: 10.1111/j.1469-7793.2000.00821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohanian V, Ohanian J, Shaw L, Scarth S, Parker PJ, Heagerty AM. Identification of Protein Kinase C Isoforms in Rat Mesenteric Small Arteries and Their Possible Role in Agonist-Induced Contraction. Circ Res. 1996;78:806–812. doi: 10.1161/01.res.78.5.806. [DOI] [PubMed] [Google Scholar]
- 9.Way KJ, Chou E, King GL. Identification of PKC-isoform-specific biological actions using pharmacological approaches. Trends Pharmacol Sci. 2000;21:181–187. doi: 10.1016/s0165-6147(00)01468-1. [DOI] [PubMed] [Google Scholar]
- 10.Guillemain I, Rossignol B. Protein kinase C and phospholipase D activation in rat parotid glands. FEBS Lett. 1995;363:13–16. doi: 10.1016/0014-5793(95)00264-a. [DOI] [PubMed] [Google Scholar]
- 11.Eckly-Michel AE, Le Bec A, Lugnier C. Chelerythrine, a protein kinase C inhibitor, interacts with cyclic nucleotide phosphodiesterases. Eur J Pharmacol. 1997;324:85–88. doi: 10.1016/s0014-2999(97)00149-0. [DOI] [PubMed] [Google Scholar]
- 12.Prekeris R, Mayhew MW, Cooper JB, Terrain DM. Identification and localization of an actin-binding motif that is unique to the epsilon isoform of protein kinase C and participates in the regulation of synaptic function. J Cell Biol. 1996;132:77–90. doi: 10.1083/jcb.132.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walsh MP, Horowitz A, Clément-Chomienne O, Andrea JE, Allen BG, Morgan KG. Protein kinase C mediation of Ca2+-independent contractions of vascular smooth muscle. Biochem Cel Biol. 1996;74:485–502. doi: 10.1139/o96-053. [DOI] [PubMed] [Google Scholar]
- 14.Leinweber B, Parissenti AM, Gallant C, Gangopadhyay S, Kirwan-Rhude A, Leavis PC, Morgan KG. Regulation of PKC by the cytoskeletal protein calponin. J Biol Chem. 2000;275:40329–40336. doi: 10.1074/jbc.M008257200. [DOI] [PubMed] [Google Scholar]
- 15.Martin JB, Young JN, Benoit JN, Dean DA. Gene Transfer to Intact Mesenteric Arteries by Electroporation. J Vasc Res. 2000;37:372–380. doi: 10.1159/000025753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ueda Y, Hirai S, Suzuki A, Mizuno K, Ohno S. Protein kinase C delta Activates the MEK-ERK Pathway in a Manner Independent of Ras and Dependent of Raf. J Biol Chem. 1996;271:23512–23519. doi: 10.1074/jbc.271.38.23512. [DOI] [PubMed] [Google Scholar]
- 17.Miranti CK, Ohno S, Brugge JS. Protein Kinase C Regulates Integrin-induced Activation of the Extracellular Regulated Kinase Pathway Upstream of Shc. J Biol Chem. 1998;274:10571–10581. doi: 10.1074/jbc.274.15.10571. [DOI] [PubMed] [Google Scholar]
- 18.Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- 19.Ali I, Sarna SK. Selective modulation of PKC isozymes by inflammation in canine colonic circular muscle cells. Gastroenterology. 2002;122:483–494. doi: 10.1053/gast.2002.31215. [DOI] [PubMed] [Google Scholar]
- 20.Schubert R, Noack T, Serebryakov VN. Protein kinase C reduces the KCa current of rat tail artery smooth muscle cells. Am J Physiol. 1999;276:C648–C658. doi: 10.1152/ajpcell.1999.276.3.C648. [DOI] [PubMed] [Google Scholar]
- 21.Woodsome TP, Eto M, Everett A, Brautigan DL, Kitazawa T. Expression of CPI-17 and myosin phosphatase correlates with Ca2+ sensitivity of protein kinase C-induced contraction in rabbit smooth muscle. J Physiol. 2001;535:553–564. doi: 10.1111/j.1469-7793.2001.t01-1-00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herbert JM, Augereau JM, Gleye J, Maffrand JP. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1990;172:993–999. doi: 10.1016/0006-291x(90)91544-3. [DOI] [PubMed] [Google Scholar]