Abstract
Objective
To augment the literature on methadone applications in pediatric oncology, the authors reviewed the use of methadone at a pediatric cancer center over a 5-year period.
Design and setting
Forty-one patients received methadone for inpatient or outpatient pain management. The authors retrospectively reviewed their demographic characteristics, diagnoses, type of pain (nociceptive, neuropathic, or mixed) and causes of pain, and the indications, dose regimens, adverse effects, and outcomes of methadone treatment.
Results
There were four types of clinical uses for methadone in 41 patients (10 patients had two): nociceptive pain unresponsive to other opioids (17 patients, 33.3 percent), neuropathic pain (20 patients, 39.2 percent), facilitation of weaning from opioids (11 patients, 21.6 percent), and end-of-life pain management (3 patients, 5.9 percent). The mean age of the 24 males (58.5 percent) and 17 females (41.5 percent) at the start of treatment was 15.7 years (range, 0.6–23 years). The most common diagnoses were leukemia (n = 10, 24.4 percent), osteosarcoma (n = 7, 17.0 percent), and rhabdomyosarcoma (n = 5, 12.2 percent). The causes of pain were bone marrow transplant (n = 13, 31.7 percent), amputation (n = 6, 14.6 percent), chemotherapy (n = 5, 12.2 percent), tumor (n = 5, 12.2 percent), limb-sparing surgery (n = 4, 9.8 percent), and other (n = 8, 19.5 percent). Efficacy was assessed at the end (or after 6 months) of methadone treatment. For many patients (43.1 percent), methadone showed efficacy in achieving the purpose for which it was prescribed, including reduction of nociceptive or neuropathic pain and prevention of opioid withdrawal. Sedation was the most common side effect (24.4 percent).
Conclusions
Methadone was effective for pediatric patients with neuropathic pain or nociceptive pain unresponsive to other opioids, and it effectively prevented opioid withdrawal.
Keywords: methadone, pain, analgesia, withdrawal, weaning, neuropathic, pediatric cancer
INTRODUCTION
Methadone has unique pharmacodynamic and pharmacokinetic properties; it may offer an advantage over other opioids in treating neuropathic pain in adults1,2 and children3,4 and is an alternative analgesic for adults5,6 and children7 with cancer who require very high doses of other opioids. As a racemic mixture, methadone is an agonist of both μ- and δ- opioid receptors, which mediate supraspinal and spinal analgesia, respectively.8 Methadone has N-methyl-d-aspartate receptor antagonist properties9; therefore, it may be useful in the management of neuropathic pain.8
In the treatment of adults with cancer, methadone was found to be rapidly absorbed in the gastrointestinal tract, yielding peak plasma levels at 90 minutes, with high oral bioavailability (mean, 79 percent; standard deviation [SD], 11.7 percent).10 Methadone is highly protein bound in the plasma; in adult healthy volunteers, the mean free fraction of racemic methadone was found to be 12.7 percent (SD 3.3 percent).11,12 Because methadone plasma binding is mostly to alpha 1-acid glycoprotein (AAG), and patients with cancer have elevated concentrations of AAG, lower free fraction of methadone levels have been reported in adult patients with cancer.12 The pharmacokinetics of methadone have been established in adults13 and children14 in the perioperative period. In studies describing the use of methadone for perioperative pain control in adults, methadone provided prolonged postoperative analgesia (mean, 21.4 hours; SD, 7 hours; median, 27 hours) consistent with the long elimination half-life of the drug (mean, 35 hours; range, 9–87 hours; SD, 22 hours).13 In studies of children and adolescents treated with methadone in the perioperative period, a long elimination half-life (mean, 19.2 hours; range, 3.8–62 hours; SD, 13.6 hours) has been described after a single dose of methadone.14,15 Methadone is metabolized via N-demethylation in the liver and is eliminated renally and fecally.16 Methadone has inactive metabolites; however, low serum levels may persist due to methadone’s considerable tissue distribution, retention in, and slow release from, the liver and other tissues.13,16 In children, methadone shows extreme variability in plasma concentration and clearance kinetics.17 Multiple drug interactions, metabolic factors, and the risk of cardiac toxicity add to the challenges of using methadone and may warrant consultation with a pain specialist.1
The body of literature reflects the use of methadone to treat nociceptive pain and neuropathic pain or to facilitate weaning of opioids and prevent withdrawal. In general, there is extensive experience with methadone for pain management in the adult population,1,18 and specifically for cancer pain,1,2,5,6,19–21 chronic noncancer pain,22 intractable neuropathic noncancer pain,23 and diabetic neuropathy.24 In adults, methadone has been reported to be effective for various types of neuropathic pain, including phantom limb pain,25 diabetic neuropathy,24 intractable neuropathic noncancer pain,23,26 burns,27 and neuropathic cancer pain.28
In children, most reports described the use of methadone for nociceptive pain, including acute procedure-related pain in burn care,29 severe30 and chronic7,31,32 cancer-related nociceptive pain, end-of-life cancer pain management,33 and noncancer pain in inpatients.34 Methadone has also been used in children to facilitate weaning of opioids and to prevent withdrawal after prolonged sedation in the intensive care unit (ICU).35–39 The use of methadone for neuropathic pain in children with cancer has been reported successfully in one series of 17 patients7; one case report noted that methadone (combined with gabapentin, oxcarbazepine, and celecoxib) provided insufficient pain control in a child with terminal malignancy, and an intrathecal pump was necessary to control severe pain.40
This retrospective study examines the clinical uses, side effects, and outcomes of treatment with methadone in children and young adults at our pediatric oncology institution over a period of 5 years.
METHODS
St. Jude Children’s Research Hospital (St. Jude) is a tertiary-care institution with the mission of advancing the cure and prevention of pediatric cancer and related hematological and infectious diseases through research and treatment. At the time of diagnosis, patients range in age from newborns to young adults. The facility consists of multiple outpatient clinics and three inpatient care areas totaling 60 beds: 38 for hematology and oncology acute care, eight for intensive care, and 14 for bone marrow transplantation. This retrospective review was approved by the St. Jude Institutional Review Board. The study population comprised all patients treated with methadone at our facility over a period of 5 years (October 2001 to September 2006).
Inpatients and outpatients who received methadone during the study period were identified by reviewing the institution’s pharmacy records. Methadone therapy is prescribed in our institution by the pain service or by the primary oncology team, or the ICU team, often in consultation with the pain service; the dosing regimens are not controlled by institutional policy, nevertheless a hospital formulary is available for reference. Using a standardized case report form developed by the investigators, we abstracted data from the medical records including age and sex, primary oncology diagnosis, type of pain experienced (nociceptive, neuropathic, or mixed) at the time of methadone prescription, and the clinical context of pain (eg, chemotherapy, bone marrow transplant, amputation, limb-sparing surgery, tumor, or other).
The clinical uses of methadone were ascertained from the medical records and were categorized as follows: 1) nociceptive pain unresponsive to other opioids; 2) neuropathic pain; 3) facilitation of opioid weaning and prevention of withdrawal; and 4) pain management at the end of life. In our institution, when methadone is used to facilitate weaning, it overlaps with the opioid regimen that is being systematically reduced. We noted the dose and route of administration of methadone and recorded pain intensity scores at the beginning of treatment with methadone, at weekly intervals during the first month, and at monthly intervals thereafter for the duration of treatment or for 6 months. Adverse effects of methadone treatment were captured by review of the outpatient clinic notes and/or inpatient nursing or physicians’ notes and recorded as present or absent in the following categories: sedation, nausea, constipation, confusion, respiratory depression, pruritus, and other. No specific scales were used to quantify these symptoms. Outcome variables analyzed were pain scores, duration of treatment, and notes in the medical record documenting the effectiveness of treatment for the specified indication; medical record notes were evaluated at weekly intervals during the first month and monthly thereafter for the duration of treatment or for 6 months. A successful outcome was defined as reduction of pain scores between the start and end of methadone treatment and/or documentation of the effectiveness of treatment for the specified indication (nociceptive pain, neuropathic pain, or facilitation of opioid weaning and prevention of withdrawal). Medical record notes were examined to complement pain score data and to capture information not reflected by pain scores (eg, notes qualitatively describing pain control, the quality of life and level of activity, or the presence or absence of withdrawal signs) or opioid side effects. Data were analyzed using descriptive statistics. An additional review of the medical records of patients with pain scores available at the beginning and end of treatment with methadone was performed to identify concurrent pain medications and tumor-directed therapies (chemotherapy and radiation therapy).
Age-appropriate pain assessment tools are used in our institution as per the pain standard of care: the FLACC (Faces, Legs, Activity, Cry, Consolability) scale for children younger than 3 years,41 the Wong-Baker FACES scale for children 4 to 6 years,42 and the numerical rating scale (NRS) for those 7 years or older.43
RESULTS
Over the 5-year study period, methadone was prescribed to 41 patients; 24 (58.5 percent) were male (Table 1). The mean age at the start of methadone treatment was 15.7 years (median, 18 years); most patients were 11 or more years of age (Table 1). The mean age of patients receiving methadone to facilitate opioid weaning and to prevent withdrawal was 10.7 years (median, 15 years). The most common diagnoses were leukemia (n = 10, 24.4 percent), osteosarcoma (n = 7, 17.0 percent), and rhabdomyosarcoma (n = 5, 12.2 percent). Thirty six of the 41 primary diagnoses were cancer; the remaining five were sickle cell disease, thalassemia major, Fanconi anemia, myelodysplastic syndrome, and osteopetrosis. The most common clinical circumstance generating pain was bone marrow transplant (13, 31.7 percent), which included abdominal pain from graft versus host disease, chemotherapy-induced neuropathy, mucositis-related pain due to conditioning regimens, or any other pain symptoms requiring methadone that developed during or after the transplant.
Table 1.
Demographics, indications, and types of pain in 41 patients prescribed methadone
| n (percent) | |
|---|---|
| Sex | |
| Male | 24 (58.5) |
| Female | 17 (41.5) |
| Age, y | |
| Range, 0.6–23 | |
| ≤10 | 7 (17) |
| 11–17 | 16 (39) |
| ≥18 | 18 (44) |
| Primary diagnosis | |
| Leukemia/lymphoma | 13 (31.2) |
| Leukemia | 10 |
| Lymphoma | 3 |
| Solid tumor | 23 (56.1) |
| Osteosarcoma | 7 |
| Rhabdomyosarcoma | 5 |
| Ewing sarcoma | 2 |
| Brain tumor | 2 |
| Other* | 7 |
| Hematologic or congenital disorders | 5 (9.8) |
| Thalassemia | 1 |
| Sickle cell disease | 1 |
| Fanconi anemia | 1 |
| Myelodysplastic syndrome | 1 |
| Osteopetrosis | 1 |
| Primary cause of pain | |
| Bone marrow transplant | 13 (31.7) |
| Amputation | 6 (14.6) |
| Chemotherapy | 5 (12.2) |
| Tumor | 5 (12.2) |
| Limb-sparing surgery | 4 (9.8) |
| Other | 8 (19.5) |
| Methadone clinical use† | n = 51† |
| Control of neuropathic pain | 20 (39.2) |
| Control of nociceptive pain | 17 (33.3) |
| Opioid weaning/prevention of withdrawal | 11 (21.6) |
| End-of-life pain management | 3 (5.9) |
Other: neuroblastoma, undifferentiated sarcoma, Wilms tumor, adrenocorticoid carcinoma, alveolar soft part sarcoma, angiosarcoma, and hepatoblastoma.
Fifty-one patients had two concurrent indications for methadone use.
Methadone was prescribed to the 41 patients for the following four types of clinical uses (10 patients had two): nociceptive pain unresponsive to other opioids (17 patients, 33.3 percent), neuropathic pain (20, 39.2 percent), facilitation of weaning from opioids (11, 21.6 percent), and end-of-life pain management (3, 5.9 percent). Of the 10 patients who had two concurrent clinical indications for methadone, seven had nociceptive pain and neuropathic pain, and one each had nociceptive pain at the end of life, neuropathic pain at the end of life, and nociceptive pain and opioid weaning and prevention of withdrawal, respectively.
Methadone dosing data were available for 37 patients; four patient records lacked baseline doses because methadone was started at another institution (Table 2). The starting dose ranged from 0.06 to 3.8 mg/kg/d (median, 0.32 mg/kg/d). The highest methadone dose used was 9.4 mg/kg/d in an 18-month-old patient with osteopetrosis, post bone marrow transplant, who weighed 8.5 kg and was given 20 mg of methadone by gastrostomy tube every 6 hours to treat generalized pain and to prevent withdrawal during opioid weaning. This patient’s methadone dose was escalated from 0.5 to 7.1 mg/kg/d over 2 weeks, and eventually to 9.4 mg/kg/d, while the patient received mechanical ventilation per tracheostomy for respiratory failure. Methadone was given enterally (orally or by nasogastric tube) to all patients, except one who received intravenous (IV) methadone; two patients received enteral and IV methadone concurrently.
Table 2.
Methadone dosing, duration, and side effects in 41 young patients with hematology/oncology
| Starting dose,* mg/kg/d | n = 37 |
|---|---|
| Mean | 0.53 |
| Median | 0.32 |
| Range | 0.06–3.8 |
| SD | 0.67 |
| Duration of treatment† | n = 31 |
| Mean | 9.1 weeks |
| Median | 8.0 |
| Range | 1 week to >6 months |
| SD | 7.4 |
| Side effects | n = 41 (percent) |
| None | 14 (34.1) |
| Sedation | 10 (24.4) |
| Other | 6 (14.6) |
| Nausea | 5 (12.2) |
| Constipation | 5 (12.2) |
| Confusion | 1 (2.4) |
| Respiratory depression | 0 |
| Pruritus | 0 |
Abbreviation: N/A: not applicable.
Not known for four patients first prescribed methadone by outside providers.
Analyzed for 31 patients who did not expire during treatment and for whom prescription dates were known.
Data were censored at 6 months.
More than one-third of the patients (14; 34.1 percent) had no documented adverse effects (Table 2). Other patients experienced sedation, nausea, constipation, or confusion. The most common adverse effect was sedation (10 patients; 24.4 percent). No respiratory depression or pruritus was documented. Other side effects included “feeling funny” and “lightheadedness” and were not conclusively attributable to methadone.
Because of the limited interim pain score data, we report pain reduction by comparing the maximum pain score on the day methadone was discontinued (or at 6 months of methadone treatment) to the maximum pain score on the day methadone was started. Fourteen of the 41 patients had documented pain scores for both time points, and nine of these patients (64.3 percent) showed reduction of the pain score; seven (50 percent) had complete resolution of pain (Table 3). The concurrent pain medications are also presented in Table 3; 12 of 14 patients received chemotherapy concurrently with the treatment with methadone, and none received concurrent radiation therapy. The effectiveness of treatment was also assessed by reviewing notes in the medical records at weekly intervals during the first month and monthly thereafter for the duration of treatment or for 6 months. Improvement of the indicating symptoms was noted in the records of 22 patients (43.1 percent; Table 4).
Table 3.
Pain score change, duration of methadone treatment, and concomitant therapies in 14 young patients
| Primary diagnosis |
Type of pain |
Clinical use for methadone therapy |
Maximum pain score* at start of therapy |
Maximum pain score* at end of therapy |
Duration of methadone therapy, wk |
Concomitant therapies† |
|
|---|---|---|---|---|---|---|---|
| 1 | Non-Hodgkin lymphoma | Mixed‡ | Nociceptive pain, neuropathic pain | 10 | 0 | 8 | CT, fentanyl, gabapentin |
| 2 | Osteosarcoma | Neuropathic | Neuropathic pain | 9 | 0 | 12 | CT, morphine, gabapentin |
| 3 | Osteosarcoma | Neuropathic | Neuropathic pain | 8 | 0 | 20 | CT, morphine, gabapentin, amitriptyline |
| 4 | Neuroblastoma | Nociceptive | Nociceptive pain | 8 | 0 | 3 | CT, morphine, fentanyl |
| 5 | AML | Undocumented | Prevention of opioid withdrawal | 8 | 0 | 4 | CT, morphine |
| 6 | Ewing sarcoma | Mixed‡ | Neuropathic pain | 7 | 0 | 8 | CT, morphine, gabapentin, amitriptyline |
| 7 | Osteosarcoma | Mixed‡ | Nociceptive pain, neuropathic pain | 6 | 0 | 2 | CT, morphine, fentanyl, amitriptyline |
| 8 | ALL | Neuropathic | End-of-life pain | 9 | 8 | 4 | Hydromorphone, fentanyl |
| 9 | Osteosarcoma | Mixed‡ | Nociceptive pain, neuropathic pain | 8 | 7 | 1 | CT, morphine, hydromorphone, gabapentin |
| 10 | Rhabdomyosarcoma | Neuropathic | Prevention of opioid withdrawal, neuropathic pain | 7 | 7 | 12 | CT, fentanyl, gabapentin |
| 11 | Sickle cell disease | Mixed‡ | Nociceptive pain, neuropathic pain | 10 | 10 | 12 | CT, fentanyl, gabapentin |
| 12 | Primitive neuroectodermal tumor | Mixed‡ | Neuropathic pain | 4 | 4 | 4 | CT, morphine, oxycodone, gabapentin |
| 13 | Myelodysplastic syndrome | Neuropathic | Neuropathic pain | 7 | 8 | 12 | Gabapentin |
| 14 | ALL | Undocumented | Prevention of opioid withdrawal | 1 | 6 | 12 | CT, fentanyl |
Abbreviations: AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; CT, chemotherapy.
Pain scale: 0–10.
Concomitant pain medications and cancer-directed treatments.
Patients had both neuropathic and nociceptive pain.
Table 4.
| Indication | Number of clinical indications‡ |
Efficacy noted, n |
Nonefficacy noted, n |
Efficacy not documented, n |
|---|---|---|---|---|
| Control of nociceptive pain | 17 | 9 (52.9) | 3 (17.6) | 5 (29.4) |
| Control of neuropathic pain | 20 | 8 (40) | 8 (40) | 4 (20) |
| Prevention of withdrawal | 11 | 5 (45.4) | 3 (27.3) | 3 (27.3) |
| End-of-life pain management | 3 | 0 | 1 (33.3) | 2 (66.7) |
| Total | 51 | 22 (43.1) | 15 (29.4) | 14 (27.5) |
Clinical notes included physicians’ notes, documentation of methadone dosing, and documentation of patient/caregiver’s reports.
Values in parenthesis are represented in percentage.
Ten patients had two concurrent clinical uses.
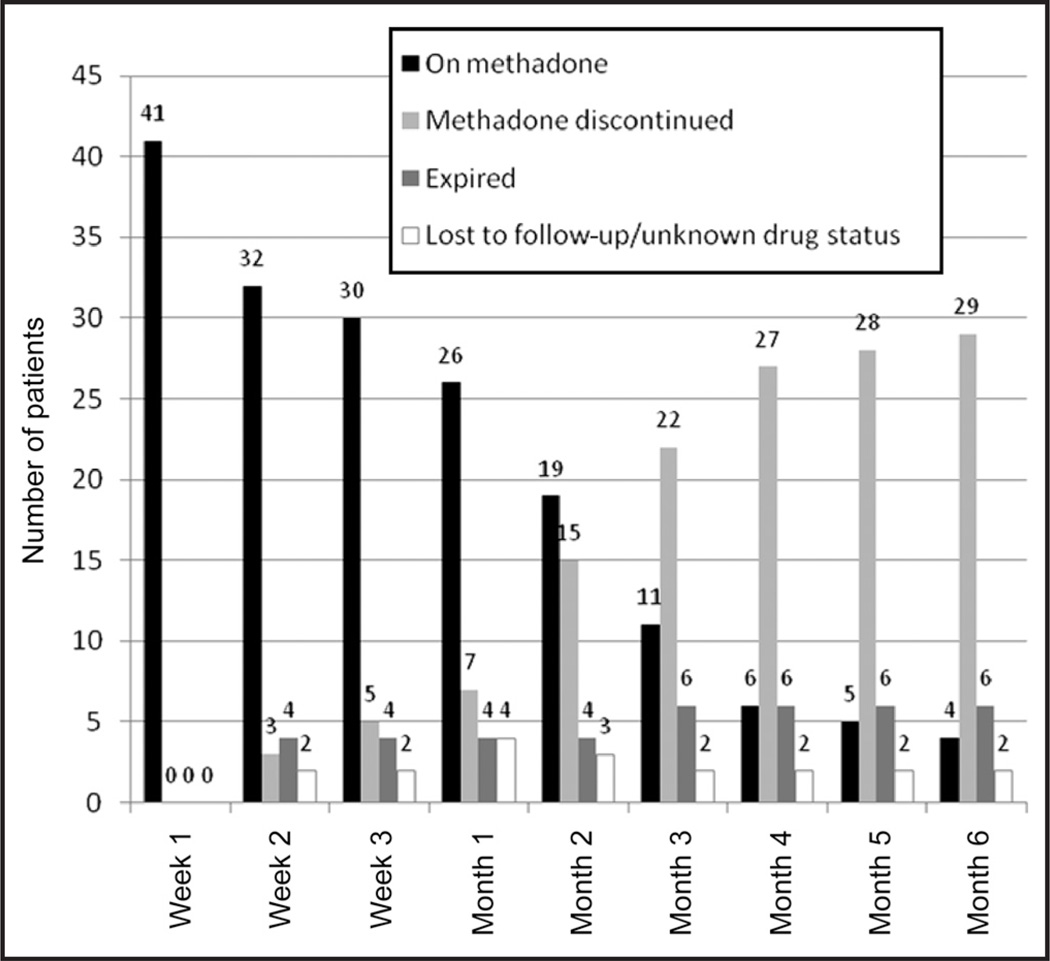
The duration of treatment with methadone ranged widely. Seven patients were treated for only 1 week, while four remained on methadone after 6 months (Figure 1).
Figure 1.
Duration of methadone treatment in 41 young patients with hematology/oncology.
DISCUSSION
This retrospective review is the first to characterize the profile of clinical uses for methadone use in pediatric hematology/oncology patients. In this group of 41 patients, the most common indication was control of neuropathic pain, followed by control of nociceptive pain unresponsive to other opioids. We found methadone to be useful and safe for both indications in our limited study sample. Clinical notes indicated efficacy in 52.9 percent of patients with nociceptive pain and 40 percent of patients with neuropathic pain, while the available pain scores revealed complete resolution of pain in seven of 14 patients and improvement in two additional patients. Methadone use is rarely reported for neuropathic pain in children with cancer, and our qualitative findings (medical record notes regarding pain control) support its value for this indication.
The highest methadone dose in our study (9.4 mg/kg/d), in an 18-month-old patient weighing 8.5 kg, is higher than any reported to date in pediatric hematology/oncology. Seven studies have investigated methadone dose regimens in a total of 108 children and young adults with cancer-related pain.7,30–34,44 One study reported the use of 0.27 to 0.89 mg/kg/d of oral methadone and 0.34 to 0.46 mg/kg/d of IV methadone in 40 children with cancer during terminal care.33 In a series of five cases, oral methadone was used in two children with cancer at doses of 0.2 to 0.6 mg/kg/d.34 In a report of 19 children and young adults with severe cancer-related pain, the authors recommended a starting dose of 0.1 mg/kg of methadone given orally every 4 hours (or smaller doses given less frequently but totaling 0.6 mg/kg/d), with escalation as required to control pain.32 In another study that examined conversion ratios for morphine and methadone in 17 children and young adults with advanced cancer, the highest dose reported was 1.4 mg/kg/d (140 mg/d in a 99-kg patient).7 The reported dose ranged from 2.5 to 40 mg orally every 4 to 12 hours in a study of 29 children with cancer who were treated at home; although these doses were not reported on the basis of body weight, a typical dose was described as 5 to 10 mg every 6 to 8 hours.31 One case report described the use of l-methadone patient-controlled analgesia at the end of life with dose escalation up to 186 mg/d for a 26-kg child (7.1 mg/kg/d).30 In our study, the starting dose ranged from 0.06 to 3.8 mg/kg/d (median, 0.32 mg/kg/d). The high end of our range of doses is higher than generally reported in the literature, which may reflect the use of methadone for opioid weaning in our series, which requires a starting dose based on the high doses of opioids in use at the time weaning begins.
Our study adds to the limited available information about the quality of analgesia achieved by methadone treatment in pediatric hematology/oncology. We found that methadone reduced the pain scores of nine of 14 (64.3 percent) patients for whom complete pain score information was available and improved the symptoms for which it was prescribed in 22 of 51 (43.1 percent) indications, without unacceptable side effects. The studies reported to date have used different measures, including pain scores,7,34 descriptive notes in the medical records,7,30,33,34 and qualitative information from structured interviews with parents.33 A study of methadone analgesia for children with advanced cancer had findings comparable to ours; pain outcomes (pain scores and/or documented comments) were available for 11 of 17 patients; in six of these patients (55 percent), positive comments about pain control and/or pain scores <5 were more frequent after methadone was substituted for other opioids.7
Our study was designed to assess methadone treatment for periods of 6 months or less. The duration of treatment with methadone ranged widely, with a mean duration of 9.1 weeks. Seven patients were treated for only 1 week, while four remained on methadone after 6 months. In another study, methadone was used in 17 pediatric patients with oncology for a total of 925 patient days (range, 1–199 days; median, 36 days).7 Although another report suggested that opioid therapy may extend for as long as 4 years in children with brain tumors, it does not clearly address the duration of treatment with methadone.33 Overall, the available results suggest that methadone therapy in pediatric patients with hematology/oncology is often significantly prolonged, which may be due to the chronic nature of the pain being treated or to the time required to titrate methadone to effect.
The small sample size and the retrospective design of our study led to limited information about the starting doses, final doses, and pain scores of patients referred from elsewhere, transferred to hospice, or lost to follow-up. Limited data regarding pain scores was also a consequence of incomplete documentation due to the outpatient setting. To augment the pain score data, we included more subjective information collected from clinicians’ notes, similar to the method used by Davies et al.7 Although this method reduces the objectivity of the measurements, narrative clinical notes can provide additional information that may be helpful in assessing factors relevant to the success of pain control (eg, activity level and quality of life). The effectiveness of methadone as reported in this study should be interpreted with caution, in the context of other concurrent factors that may have contributed to pain control (short-acting opioids for breakthrough pain, gabapentin and amitriptyline for neuropathic pain, and administration of chemotherapy as per specific oncology protocols).
CONCLUSIONS
We report the clinical applications for the use of methadone at our pediatric oncology institution for three indications: 1) nociceptive pain unresponsive to other opioids; 2) neuropathic pain; and 3) to facilitate weaning of opioids to prevent withdrawal. Methadone should be considered as an alternative analgesic for pediatric patients with hematology/oncology diagnoses whose pain is difficult to control with conventional analgesic regimens. Prospective studies are needed to evaluate specific methadone regimens for each of the clinical entities described here and to determine opioid conversion scales to and from methadone in the pediatric population.
ACKNOWLEDGMENTS
This work was supported in part by the NIH Cancer Center Support Core Grant P30CA021765-32, and by the American Lebanese Syrian Associated Charities (ALSAC). The authors thank Sharon Naron for editorial support.
Contributor Information
Doralina L. Anghelescu, Division of Anesthesia and Pain Management Service, St. Jude Children’s Research Hospital, Memphis, Tennessee.
Lane G. Faughnan, Division of Anesthesia and Pain Management Service, St. Jude Children’s Research Hospital, Memphis, Tennessee.
Gisele M. Hankins, Division of Anesthesia and Pain Management Service, St. Jude Children’s Research Hospital, Memphis, Tennessee.
Deborah A. Ward, Department of Pharmaceutical Sciences, St. Jude Children’s Research Hospital, Memphis, Tennessee.
Linda L. Oakes, Department of Patient Care Services, St. Jude Children’s Research Hospital, Memphis, Tennessee.
REFERENCES
- 1.Nicholson AB. Methadone for cancer pain. Cochrane Database Syst Rev. 2007;(4) doi: 10.1002/14651858.CD003971.pub3. CD003971. [DOI] [PubMed] [Google Scholar]
- 2.Bruera E, Palmer JL, Bosnjak S, et al. Methadone versus morphine as a first-line strong opioid for cancer pain: A randomized, double-blind study. J Clin Oncol. 2004;22(1):185–192. doi: 10.1200/JCO.2004.03.172. [DOI] [PubMed] [Google Scholar]
- 3.Jacob E. Neuropathic pain in children with cancer. J Pediatr Oncol Nurs. 2004;21(6):350–357. doi: 10.1177/1043454204270251. [DOI] [PubMed] [Google Scholar]
- 4.Anghelescu D, Oakes L, Popenhagen M. Management of cancer pain in neonates, children and adolescents. In: de Leon-Casasola OA, editor. Cancer Pain: Pharmacological, Interventional and Palliative Care Approaches. 1st ed. Philadelphia: Saunders Elsevier; 2006. pp. 509–521. [Google Scholar]
- 5.Manfredi PL, Houde RW. Prescribing methadone, a unique analgesic. J Support Oncol. 2003;1(3):216–220. [PubMed] [Google Scholar]
- 6.Ripamonti C, Bianchi M. The use of methadone for cancer pain. Hematol Oncol Clin North Am. 2002;16(3):543–555. doi: 10.1016/s0889-8588(02)00017-5. [DOI] [PubMed] [Google Scholar]
- 7.Davies D, DeVlaming D, Haines C. Methadone analgesia for children with advanced cancer. Pediatr Blood Cancer. 2008;51(3):393–397. doi: 10.1002/pbc.21584. [DOI] [PubMed] [Google Scholar]
- 8.Moulin DE, Clark AJ, Gilron I, et al. Pharmacological management of chronic neuropathic pain—Consensus statement and guidelines from the Canadian Pain Society. Pain Res Manag. 2007;12(1):13–21. doi: 10.1155/2007/730785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorman AL, Elliott KJ, Inturrisi CE. The d- and l-isomers of methadone bind to the non-competitive site on the N-methyl-d-aspartate (NMDA) receptor in rat forebrain and spinal cord. Neurosci Lett. 1997;223(1):5–8. doi: 10.1016/s0304-3940(97)13391-2. [DOI] [PubMed] [Google Scholar]
- 10.Gourlay GK, Cherry DA, Cousins MJ. A comparative study of the efficacy and pharmacokinetics of oral methadone and morphine in the treatment of severe pain in patients with cancer. Pain. 1986;25(3):297–312. doi: 10.1016/0304-3959(86)90234-4. [DOI] [PubMed] [Google Scholar]
- 11.Eap CB, Cuendet C, Baumann P. Binding of d-methadone, l-methadone, and dl-methadone to proteins in plasma of healthy volunteers: Role of the variants of alpha 1-acid glycoprotein. Clin Pharmacol Ther. 1990;47(3):338–346. doi: 10.1038/clpt.1990.37. [DOI] [PubMed] [Google Scholar]
- 12.Abramson FP. Methadone plasma protein binding: Alterations in cancer and displacement from alpha 1-acid glycoprotein. Clin Pharmacol Ther. 1982;32(5):652–658. doi: 10.1038/clpt.1982.217. [DOI] [PubMed] [Google Scholar]
- 13.Gourlay GK, Wilson PR, Glynn CJ. Pharmacodynamics and pharmacokinetics of methadone during the perioperative period. Anesthesiology. 1982;57(6):458–467. doi: 10.1097/00000542-198212000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Berde CB, Sethna NF, Holzman RS, et al. Pharmacokinetics of methadone in children and adolescents in the perioperative period. Anesthesiology. 1987;67(3):A519. [Google Scholar]
- 15.Berde CB, Beyer JE, Bournaki MC, et al. Comparison of morphine and methadone for prevention of postoperative pain in 3- to 7-year-old children. J Pediatr. 1991;119(1, Part 1):136–141. doi: 10.1016/s0022-3476(05)81054-6. [DOI] [PubMed] [Google Scholar]
- 16.Sawe J. High-dose morphine and methadone in cancer patients. Clinical pharmacokinetic considerations of oral treatment. Clin Pharmacokinet. 1986;11(2):87–106. doi: 10.2165/00003088-198611020-00001. [DOI] [PubMed] [Google Scholar]
- 17.Yang F, Tong X, McCarver DG, et al. Population-based analysis of methadone distribution and metabolism using an age-dependent physiologically based pharmacokinetic model. J Pharmacokinet Pharmacodyn. 2006;33(4):485–518. doi: 10.1007/s10928-006-9018-0. [DOI] [PubMed] [Google Scholar]
- 18.Shaiova L, Berger A, Blinderman CD, et al. Consensus guideline on parenteral methadone use in pain and palliative care. Palliat Support Care. 2008;6(2):165–176. doi: 10.1017/S1478951508000254. [DOI] [PubMed] [Google Scholar]
- 19.Mercadante S. Cancer pain management in children. Palliat Med. 2004;18(7):654–662. doi: 10.1191/0269216304pm945rr. [DOI] [PubMed] [Google Scholar]
- 20.Parsons HA, de la Cruz M, El Osta B, et al. Methadone initiation and rotation in the outpatient setting for patients with cancer pain. Cancer. 2010;116(2):520–528. doi: 10.1002/cncr.24754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leppert W. The role of methadone in cancer pain treatment—A review. Int J Clin Pract. 2009;63(7):1095–1109. doi: 10.1111/j.1742-1241.2008.01990.x. [DOI] [PubMed] [Google Scholar]
- 22.Lynch ME. A review of the use of methadone for the treatment of chronic noncancer pain. Pain Res Manag. 2005;10(3):133–144. doi: 10.1155/2005/286713. [DOI] [PubMed] [Google Scholar]
- 23.Moulin DE, Palma D, Watling C, et al. Methadone in the management of intractable neuropathic noncancer pain. Can J Neurol Sci. 2005;32(3):340–343. doi: 10.1017/s0317167100004236. [DOI] [PubMed] [Google Scholar]
- 24.Hays L, Reid C, Doran M, et al. Use of methadone for the treatment of diabetic neuropathy. Diabetes Care. 2005;28(2):485–487. doi: 10.2337/diacare.28.2.485. [DOI] [PubMed] [Google Scholar]
- 25.Bergmans L, Snijdelaar DG, Katz J, et al. Methadone for phantom limb pain. Clin J Pain. 2002;18(3):203–205. doi: 10.1097/00002508-200205000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Altier N, Dion D, Boulanger A, et al. Management of chronic neuropathic pain with methadone: A review of 13 cases. Clin J Pain. 2005;21(4):364–369. doi: 10.1097/01.ajp.0000125247.95213.53. [DOI] [PubMed] [Google Scholar]
- 27.Altier N, Dion D, Boulanger A, et al. Successful use of methadone in the treatment of chronic neuropathic pain arising from burn injuries: A case-study. Burns. 2001;27(7):771–775. doi: 10.1016/s0305-4179(01)00032-8. [DOI] [PubMed] [Google Scholar]
- 28.Makin MK, Ellershaw JE. Substitution of another opioid for morphine. Methadone can be used to manage neuropathic pain related to cancer. BMJ. 1998;317(7150):81. [PubMed] [Google Scholar]
- 29.Williams PI, Sarginson RE, Ratcliffe JM. Use of methadone in the morphine-tolerant burned paediatric patient. Br J Anaesth. 1998;80(1):92–95. doi: 10.1093/bja/80.1.92. [DOI] [PubMed] [Google Scholar]
- 30.Sabatowski R, Kasper SM, Radbruch L. Patient-controlled analgesia with intravenous l-methadone in a child with cancer pain refractory to high-dose morphine. J Pain Symptom Manage. 2002;23(1):3–5. doi: 10.1016/s0885-3924(01)00389-x. [DOI] [PubMed] [Google Scholar]
- 31.Martinson IM, Nixon S, Geis D, et al. Nursing care in childhood cancer: Methadone. Am J Nurs. 1982;82(3):432–435. [PubMed] [Google Scholar]
- 32.Miser AW, Miser JS. The use of oral methadone to control moderate to severe pain in children and young adults with malignancy. Clin J Pain. 1985;1:243–248. [Google Scholar]
- 33.Sirkia K, Hovi L, Pouttu J, et al. Pain medication during terminal care of children with cancer. J Pain Symptom Manage. 1998;15(4):220–226. doi: 10.1016/s0885-3924(98)00366-2. [DOI] [PubMed] [Google Scholar]
- 34.Shir Y, Shenkman Z, Shavelson V, et al. Oral methadone for the treatment of severe pain in hospitalized children: A report of five cases. Clin J Pain. 1998;14(4):350–353. doi: 10.1097/00002508-199812000-00013. [DOI] [PubMed] [Google Scholar]
- 35.Lugo RA, MacLaren R, Cash J, et al. Enteral methadone to expedite fentanyl discontinuation and prevent opioid abstinence syndrome in the PICU. Pharmacotherapy. 2001;21(12):1566–1573. doi: 10.1592/phco.21.20.1566.34471. [DOI] [PubMed] [Google Scholar]
- 36.Meyer MM, Berens RJ. Efficacy of an enteral 10-day methadone wean to prevent opioid withdrawal in fentanyl-tolerant pediatric intensive care unit patients. Pediatr Crit Care Med. 2001;2(4):329–333. doi: 10.1097/00130478-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 37.Robertson RC, Darsey E, Fortenberry JD, et al. Evaluation of an opiate-weaning protocol using methadone in pediatric intensive care unit patients. Pediatr Crit Care Med. 2000;1(2):119–123. doi: 10.1097/00130478-200010000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Siddappa R, Fletcher JE, Heard AM, et al. Methadone dosage for prevention of opioid withdrawal in children. Paediatr Anaesth. 2003;13(9):805–810. doi: 10.1046/j.1460-9592.2003.01153.x. [DOI] [PubMed] [Google Scholar]
- 39.Tobias JD, Deshpande JK, Gregory DF. Outpatient therapy of iatrogenic drug dependency following prolonged sedation in the pediatric intensive care unit. Intensive Care Med. 1994 Aug;20(7):504–507. doi: 10.1007/BF01711905. [DOI] [PubMed] [Google Scholar]
- 40.Saroyan JM, Schechter WS, Tresgallo ME, et al. Role of intraspinal analgesia in terminal pediatric malignancy. J Clin Oncol. 2005;23(6):1318–1321. doi: 10.1200/JCO.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 41.Merkel SI, Voepel-Lewis T, Shayevitz JR, et al. The FLACC: A behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997;23(3):293–297. [PubMed] [Google Scholar]
- 42.Hockenberry M, Wilson D. Wong’s Essentials of Pediatric Nursing. 8th ed. St. Louis: Mosby; 2009. [Google Scholar]
- 43.von Baeyer CL. Children’s self-reports of pain intensity: Scale selection, limitations and interpretation. Pain Res Manag. 2006;11(3):157–162. doi: 10.1155/2006/197616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zernikow B, Michel E, Craig F, et al. Pediatric palliative care: Use of opioids for the management of pain. Paediatr Drugs. 2009;11(2):129–151. doi: 10.2165/00148581-200911020-00004. [DOI] [PubMed] [Google Scholar]