Abstract
IL-1β and TNF-α are important proinflammatory cytokines that respond to mutated self-antigens of tissue damage and exogenous pathogens. The endoplasmic reticulum (ER) stress and unfolded protein responses are related to the induction of proinflammatory cytokines. However, the detailed molecular pathways by which ER stress mediates cytokine gene expression have not been investigated. In this study, we found that ER stress–induced inositol-requiring enzyme (IRE)1α activation differentially regulates proinflammatory cytokine gene expression via activation of glycogen synthase kinase (GSK)-3β and X-box binding protein (XBP)-1. Surprisingly, IL-1β gene expression was modulated by IRE1α-mediated GSK-3β activation, but not by XBP-1. However, IRE1α-mediated XBP-1 splicing regulated TNF-α gene expression. SB216763, a GSK-3 inhibitor, selectively inhibited IL-1β gene expression, whereas the IRE1α RNase inhibitor STF083010 suppressed only TNF-α production. Additionally, inhibition of GSK-3β greatly increased IRE1α-dependent XBP-1 splicing. Our results identify an unsuspected differential role of downstream mediators GSK-3β and XBP-1 in ER stress–induced IRE1α activation that regulates cytokine production through signaling cross-talk. These results have important implications in the regulation of inflammatory pathways during ER stress, and they suggest novel therapeutic targets for diseases in which meta-inflammation plays a key role.
Introduction
Inflammation, representing a primary response of the immune system following exposure to various stimuli (1, 2), is regulated by cytokines that are involved in host defense responses (3).
In recent years, endoplasmic reticulum (ER) stress has been considered as the underlying pathology of various inflammatory diseases, including obesity-induced type 2 diabetes, cardiovascular diseases, and cancer (4–6). The ER stress response is important for normal cellular homeostasis and may play a key role in the pathogenesis of many diseases (7). As a protective mechanism during ER stress, the unfolded protein response (UPR) is initiated through three distinct transmembrane signaling proteins, namely inositol-requiring enzyme (IRE)1, pancreatic ER kinase (PERK), and activating transcription factor (ATF)6 (8–11). The most conserved signaling pathway consists of IRE1, which activates the X-box binding protein (XBP)-1 via an unconventional cytosolic mRNA-splicing event. In response to ER stress, IRE1α autophosphorylation activates an endoribonuclease activity that cleaves a 26-base intron from the mRNA encoding XBP-1, resulting in the expression of an active form of XBP-1 with potent transcriptional activity. Active XBP-1 in turn induces the expression of UPR target genes, stimulates the production of inflammatory cytokine genes by enhancing TLR signaling, and promotes the differentiation of B lymphocytes and dendritic cells (12, 13).
Glycogen synthase kinase (GSK)-3, a multifunctional Ser/Thr kinase, is negatively regulated by the phosphorylation of serine in either of its two isoforms (Ser9 in GSK-3β or Ser21 in GSK-3α). Conversely, the enzymatic activity of GSK-3 is enhanced by phosphorylation of Tyr216 in GSK-3β and Tyr279 in GSK-3α (14). The recent identification of GSK-3 as a major regulator of peripheral inflammatory responses revealed that this protein promotes the production of several cytokines in animal models of inflammation (12, 14, 15). Recent studies indicate that GSK-3 is an important positive regulator of the inflammatory process, and that ER stress conditions can increase the activity of GSK-3β (16, 17). In this study, we identify that ER stress–induced IRE1α activation can mediate the cross-talk of GSK-3β and XBP-1 to differentially regulate proinflammatory cytokine production. We report that GSK-3β activation through ER stress–induced IRE1α activation regulated IL-1β production. However, we show that IRE1α-dependent splicing of XBP-1 regulates only TNF-α, suggesting that ER stress–induced IRE1α activation can operate independently of the production of inflammatory mediators, including IL-1β. In contrast, GSK-3β activation inhibited XBP-1 splicing, resulting in the downregulation of TNF-α production. Our data indicate that a heretofore unsuspected differential role of ER stress–induced IRE1α activation may explain how ER stress and TLR signaling can cross-talk to regulate the production of innate immune mediators.
Materials and Methods
Reagents
Tunicamycin (TM), thapsigargin (TG), 4-phenylbutyric acid (4-PBA), LPS, and SB216763 were from Sigma-Aldrich (St. Louis, MO). STF083010 was purchased from Tocris Biosciences (Ellisville, MO). Abs against GSK-3β were from Santa Cruz Biotechnology (Santa Cruz, CA). Abs against α-tubulin, phospho–GS (Ser641), GS, and p-PERK were from Cell Signaling Technology (Danvers, MA). The phospho-specific Ab to GSK-3α (pY279)/GSK-3β (pY216) was from Invitrogen. XBP-1 Ab was purchased from BioLegend (San Diego, CA). GRP78 Ab was purchased from Assay Design (Ann Arbor, MI). All other chemicals were from Sigma-Aldrich.
Mice
C57BL/6 mice (6 wk old, male) were purchased from Orient (Busan, Korea). The mice were maintained under specific pathogen-free conditions at 22°C and given access to food and water ad libitum. Two hours after SB216763 treatment, mice were injected with TM (3 mg/kg, i.p.) dissolved in 0.5% (v/v) DMSO/saline solution. The control mice received identical amounts of vehicle (0.5% DMSO/saline solution). At 6 h postinjection with TM, mice were sacrificed by cervical dislocation, and then serum and liver tissues were obtained for experiments.
Cell culture
Cell cultures were maintained at 37°C in humidified incubators containing an atmosphere of 95% air, 5% CO2 mouse RAW 264.7 cells were cultured in DMEM medium supplemented with 10% FBS and 1% penicillin/streptomycin (100 U/100 μg) solution (Life Technologies, Grand Island, NY). Mouse embryonic fibroblasts (MEFs) from wild-type, PERK, IRE1α, XBP-1, ATF6, and GSK-3β knockout mice were grown in DMEM medium supplemented with 10% FBS, 1% penicillin/streptomycin solution, and 1% MEM nonessential amino acid solution (Life Technologies).
Primary macrophage cells
C57BL/6 mice (6 wk old, male) were purchased from Orient. Murine peritoneal macrophage cells were isolated from the peritoneal lavage of mice 3 d after injection with 4% sterile thioglycollate. Bone marrow–derived macrophages (BMDMs) were isolated from the femurs of mice. Femurs were flushed out with PBS using a sterile needle. The bone marrow–derived cells were cultivated in DMEM medium (Life Technologies) containing 10% FBS and 10 ng/ml M-CSF. After 4–5 d, fully differentiated BMDMs had formed on the bottom of the culture plates. The isolated macrophage cells were then incubated at 37°C in DMEM medium supplemented with 10% FBS and 1% penicillin/streptomycin solution.
Transfection
Small interfering RNA (siRNA) against mouse IRE1α, XBP-1, and GSK-3β were purchased from Santa Cruz Biotechnology. RAW 264.7 cells were transfected with siRNA (100 nM) for 48 h using the Lipofectamine method according to the manufacturer’s protocol (Biontex Laboratories, Planegg, Germany). The interference of IRE1α and XBP-1 expression was confirmed by RT-PCR. Corresponding scrambled siRNAs were used as transfection controls.
Reverse-transcription PCR
Total RNA was isolated with TRIzol reagent (Invitrogen, Carlsbad, CA). First-strand cDNA was synthesized using 2 μg total RNA using Moloney murine leukemia virus reverse transcriptase and oligo(dT) 15 primer (Promega, Madison, WI). cDNA was amplified using primers specific for the human or the mouse genes corresponding to TNF-α, pro–IL-1β, C/EPB homologous protein (CHOP), and XBP-1(s) using RT-PCR. Specific primer sequences are as follows: mouse TNF-α, sense, 5′-AGC CCA CGT CGT AGC AAA CCA CCA A-3′, antisense, 5′-ACA CCC ATT CCC TTC ACA GAG CAA T-3′; mouse CHOP, sense, 5′-CCC AGG AAA CGA AGA GGA AG-3′, antisense, 5′-AGT GCA GTG CAG GGT CAC AT-3′; mouse XBP-1(s), sense, 5′-GAA CCA GGA GTT AAG AAC ACG-3′, antisense, 5′-AGG CAA CAG TGT CAG AGT CC-3′; mouse 18S, sense, 5′-CAG TGA AAC TGC GAA TGG CT-3′, antisense, 5′-TGC CTT CCT TGG ATG TGG TA-3′; mouse GAPDH, sense, 5′-AGG CCG GTG CTG AGT ATG TC-3′, antisense, 5′-TGC CTG CTT CAC CTT CT-3′. Primers for mouse IL-1β was supplied by Bioneer (N_4009). The PCR products were detected on 2% agarose gels. Gene expression data from RT-PCR was quantified relative to GAPDH or 18S.
Real-time quantitative RT-PCR
Real-time quantitative RT-PCR was performed using SYBR Green qPCR Master Mix (2×; USB, Affymetrix) on an ABI 7500 fast real-time PCR system (Applied Biosystems). PCR primer pairs were as follows: IL-1β, 5′-TCGCTCAGGGTCACAAGAAA-3′, 5′-ATCAGAGGCAAGGAGGAAACAC-3′; TNF-α, 5′-GACCCTCACACTCAGATCATCTTC-3′, 5′-TTGCTACGACGTGGGCTAC A-3′; GAPDH, 5′-GGGAAGCCCATCACCATCT-3′, 5′-CGGCCTCACCCCATTTG-3′; IL-6, 5′-CCAGAGATACAAAGA AATGATGG-3′, 5′-ACTCCAGAAGACCAGAGGAAAT-3′; splicing XBP-1, 5′-GAGTCCGCAGCAGGTG-3′, 5′-AGG CTTGGTGTATACATGG-3′.
Immunoblot analysis
Following experimental treatments, cells were harvested and washed twice with ice-cold PBS. Cells were lysed with 1× RIPA buffer containing protease and phosphatase inhibitors. Equal amounts of cell lysates were measured using a BCA protein assay reagent (Pierce Biotechnology, Rockford, IL). The samples were diluted with 2× sample buffer containing 5% 2-ME, and then an equal amount of protein was resolved on SDS-PAGE gels followed by transfer to polyvinylidene difluoride membranes. The membranes were blocked with 5% nonfat milk in PBS containing 0.1% Tween 20 (PBS-T) for 20 min and then incubated overnight with Abs in PBS-T containing 1% nonfat milk. The blots were developed with a peroxidase-conjugated secondary Ab and reacted proteins were visualized using the ECL Plus Western blotting setection aystem (GE Healthcare Life Sciences, Buckinghamshire, U.K.). The relative signal intensity of the bands was determined and standardized using Scion Image Software (Scion, Frederick, MD).
ELISA
Mouse sera were assayed for cytokine production by ELISA using a commercial cytokine ELISA DuoSet kit for mouse IL-1β (DY401, R&D Systems), mouse TNF-α (DY410, R&D Systems), and mouse IL-6 (DY406, R&D Systems).
Statistical analyses
Data were expressed as means ± SD. Statistical analysis was performed by nonparametric t tests for paired data using GraphPad Prism (GraphPad Software, La Jolla, CA). A p value <0.05 was considered statistically significant.
Results
ER stress–induced GSK-3β activation differently regulates IL-1β and TNF-α mRNA expression in cultured macrophages
ER stress–mediated UPRs have been suggested to be involved in the activation of NF-κB, which regulates genes encoding proinflammatory cytokines such as IL-1β, TNF-α, and IL-6 (18–22). Moreover, activation of NF-κB and expression of the proinflammatory cytokines have been observed in the liver of mice challenged with the pharmacologic ER stress inducer TM (23).
To test whether ER stress–mediated UPR activation can induce inflammatory responses, we treated RAW 264.7 macrophages with the chemical ER stress–inducing compound TM and measured proinflammatory cytokine gene expression by RT-PCR. As shown in Supplemental Fig. 1A, ER stress induced the upregulation of IL-1β and TNF-α gene expression in a manner similar to LPS, indicating that an ER stress–induced downstream event can regulate the expression of proinflammatory cytokines. Consistent with previous reports (19), the expression of CHOP, a classic ER stress response, was induced by TM treatment, but not by LPS treatment (Supplemental Fig. 1A).
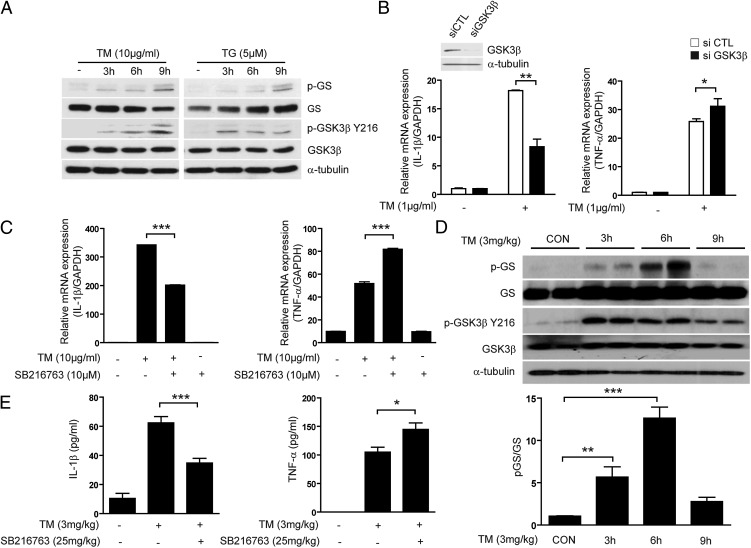
Several studies have identified that ER stress can activate GSK-3β, which in turn can accelerate inflammation and lipid accumulation (16, 20). Moreover, GSK-3β activation can play a role in NF-κB activation in vitro and in vivo (24, 25), implying that during ER stress, GSK-3β activation may be involved in activation of the NF-κB–mediated inflammatory pathway. Therefore, we sought to determine whether ER stress can modulate GSK-3β activation in RAW 264.7 macrophages. We confirmed that TM and TG stimulated GSK-3β expression, as determined by immunoblot analysis (Fig. 1A). As expected, ER stress–inducing compounds significantly increased GSK-3β activation, leading to the time-dependent phosphorylation of GS and Tyr216 GSK-3β (Fig. 1A). We hypothesized that ER stress–induced upregulation of proinflammatory cytokine gene expression may occur downstream of GSK-3β activation. To establish the function of GSK-3β in ER stress–induced proinflammatory cytokine gene expression, we used siRNA specific for GSK-3β. Surprisingly, knockdown of GSK-3β in RAW 264.7 macrophages resulted in reduced gene expression of IL-1β in response to TM, whereas the gene expression of TNF-α was increased (Fig. 1B). To further confirm these distinct cytokine gene expression patterns, RAW 264.7 macrophages were treated with SB216763, a cell-permeable–specific inhibitor of GSK-3. Pretreatment with SB216763 completely inhibited TM-induced GSK-3β kinase activity (Supplemental Fig. 1B). Similar to the effects of GSK-3β siRNA on TM-induced cytokine production, SB216763 significantly inhibited gene expression and production of IL-1β (Fig. 1C, Supplemental Fig. 1C). Interestingly, pretreatment with SB216763 enhanced the gene and protein expression of TNF-α (Fig. 1C, right panel). Additionally, GSK-3β knockout MEFs inhibit TM-induced IL-1β mRNA via absence of GSK-3β, whereas TNF-α mRNA was increased (Supplemental Fig. 1D).
FIGURE 1.
ER stress–induced GSK-3β activation regulates proinflammatory cytokine IL-1β gene expression in vitro and vivo. (A) RAW 264.7 cells were treated with TM (10 μg/ml) and TG (5 μM) for the indicated times and then protein expression for p-GS, GS, GSK-3α (pY279)/β (pY216), and GSK-3β were determined by Western immunoblot analysis. (B) RAW 264.7 cells were transfected with specific siRNA against GSK-3β or control siRNA (scramble) for 24 h and the level of endogenous GSK-3β was measured by Western immunoblot analysis (upper panel). Under these conditions, the mRNA expression of TNF-α and IL-1β was measured by quantitative real-time PCR from cells treated with TM (1 μg/ml) for 3 h (lower panels). (C) RAW 264.7 cells were treated with TM (10 μg/ml) in the absence or presence of SB216763 treatment for 3 h and the level of IL-1β and TNF-α mRNA was determined by quantitative real-time PCR. (D) C57BL/6 mice (n = 20) were injected with TM (3 mg/kg, i.p.). Mice were sacrificed at the indicated times, and then liver tissue was collected for the analysis of GSK-3β activity by Western immunoblotting (upper panel) and bar graphs representing the relative density of p-GS (lower panel). α-Tubulin was served as the standard. (E) C57BL/6 mice were preinjected with SB216763 (25 mg/kg) and then injected 2 h later with TM (3 mg/kg, i.p.). Mice were sacrificed at 6 or 12 h postinjection and then blood was collected for the analysis of serum IL-1β (12 h) and TNF-α (6 h) by ELISA. Data represent means ± SD of three independent determinations. Blots shown are representative of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
ER stress–induced GSK-3β activation differently regulates transcription of IL-1β and TNF-α in vivo
We sought to determine whether ER stress can induce GSK-3β activation and increase inflammatory responses in vivo, and whether pharmacological inhibition of GSK-3β could inhibit this effect. C57BL/6 mice were randomized into three groups: 1) saline-treated control, 2) treatment with TM (3 mg/kg), or 3) cotreatment with TM (3 mg/kg) and SB216763 (25 mg/kg). Mouse liver sections were examined to determine GSK-3β activation and levels of IL-1β mRNA and TNF-α mRNA. The results indicate that TM treatment resulted in significantly increased activity of GSK-3β (Fig. 1D, upper panel) and bar graphs representing the relative density of p-GS that was normalized with total GS (Fig. 1D, lower panel). The GSK-3 inhibitor SB216763 abolished the ability of TM to induce activation of GSK-3β (Supplemental Fig. 1E). Additionally, preinjection of SB216763 resulted in the inhibition of IL-1β and activation of caspase-1, whereas TNF-α production was enhanced in the serum (Fig. 1E, Supplemental Fig. 1F) and liver (Supplemental Fig. 1G) of mice injected with TM. To examine the stability of cytokine mRNA, we inhibited transcription in RAW264.8 cells with actinomycinD and measured mRNA levels using real-time PCR (Supplemental Fig. 1H). At both the 20 min time point and the 40 min time point after treatment of actinomycin D, the remaining IL-1β mRNA in SB216763-treated cells was significantly reduced compared with that in the control cells, but the gene expression of TNF-α was increased. However, we did not observe a significant difference in half-life of cytokine mRNA between the TM-treated cells and TM with SB216763-treated cells. Based on our in vitro and in vivo results, ER stress can induce proinflammatory cytokine gene expression, and GSK-3β activation could be associated with the upregulation of IL-1β and downregulation TNF-α gene expression.
ER stress–induced IRE1α activation regulates two different downstream molecules, GSK-3β and XBP-1
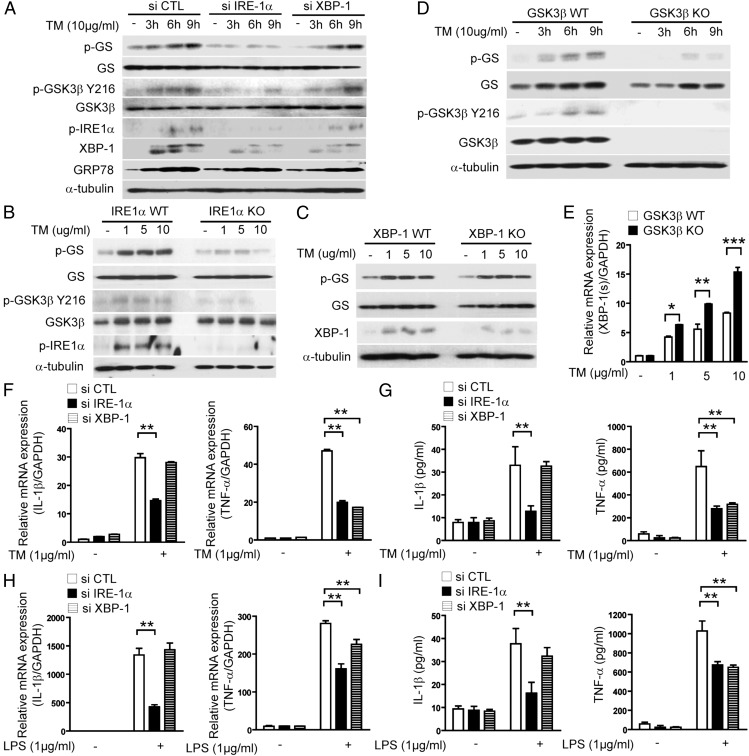
Upon examining the interactions between GSK-3β and ER stress–induced signaling pathways, we then sought to determine whether the expression of IRE1α was required for GSK-3β activation. In RAW 264.7 macrophages, knockdown of IRE1α significantly inhibited TM-induced GSK-3β activation, whereas knockdown of XBP-1 did not affect GSK-3β activation (Fig. 2A). Furthermore, knockout of the IRE and GSK-3β gene in wild-type MEFs completely blocked TM-induced GSK-3β activation (Fig. 2B, 2D). In contrast, TM-induced GSK-3β activation was not impaired in XBP-1–, PERK-, and ATF6-deficient MEFs (Fig. 2C, Supplemental Fig. 2A, 2B). These experiments suggested that activation of the IRE1α signaling pathway by ER stress was required for GSK-3β activation, which differently regulated IL-1β mRNA and TNF-α mRNA expression.
FIGURE 2.
ER stress–induced IRE1α activation regulates differential proinflammatory cytokine gene expression via activation of GSK-3β and XBP-1. (A) RAW 264.7 cells were transfected with specific siRNA against IRE1α, XBP-1, or control siRNA (scramble). Under these conditions, the activation of GSK-3β in TM-treated cells was determined by Western immunoblot analysis. (B) Wild-type (WT) or IRE1α-, (C) XBP-1–, and (D) GSK-3β–deficient MEFs were treated with TM for 9 h or indicated times and then activation of GSK-3β was determined by Western immunoblot analysis. α-Tubulin served as the standard. (E) GSK-3β–deficient MEFs were treated with TM for 3 h and then the level of XBP-1(s) mRNA was determined by quantitative real-time PCR. (F–I) RAW 264.7 cells were transfected with specific siRNA against IRE1α, XBP-1, or control siRNA (scramble), and the level of TNF-α and IL-1β was determined by quantitative real-time PCR (F and H) and ELISA (G and I) from RAW 264.7 cells treated with (F and G) TM (1 μg/ml) or (H and I) LPS (1 μg/ml). GAPDH served as the standard. Data represent means ± SD of three independent determinations. Blots shown are representative of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
We next examined whether knockdown of IRE1α and XBP-1 in macrophages could prevent IL-1β mRNA and TNF-α mRNA expression in response to TM and LPS via inactivation of GSK-3β. As shown in Fig. 2F–I, IRE1α siRNA, but not XBP-1 siRNA, inhibited TM- and LPS-induced IL-1β mRNA and protein levels, suggesting that IRE1α-mediated GSK-3β activation may be responsible for TM- and LPS-induced IL-1β expression. Interestingly, both IRE1α siRNA and XBP-1 siRNA inhibited TM- and LPS-induced TNF-α mRNA and protein levels (Fig. 2F–I). Furthermore, knockout of the IRE1α, but not XBP-1, in MEFs reduced TM-induced IL-1β mRNA expression (Supplemental Fig. 2C, 2D, left panel) and both knockout of the IRE1α and XBP-1 in MEFs reduced TM-induced TNF-α mRNA expression (Supplemental Fig. 2C, 2D, right panel). These results suggest that endonuclease activity of IRE1α is associated with regulation of TNF-α transcription.
GSK-3β activation differently regulates transcription of IL-1β and TNF-α in response to ER stress and TLR4 signaling
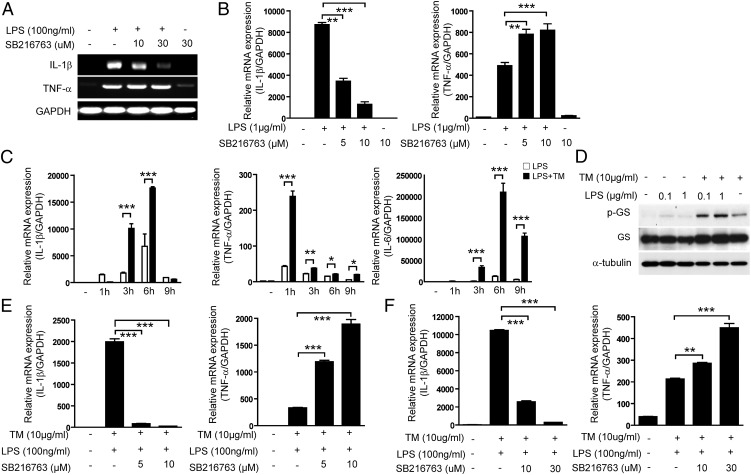
We also speculated that GSK-3β activation in macrophages might be involved in differential regulation of LPS-induced transcription of IL-1β and TNF-α. As expected, SB216763 treatment significantly reduced LPS-induced IL-1β mRNA expression and enhanced LPS-induced TNF-α mRNA expression in primary BMDMs and RAW 264.7 cells (Fig. 3A, 3B). These results are similar to the observed effects of SB216763 on TM-induced expression of IL-1β and TNF-α mRNA (Fig. 1C).
FIGURE 3.
Effects of GSK-3β on the cross-talk between ER stress and TLR signaling. (A) Primary BMDMs were treated with LPS in the absence or presence of SB216763 treatment for 9 h and the levels of IL-1β and TNF-α mRNA were determined by RT-PCR. (B) RAW 264.7 cells were treated with LPS in the absence or presence of SB216763 treatment for 3 h and the levels of IL-1β and TNF-α mRNA were determined by quantitative real-time PCR. (C) RAW 264.7 cells were treated with TM (10 μg/ml) in the presence or absence of LPS (100 ng/ml). Expression of IL-1β, TNF-α, and IL-6 mRNA was determined by quantitative real-time PCR. (D) RAW 264.7 cells were treated with TM (10 μg/ml) and/or LPS for 9 h and then protein expression for p-GS and GS was determined by Western immunoblot analysis. α-Tubulin served as the standard. (E) Mouse primary peritoneal macrophages and (F) RAW 264.7 cells were treated with TM (10 μg/ml) and LPS for 3 h in the absence or presence of SB216763, an inhibitor of GSK. The expression of IL-1β and TNF-α mRNA was determined by quantitative real-time PCR, with GAPDH as the standard. Data represent means ± SD of three independent determinations. Blots shown are representative of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
ER stress–mediated XBP-1 activation can enhance TLR signaling to promote the secretion of selected proinflammatory cytokines, and it has also been implicated in GSK-3β activation (18).
Indeed, treatment of RAW 264.7 cells with TM significantly augmented LPS-induced production and transcription of TNF-α, IL-1β, and IL-6 (Fig. 3C), which resulted, in part, from increased GSK-3β activation observed during concurrent LPS and TM simulation (Fig. 3D).
We further asked whether GSK-3β activation can regulate the transcription of IL-1β and TNF-α by cotreatment with TM and LPS in macrophages. Inhibition of GSK-3β activation by SB216763 in primary BMDMs and RAW 264.7 macrophages stimulated with LPS and TM markedly downregulated IL-1β transcription and upregulated TNF-α transcription (Fig. 3E, 3F).
GSK-3β inhibits XBP-1 splicing triggered by ER stress and/or TLR4 signaling that mediates TNF-α gene expression
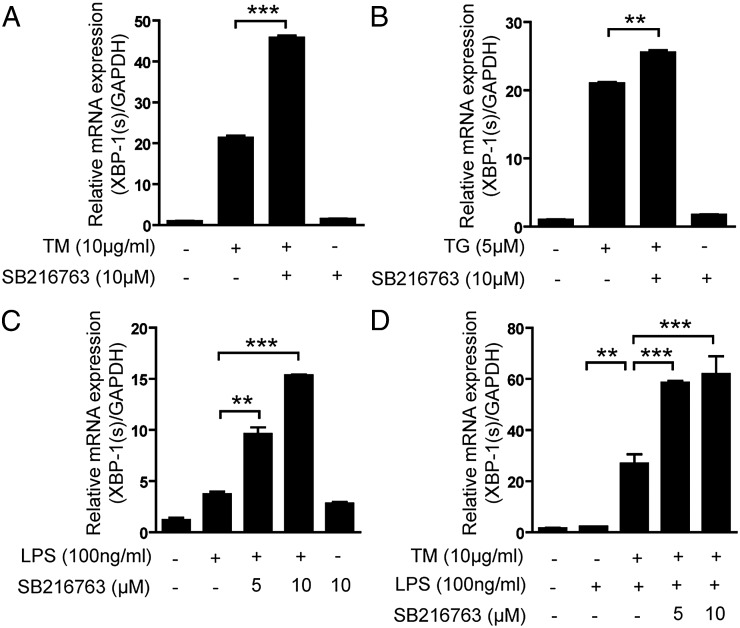
During ER stress, XBP-1 is a key effector of the UPR (26). IRE1α-mediated splicing of XBP-1 mRNA generates XBP-1s, a potent transcriptional activator, whereas the unspliced XBP-1u contains the DNA binding N-terminal domain, but not the trans-activating C-terminal domain. A previous report has shown that macrophages isolated from XBP-1 mutant mice stimulated with ER stress inducers and TLR4 agonists displayed impaired proinflammatory cytokine gene expression (e.g., TNF-α, IL-6, and COX2). However, other cytokines such as IL-1β and RANTES were unaffected by XBP-1 deficiency (27). We sought to elucidate the role of GSK-3β in XBP-1 splicing. As shown in Fig. 4, pharmacologic inhibition of GSK-3β activation by SB216763 in RAW 264.7 macrophages stimulated with TM (Fig. 4A), TG (Fig. 4B), or LPS alone (Fig. 4C), or TM in combination with LPS (Fig. 4D), resulted in increased XBP-1s production. Furthermore, knockout of GSK-3β in MEFs significantly increased XBP-1s production compared with WT cells by TM treatment in a dose-dependent (Fig. 2E) or time-dependent manner (Supplemental Fig. 2E), suggesting that IRE1α-dependent GSK-3β activation participated in a pathway leading to inhibition of the endonuclease activity of IRE1α. This finding may provide an explanation for why pharmacologic and genetic inhibition of GSK-3β resulted in enhanced TNF-α transcription that was dependent on the endonuclease activity of IRE1α.
FIGURE 4.
GSK-3β inhibits ER stress– or LPS-induced XBP-1 splicing that mediates TNF-α gene expression. RAW 264.7 cells were treated with (A) TM (10 μg/ml), (B) TG (5 μM), (C) LPS (100 ng/ml), and (D) TM with LPS in the presence or absence of SB216763, and the activation of XBP-1 was determined by quantitative real-time PCR. Data represent means ± SD of three independent determinations. **p < 0.01, ***p < 0.001.
IRE1α-dependent XBP-1 splicing is involved in ER stress–induced TNF-α gene activation
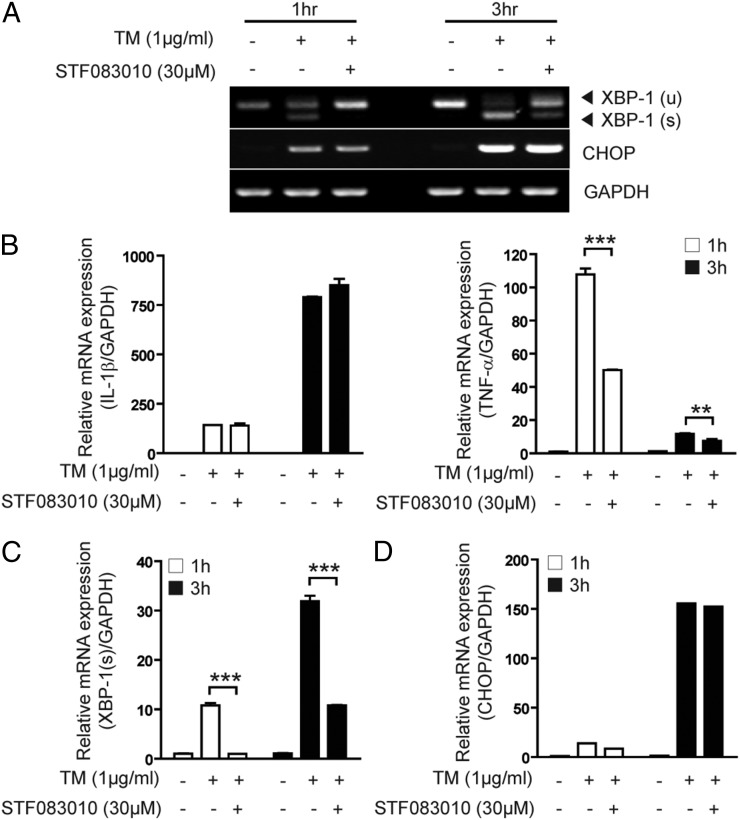
To further elucidate the potential signaling mechanisms involved in ER stress–induced TNF-α transcription in macrophages, we used STF-083010, a novel small-molecule inhibitor of IRE1α that inhibits IRE1α endonuclease activity (28, 29). Treatment of RAW 264.7 macrophages with STF-083010 resulted in specific inhibition of TM-induced XBP-1 splicing (Fig. 5A, 5C), with no significant change in CHOP expression (Fig. 5A, 5D) and GSK-3β and IRE1α activation (Supplemental Fig. 3). Moreover, STF-083010 prevented TM-induced TNF-α transcription, whereas TM-induced IL-1β transcription was not affected by this inhibitor (Fig. 5B). These results suggest that ER stress might induce TNF-α expression through IRE1α-dependent XBP-1 splicing.
FIGURE 5.
The expression of TNF-α, but not of IL-1β, is dependent on IRE1α endonuclease activity. RAW 264.7 cells were treated with TM (10 μg/ml) for 1 or 3 h in the absence or presence of STF083010. (A) Activation of XBP-1 and CHOP mRNA expression were determined by RT-PCR. GAPDH served as the standard. The expression of (B) IL-1β and TNF-α, (C) XBP-1 splicing, and (D) CHOP mRNA was determined by quantitative real-time PCR. GAPDH served as the standard. Data represent means ± SD of three independent determinations. **p < 0.01, ***p < 0.001.
A chemical chaperone reduces IL-1β and TNF-α transcription induced by ER stress
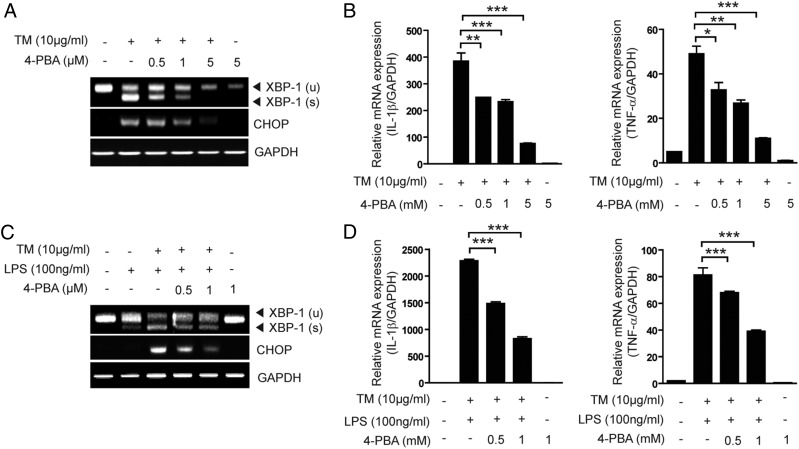
Finally, we hypothesized that 4-PBA, a chemical chaperone, would prevent TM-induced transcription of IL-1β and TNF-α through inhibition of ER stress–induced IRE1α activation. Therefore, we examined whether 4-PBA could inhibit TM-induced splicing of XBP-1 and transcription of IL-1β and TNF-α in RAW 264.7 macrophages. 4-PBA, through its action as an ER stress inhibitor, repressed TM-induced CHOP expression and also reduced TM-induced production of XBP-1 (Fig. 6A, Supplemental Fig. 4A). This was accompanied by a dose-dependent decrease in TM-induced transcription of IL-1β and TNF-α (Fig. 6B). We also examined whether 4-PBA could prevent XBP-1 splicing and IL-1β and TNF-α transcription induced by cotreatment with TM and LPS. XBP-1s production induced by cotreatment with TM and LPS was reduced by 4-PBA treatment (Fig. 6C). Transcription of IL-1β and TNF-α by cotreatment of RAW 264.7 macrophages and primary BMDM with TM and LPS was also reduced by 4-PBA treatment (Fig. 6D, Supplemental Fig. 4B).
FIGURE 6.
4PBA, a chemical chaperon, reduces ER stress inducer-mediated proinflammatory cytokine gene expression. (A and B) RAW 264.7 cells were treated with TM (10 μg/ml) for 3 h in the absence or presence of 4-PBA. (A) XBP-1 splicing and CHOP mRNA expression were determined by RT-PCR. (B) IL-1β and TNF-α mRNA expression were determined by quantitative real-time PCR. (C and D) RAW 264.7 cells were treated with TM (10 μg/ml) and/or LPS for 3 h in the absence or presence of 4-PBA. (C) XBP-1 splicing and CHOP mRNA expression were determined by RT-PCR. GAPDH served as the standard. (D) IL-1β and TNF-α mRNA expression were determined by quantitative real-time PCR. GAPDH served as the standard. Data represent means ± SD of three independent determinations. *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
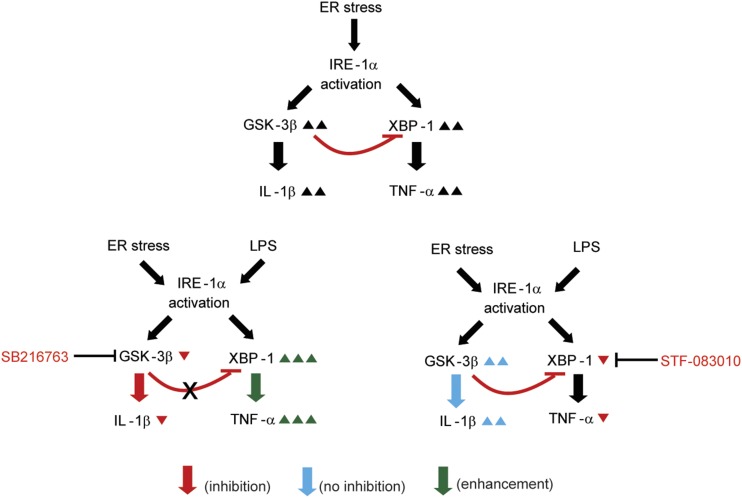
The results of the present study provide mechanistic insights regarding the relative roles of GSK-3β and XBP-1 in the regulation of proinflammatory cytokines during ER stress. The principal findings of this study indicate that: 1) IL-β and TNF-α transcription and GSK-3β activation increase during ER stress in vitro and in vivo; 2) pharmacological or genetic inhibition of GSK-3 attenuates IL-1β transcription (but not TNF-α transcription); 3) IRE1α induces GSK-3β activation; 4) IRE1α-mediated GSK-3β activation results in the suppression of XBP-1 splicing; and 5) TNF-α transcription is regulated by IRE1α-dependent XBP-1 splicing. A scheme is provided to illustrate each of these results (Fig. 7).
FIGURE 7.
Schematic representation of the proposed pathways for the transcriptional regulation of IL-1β and TNF-α during IRE1α activation in response to ER stress. IRE1α-mediated GSK-3β activation results in the selective transcription of IL-1β and inhibition of XBP-1 splicing. In contrast, IRE1α-mediated XBP-1 activation results in the transcription of TNF-α.
Our findings support the hypothesis that ER stress can trigger inflammation. In fact, all three arms of the UPR have been suggested to be involved in the activation of NF-κB, which regulates genes encoding proinflammatory cytokines such as IL-1β, TNF-α, and IL-6 (16, 18, 19). For instance, IRE1α together with TNFR-associated factor 2 binds to and activates I-κB kinase, which induces I-κB degradation, thereby leading to NF-κB activation (20–22). The PERK–eIF2α pathway promotes NF-κB activation by suppressing I-κB translation (26, 30, 31). ATF6 can induce NF-κB activation through Akt phosphorylation (30). Moreover, activation of NF-κB and expression of the genes encoding TNF-α and IL-6 have been observed in the liver of mice challenged with the pharmacologic ER stress inducer TM, suggesting the activation of the NF-κB–mediated inflammatory pathway (23). In our study, we found that treatment of mice with TM significantly increased the transcription of IL-1β and TNF-α in the liver and increased the plasma levels of these inflammatory cytokines. We also found that TM challenge induced GSK-3β activation in the liver. Based on our in vitro and in vivo results, GSK-3β activation was associated with the transcription of IL-1β and TNF-α and secretion of these cytokines.
ER stress conditions have been shown to increase the activity of GSK-3β in cultured cells (16). Moreover, GSK-3β activation can play a role in NF-κB activation in vivo and in vitro (24, 25), implying that during ER stress, GSK-3β activation may be involved in activation of the NF-κB–mediated inflammatory pathway. A number of recent findings have linked GSK-3β to apoptosis (32, 33). Our finding that GSK-3β activation is involved in the regulation of IL-1β and TNF-α transcription in response to ER stress is novel and important. In our study, GSK-3β activation by TM treatment, along with the coincident expression of IL-1β and TNF-α genes, was evident in cultured macrophages as well as the liver of mice. In vitro and in vivo inhibition of GSK-3 activity with the GSK-3 inhibitor SB216763 reduced IL-1β transcription and enhanced TNF-α transcription, suggesting that GSK-3β activation is a regulatory step in inflammation caused by ER stress. This conclusion was further substantiated by the finding that knockdown of GSK-3β resulted in reduced IL-1β transcription and enhanced TNF-α transcription in cultured macrophages stimulated with TM.
In examining the hypothesis that ER stress–induced signaling pathways may influence the activation of GSK-3β, we made the unexpected observation that the UPR transducer IRE1α regulated GSK-3β activation in response to ER stress. Interestingly, the ability of ER stress to activate GSK-3β was completely abolished in IRE1α-deficient MEFs, whereas GSK-3β activation was normal in MEFs deficient in PERK, XBP-1, or ATF6. Moreover, inhibition of IRE1α expression using siRNA markedly suppressed TM-induced activation of GSK-3β in macrophages, whereas siRNA targeting of XBP-1 had no effect. These results suggest a novel signaling role for IRE1α in GSK-3β activation in response to ER stress.
Interestingly, we found that the endonuclease activity of IRE1α was associated with regulation of TNF-α transcription. Knockdown of either IRE1α or XBP-1 expression, using two independent siRNAs for each target, markedly suppressed TM-induced TNF-α transcription in macrophages. Moreover, inhibition of the endonuclease activity of IRE1α by a pharmacologic inhibitor (STF083010) significantly reduced TM-induced TNF-α transcription. Although these findings suggest a regulatory role of IRE1α, through its endonuclease activity, in TNF-α transcription, the endonuclease activity of IRE1α was not associated with regulation of IL-1β transcription. Both siRNA targeting of XBP-1 and STF083010 were inactive against IL-1β transcription in response to ER stress. Thus, it is most likely that IRE1α may play differential roles in expression of IL-1β and TNF-α genes in response to ER stress. Whereas TNF-α transcription was regulated through both IRE1α-dependent GSK-3β activation and endonuclease activation of IRE1α, IL-1β transcription was preferentially regulated through IRE1α-dependent GSK-3β activation. Interestingly, IRE1α-dependent GSK-3β activation participated in a pathway leading to inhibition of the endonuclease activity of IRE1α. Pharmacologic inhibition of GSK-3β significantly increased XBP-1 splicing induced by TM, LPS, or TM and LPS, indicating that the endonuclease activity of IRE1α was downregulated by GSK-3β activation. This finding may provide an explanation for why pharmacologic and genetic inhibition of GSK-3β resulted in enhanced TNF-α transcription that was dependent on the endonuclease activity of IRE1α.
Supplementary Material
This work was supported by a Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2014R1A6A1030318) and by the Bio and Medical Technology Development Program of the NRF funded by the Ministry of Science, ICT and Future Planning (2012M3A9C3048687).
The online version of this article contains supplemental material.
- ATF
- activating transcription factor
- BMDM
- bone marrow–derived macrophage
- CHOP
- C/EPB homologous protein
- ER
- endoplasmic reticulum
- GS
- glycogen synthase
- GSK
- glycogen synthase kinase
- IRE
- inositol-requiring enzyme
- MEF
- mouse embryonic fibroblast
- 4-PBA
- 4-phenylbutyric acid
- PERK
- pancreatic endoplasmic reticulum kinase
- siRNA
- small interfering RNA
- TG
- thapsigargin
- TM
- tunicamycin
- UPR
- unfolded protein response
- XBP
- X-box binding protein.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Medzhitov R. 2007. Recognition of microorganisms and activation of the immune response. Nature 449: 819–826. [DOI] [PubMed] [Google Scholar]
- 2.Chen K., Huang J., Gong W., Iribarren P., Dunlop N. M., Wang J. M. 2007. Toll-like receptors in inflammation, infection and cancer. Int. Immunopharmacol. 7: 1271–1285. [DOI] [PubMed] [Google Scholar]
- 3.Wellen K. E., Hotamisligil G. S. 2005. Inflammation, stress, and diabetes. J. Clin. Invest. 115: 1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolattukudy P. E., Niu J. 2012. Inflammation, endoplasmic reticulum stress, autophagy, and the monocyte chemoattractant protein-1/CCR2 pathway. Circ. Res. 110: 174–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cnop M., Foufelle F., Velloso L. A. 2012. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol. Med. 18: 59–68. [DOI] [PubMed] [Google Scholar]
- 6.Gotoh T., Endo M., Oike Y. 2011. Endoplasmic reticulum stress-related inflammation and cardiovascular diseases. Int. J. Inflam. 2011: 259462.doi:10.4061/2011/259462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaufman R. J. 1999. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 13: 1211–1233. [DOI] [PubMed] [Google Scholar]
- 8.Schröder M., Kaufman R. J. 2005. The mammalian unfolded protein response. Annu. Rev. Biochem. 74: 739–789. [DOI] [PubMed] [Google Scholar]
- 9.Zhang K., Kaufman R. J. 2004. Signaling the unfolded protein response from the endoplasmic reticulum. J. Biol. Chem. 279: 25935–25938. [DOI] [PubMed] [Google Scholar]
- 10.Harding H. P., Zhang Y., Ron D. 1999. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397: 271–274. [DOI] [PubMed] [Google Scholar]
- 11.Haze K., Yoshida H., Yanagi H., Yura T., Mori K. 1999. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell 10: 3787–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin M., Rehani K., Jope R. S., Michalek S. M. 2005. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat. Immunol. 6: 777–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Todd D. J., McHeyzer-Williams L. J., Kowal C., Lee A. H., Volpe B. T., Diamond B., McHeyzer-Williams M. G., Glimcher L. H. 2009. XBP1 governs late events in plasma cell differentiation and is not required for antigen-specific memory B cell development. J. Exp. Med. 206: 2151–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jope R. S., Yuskaitis C. J., Beurel E. 2007. Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem. Res. 32: 577–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cortés-Vieyra R., Bravo-Patiño A., Valdez-Alarcón J. J., Juárez M. C., Finlay B. B., Baizabal-Aguirre V. M. 2012. Role of glycogen synthase kinase-3 beta in the inflammatory response caused by bacterial pathogens. J. Inflamm. (Lond.) 9: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McAlpine C. S., Bowes A. J., Khan M. I., Shi Y., Werstuck G. H. 2012. Endoplasmic reticulum stress and glycogen synthase kinase-3β activation in apolipoprotein E-deficient mouse models of accelerated atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 32: 82–91. [DOI] [PubMed] [Google Scholar]
- 17.Huang W. C., Lin Y. S., Chen C. L., Wang C. Y., Chiu W. H., Lin C. F. 2009. Glycogen synthase kinase-3beta mediates endoplasmic reticulum stress-induced lysosomal apoptosis in leukemia. J. Pharmacol. Exp. Ther. 329: 524–531. [DOI] [PubMed] [Google Scholar]
- 18.Robertson L. A., Kim A. J., Werstuck G. H. 2006. Mechanisms linking diabetes mellitus to the development of atherosclerosis: a role for endoplasmic reticulum stress and glycogen synthase kinase-3. Can. J. Physiol. Pharmacol. 84: 39–48. [DOI] [PubMed] [Google Scholar]
- 19.Tabata Y., Takano K., Ito T., Iinuma M., Yoshimoto T., Miura H., Kitao Y., Ogawa S., Hori O. 2007. Vaticanol B, a resveratrol tetramer, regulates endoplasmic reticulum stress and inflammation. Am. J. Physiol. Cell Physiol. 293: C411–C418. [DOI] [PubMed] [Google Scholar]
- 20.Kim A. J., Shi Y., Austin R. C., Werstuck G. H. 2005. Valproate protects cells from ER stress-induced lipid accumulation and apoptosis by inhibiting glycogen synthase kinase-3. J. Cell Sci. 118: 89–99. [DOI] [PubMed] [Google Scholar]
- 21.Kaneko M., Niinuma Y., Nomura Y. 2003. Activation signal of nuclear factor-κB in response to endoplasmic reticulum stress is transduced via IRE1 and tumor necrosis factor receptor-associated factor 2. Biol. Pharm. Bull. 26: 931–935. [DOI] [PubMed] [Google Scholar]
- 22.Hu P., Han Z., Couvillon A. D., Kaufman R. J., Exton J. H. 2006. Autocrine tumor necrosis factor α links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1α-mediated NF-κB activation and down-regulation of TRAF2 expression. Mol. Cell. Biol. 26: 3071–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim S., Joe Y., Jeong S. O., Zheng M., Back S. H., Park S. W., Ryter S. W., Chung H. T. 2014. Endoplasmic reticulum stress is sufficient for the induction of IL-1β production via activation of the NF-κB and inflammasome pathways. Innate Immun 20: 799–815. [DOI] [PubMed] [Google Scholar]
- 24.Demarchi F., Bertoli C., Sandy P., Schneider C. 2003. Glycogen synthase kinase-3β regulates NF-κB1/p105 stability. J. Biol. Chem. 278: 39583–39590. [DOI] [PubMed] [Google Scholar]
- 25.Schwabe R. F., Brenner D. A. 2002. Role of glycogen synthase kinase-3 in TNF-α-induced NF-κB activation and apoptosis in hepatocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 283: G204–G211. [DOI] [PubMed] [Google Scholar]
- 26.Kim H. P., Pae H. O., Back S. H., Chung S. W., Woo J. M., Son Y., Chung H. T. 2011. Heme oxygenase-1 comes back to endoplasmic reticulum. Biochem. Biophys. Res. Commun. 404: 1–5. [DOI] [PubMed] [Google Scholar]
- 27.Martinon F., Chen X., Lee A. H., Glimcher L. H. 2010. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat. Immunol. 11: 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang S., Kaufman R. J. 2012. The impact of the unfolded protein response on human disease. J. Cell Biol. 197: 857–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reinhardt S., Schuck F., Grösgen S., Riemenschneider M., Hartmann T., Postina R., Grimm M., Endres K. 2014. Unfolded protein response signaling by transcription factor XBP-1 regulates ADAM10 and is affected in Alzheimer’s disease. FASEB J. 28: 978–997. [DOI] [PubMed] [Google Scholar]
- 30.Kitamura M. 2009. Biphasic, bidirectional regulation of NF-κB by endoplasmic reticulum stress. Antioxid. Redox Signal. 11: 2353–2364. [DOI] [PubMed] [Google Scholar]
- 31.Jiang H. Y., Wek S. A., McGrath B. C., Lu D., Hai T., Harding H. P., Wang X., Ron D., Cavener D. R., Wek R. C. 2004. Activating transcription factor 3 is integral to the eukaryotic initiation factor 2 kinase stress response. Mol. Cell. Biol. 24: 1365–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Z., Havasi A., Gall J., Bonegio R., Li Z., Mao H., Schwartz J. H., Borkan S. C. 2010. GSK3β promotes apoptosis after renal ischemic injury. J. Am. Soc. Nephrol. 21: 284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobs K. M., Bhave S. R., Ferraro D. J., Jaboin J. J., Hallahan D. E., Thotala D. 2012. GSK-3β: a bifunctional role in cell death pathways. Int. J. Cell Biol. 2012: 930710. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.