Abstract
Background
Roughly 3000 new cases of Barrett’s carcinoma arise in Germany each year. In view of recent advances in the epidemiology, diagnosis, and treatment of this disease, an update of the clinical recommendations is in order.
Methods
This review is based on selected relevant publications, including current reviews, meta-analyses, and guidelines.
Results
The risk of progression of Barrett’s esophagus to carcinoma lies between 0.10% and 0.15% per year. Risk factors for progression include male sex, age over 50 years, obesity, longstanding and frequent reflux symptoms, smoking, length of the Barrett’s esophagus, and intraepithelial neoplasia. Well-differentiated carcinomas that are confined to the esophageal mucosa can be resected endoscopically with a cure rate above 90%. For more advanced, but still locally confined tumors, surgical resection is the treatment of choice. In stages cT3/4, the prognosis can be improved with neo-adjuvant chemotherapy or combined radiotherapy and chemotherapy. Metastatic Barrett’s carcinoma can be treated by endoscopic, chemotherapeutic, radiotherapeutic, and palliative methods.
Conclusion
Early carcinoma can often be cured by endoscopic resection. Locally advanced carcinoma calls for multimodal treatment. Current research focuses on means of preventing the progression of Barrett’s esophagus, the scope of applicability of endoscopic techniques, and the optimization of multimodal treatment strategies for advanced disease.
Barrett’s carcinoma is one of the fastest-increasing cancers in the western world (average rate of increase 3.5% to 8.1% per year) (1). Despite this, at about 3000 new cases per year in Germany, it is relatively rare in relation to other cancers (e1). Barrett’s carcinoma develops from Barrett’s esophagus, a metaplastic condition of the esophageal mucosa associated with gastroesophageal reflux disease (2). This means that gastroesophageal reflux disease—a very common condition that affects one in five adults—is associated with the possibility of development of a life-threatening disease ((e2, (e3). However, Barrett’s metaplasia is a change in the mucosa that, although associated with an increased risk of cancer, is also easily accessible to monitoring and indeed to endoscopic resection. This means that it is possible to improve the poor prognosis of this disease, with 5-year survival rates below 20%, by early recognition or even by preventing the tumor from developing at all. Data from the Netherlands showing an improvement in 5-year survival rates from 17% to 74% confirm this assumption (e4).
For this CME article, a PubMed literature search (limited to the past 10 years) was carried out. Current guidelines and reviews were also taken into account.
Learning goals
After studying this article, the reader should
be familiar with the epidemiology and pathogenesis of Barrett’s carcinoma, including its risk factors
understand the role of the various diagnostic options and know how to use them correctly, and
understand the main principles of stage-based treatment.
Definition.
In Germany, Barrett’s esophagus is defined as columnar cell metaplasia visible on endoscopy with histological confirmation of specialized intestinal metaplasia.
Definition
“Barrett’s carcinoma” is the term used to refer to esophageal adenocarcinoma that has developed on the site of Barrett’s esophagus. Barrett’s esophagus is variously defined in the literature. An international consensus in 2006 determined that endoscopic evidence of columnar epithelial metaplasia justifies a diagnosis of Barrett’s esophagus (2). On the basis of the histology, information should be added as to whether the metaplasia is gastric (GM) or specialized intestinal metaplasia (SIM) (2). A substantial proportion of patients with gastric metaplasia (29%) show specialized intestinal metaplasia in the course of their disease (e5). The current German guidelines still require evidence of specialized intestinal metaplasia for a diagnosis of Barrett’s esophagus. According to these guidelines, patients with gastric metaplasia should be followed up 1 year later (3). In the surgical literature, Barrett’s carcinoma corresponds to type 1 adenocarcinoma of the esophagogastric junction (e6).
Progression rate.
Barrett’s esophagus is frequent, but at 0.10% to 0.15% the overall rate of progression to carcinoma is lower than previously assumed.
Epidemiology
The incidence of Barrett’s carcinoma has been steadily increasing in the West over the past 50 years, and in many countries has overtaken the incidence of squamous cell carcinoma of the esophagus (1). In England and the Netherlands, however, the most recent statistics are showing a leveling off of the numbers, indicating that there is no need to fear a runaway increase in the number of cases of this cancer (4). Compared to the most frequent cancers (breast cancer, prostate cancer, bronchial carcinoma, colorectal cancer), Barrett’s carcinoma is still quite rare in Germany. Figures predicted by the Robert Koch Institute for 2014 are 5400 cases of esophageal carcinoma in men and 1500 in women. Barrett’s carcinoma is slightly less common than squamous cell carcinoma. The lifetime risk is 0.9% for men and 0.3% for women (e1).
Compared to this, Barrett’s esophagus is much more common. On the basis of a population-based endoscopy study, at least 1% to 2% of adults in a Western population are affected (5). A systematic analysis of the literature showed the prevalence of long-segment Barrett’s esophagus (metaplastic segment >3 cm longitudinal extent) in the population to be 1%, that of short-segment Barrett’s esophagus (1–3 cm) to be 8%, and that of ultra-short-segment Barrett’s esophagus (<1 cm) to be 15% (e7). Since Barrett’s esophagus is much more common than previously thought and Barrett’s carcinoma rarer than was feared, the risk for the individual patient that Barrett’s esophagus will progress to carcinoma is lower than has been assumed. Population studies have shown that the carcinoma risk associated with Barrett’s esophagus is between 0.10% and 0.15% per year, and that patients with Barrett’s esophagus rarely die of Barrett’s carcinoma (6).
Lifetime risk.
The lifetime risk of developing esophageal carcinoma is 0.9% for men and 0.3% for women.
Etiology and pathogenesis
Reflux disease is an important risk factor for Barrett’s esophagus and its associated carcinoma. This means that all factors that favor the occurrence of reflux disease (e.g., overweight) are also involved in the pathogenesis of Barrett’s carcinoma. However, it is also a fact that at least 40% of patients with Barrett’s carcinoma have no clinical signs of gastroesophageal reflux disease (7).
Increased risk.
Men over 50 years of age with a long history of frequent reflux symptoms and abdominal obesity are at increased risk of Barrett’s carcinoma. Smoking further increases this risk.
It is generally assumed that Barrett’s esophagus is a precondition for the development of Barrett’s carcinoma, and that the development occurs in several stages. Figure 1 illustrates the extrapolated risk of progression (8). The question of when and why the precursor lesion Barrett’s esophagus occurs has not yet been conclusively answered. Theories put forward in the literature include congenital alteration of the mucosa, occurrence in the early stage of gastroesophageal reflux disease, and occurrence as a late complication of the same. The stepwise process of progression is accompanied by a number of molecular changes. At present it is unknown whether these changes are a cause or an effect of the progression (8). It was long assumed that both gastroesophageal reflux disease and Barrett’s esophagus are purely acquired diseases. However, studies in families and twins have shown clearly that often a genetic predisposition also exists, and so the pathogenesis of Barrett’s esophagus is understood today as a multifactorial process that includes genetic factors, chemical triggers, and immunological and structural changes (9).
Risk factors
A family history of Barrett’s carcinoma is one risk factor (10– (12). Overall, about 5% to 10% of patients with Barrett’s carcinoma have a familial (genetic) predisposition (10). After that, the next most important risk factor for the occurrence of Barrett’s carcinoma is reflux disease (11, 12) (Table 1). Patients with erosive reflux esophagitis have seven times the risk of those with non-erosive disease (13). Men are affected by the disease more often than women. This is primarily because both Barrett’s esophagus and adenocarcinoma occur almost 20 years later in women (14, (e8). Another important risk factor is abdominal obesity (11, 12). In addition to the mechanical favoring of reflux by the increased pressure in the abdomen, other mechanisms (e.g., leptin) appear to raise the cancer risk irrespective of body mass index. Smokers have a two- to four-fold increased risk (11). Alcohol consumption, on the other hand, does not appear to play any important part. Helicobacter pylori is associated with an approximately 45% reduction in the risk of Barrett’s esophagus and carcinoma (15, (e9). However, it is not clear whether eradicating H. pylori increases the risk of these conditions.
Table 1. Risk factors for Barrett’s carcinoma (source: 11, 12).
| Risk factors | Additional influences |
|---|---|
| Reflux symptoms | Frequency, nocturnal reflux, duration (years) |
| Reflux esophagitis | Severity |
| Barrett’s esophagus | Length (surrogate for area) |
| Overweight | BMI 30+ >BMI 25–30 |
| Smoking | |
| Chest irradiation | |
| Low intake of fruit and vegetables | |
| Medications that relax the lower esophageal sphincter (LES) | |
| Male sex | |
| Age | ↑ per year of life |
| Family history of Barrett carcinoma | |
BMI, body mass index
Histology.
Histologically, evidence of intraepithelial neoplasia is the most important risk indicator.
Patients with Barrett’s esophagus have a 30- to 125-fold increased risk of developing esophageal adenocarcinoma (16). So far, however, no treatment (e.g., endoscopic ablation) has become established for non-dysplastic Barrett’s esophagus (3). The risk of progression to Barrett’s carcinoma increases with the length of the Barrett’s esophagus (length as a surrogate marker for surface area) (17). Evidence of ulceration in the Barrett’s segment is also associated with increased risk of progression (18). Histologically, evidence of intraepithelial neoplasia (IEN; previously called dysplasia) is the most important risk indicator (19). However, it should be borne in mind that diagnosing this change securely is difficult and requires special expertise (20), and for this reason, when there is evidence of intraepithelial neoplasia, a second opinion should always be sought from a pathologist experienced in Barrett-related diagnostic investigations (3). In recent years, a mass of genetic and epigenetic risk markers analyzed on the basis of tissue biopsies have been assessed for their value as predictors of cancer risk. The candidate markers are changes to genes that also play a role in other cancers (e.g., p53, p16). The analyses have thrown up some promising leads. However, since risk indicators need to be validated in a step-by-step process with population studies as the last step, it is too early at present to make a final judgment about their possible clinical value (e10). Only in a few individual cases can, for example, p53 analysis help in grading the intraepithelial neoplasia.
Prevention and early recognition
Theoretically, steps taken to avoid gastroesophageal reflux disease also have the potential to reduce the risk of Barrett’s carcinoma. Smoking increases the risk that Barrett’s esophagus will progress and should (not for that reason alone) be given up. A diet rich in fruit and vegetables can have a protective effect (11, 12, (e11, (e12). It has not yet been conclusively proven that treating gastroesophageal reflux disease with proton pump inhibitors or fundoplication can reduce the risk of cancer. The results of various studies are at best controversial. Currently ongoing is a large randomized controlled study investigating the effects of a proton pump inhibitor with or without acetylsalicylic acid on the incidence of Barrett’s carcinoma in men over the age of 50 with Barrett’s esophagus (ASPECT study). Case–control studies indicate that acetylsalicylic acid, non-steroidal anti-inflammatory drugs, and statins all have cancer-preventing effects (11). No controlled studies have been carried out for these drugs, and there are no benefit–harm analyses for them.
Prevention.
It has not yet been conclusively proven that treating the gastroesophageal reflux disease with proton pump inhibitors or fundoplication can reduce the risk of carcinoma.
Most Barrett’s carcinomas are discovered at the first endoscopy (19). Since the risk associated with non-dysplastic Barrett’s esophagus is lower than was for a long time assumed, the usefulness of regular endoscopic surveillance must be called into question. The updated German guideline recommends endoscopic surveillance with biopsy of non-dysplastic Barrett’s esophagus 1 year after first diagnosis; after that, surveillance is optional. It does seem sensible to carry out follow-up investigations in patients at increased risk of progression to carcinoma. The more risk factors the patient has, the higher the cancer risk (e13). Ablation of the non-dysplastic Barrett’s esophagus should not be performed (3). Radiofrequency ablation, on the other hand, is an alternative to frequent follow-ups in patients with intraepithelial neoplasia that has been proven to be low grade and that cannot be located endoscopically ((e14, (e15).
Protective effect against carcinoma.
Case–control studies indicate that acetylsalicylic acid, nonsteroidal anti-inflammatory drugs, and statins have a protective effect against carcinoma.
Diagnosis
Barrett’s esophagus and Barrett’s carcinoma are diagnosed endoscopically. The standard investigation today is esophagogastroduodenoscopy using high-resolution, high-definition video endoscopy (3, (e16). Chemical (e.g., acetic acid) and technical aids (e.g., electronic image processing, magnification, autofluorescence, endomicroscopy) make it possible to detect early neoplasias (high-grade intraepithelial neoplasia, intramucosal carcinoma) better and to distinguish them from non-dysplastic Barrett’s epithelium (21) (Figure 2). The clinical value of these new technologies has not yet been finally determined (e16), so systematic quadrant biopsy every 1 to 2 cm is still mandatory (22). If an identifiable early neoplasia is found, it should be resected using the technique of endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD). In Barrett’s carcinoma, submucosal dissection must still be regarded as experimental (e17). The depth of tumor invasion into the mucosa or submucosa and its differentiation grade are determined histologically from the resected specimen. At the same time, it can also be determined whether the resection margins (basal; lateral only for en bloc resections) are disease-free. Various proposals exist for the classification of early carcinomas (with invasion of mucosa and submucosa). Where the current TNM classification distinguishes only between pT1a (mucosal invasion) and pT1b (submucosal invasion), several proposed classifications make a case for further subdivision of these early lesions ((e18, (e19). For example, Japanese researchers have proposed subdividing both mucosal and submucosal invasion into three subclasses. These systems are currently undergoing clinical testing (Figure 3).
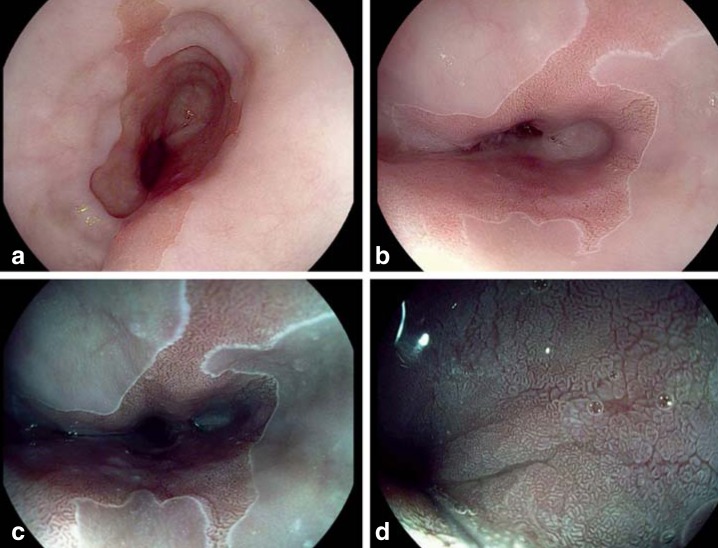
Figure 2.
Endoscopic images of Barrett’s esophagus made by a high-definition endoscope with acetic acid for contrast enhancement and with electronic image processing (iScan)
a+b) Red columnar epithelial metaplasia surrounded by pale squamous epithelium
c+d) After electronic image processing
Figure 3.
TNM (2010) classification of early carcinomas—left) subclassification of early carcinomas pT1a and pT1b in m1–3 (blue) and right) sm1–3 (green), according to the Japanese classification. HG-IEN, high-grade intraepithelial neoplasia; Cis, carcinoma in situ; HGD, high-grade dysplasia
High-resolution video endoscopy.
The standard investigation today is esophagogastroduodenoscopy using high-definition video endoscopy.
Resection technique.
Once identified, an early-stage neoplasia should be resected using the technique of endoscopic mucosal resection or endoscopic submucosal dissection.
Staging involves ultrasonography of the liver and CT of the chest and abdomen in the search for distant metastases. The local T and N stage are determined endosonographically. In our experience, patients with locally advanced disease may already have peritoneal carcinosis, which can be recognized at laparoscopy (which is optional). In some cases, PET-CT may be valuable, as in up to 28% of cases this technique can reveal unknown distant metastases and sometimes even a second carcinoma, leading to a change in treatment strategy (23). Coverage of the costs must be clarified beforehand with the patient’s insurance company.
Treatment
The published research on the treatment of Barrett’s carcinoma is limited; especially, there are no randomized studies of competing treatment procedures. The basic therapeutic options for Barrett’s carcinoma are endoscopic procedures, surgical resection, and chemo- and radiotherapy. For optimal treatment, a single- or multi-mode treatment program is developed individually for each patient. Treatment decisions depend on disease stage (Table 2). Invasion depth and TNM stage are decisive factors (Figure 2).
Table 2. Stage-based treatment of Barrett’s carcinoma (source: 3, 23, 24).
| Tumor stage | Treatment |
|---|---|
| High-grade intraepithelial neoplasiaT1a carcinoma | EMR/ESD followed by radiofrequency ablation of the non-dysplastic Barrett’s mucosa (two-stage treatment) |
| T1b and T2 carcinoma | Esophageal resection |
| T3 to T4a M0 carcinoma (possibly also for T2) | Neoadjuvant chemotherapy or radiochemotherapy → esophageal resection |
| M1 carcinoma | Palliation (occasionally, in patients with limited metastases, resection of primary tumor and metastases) |
EMR, endoscopic mucosal resection; ESD, endoscopic submucosal dissection
Locally limited carcinoma (T1)
Barrett’s carcinomas that are restricted to the mucosa (T1a) are resected endoscopically (Figure 4). Since the risk of lymph node metastasization is extremely low for a tumor of this stage, this is a curative treatment (25). Despite a lack of randomized controlled trials, this strategy has been validated by extensive long-term observations and comparison with surgical treatment series and is now generally accepted (24, 26, 27). When tumor growth is restricted to the upper submucosa (T1b), endoscopic resection can also be curative (28).
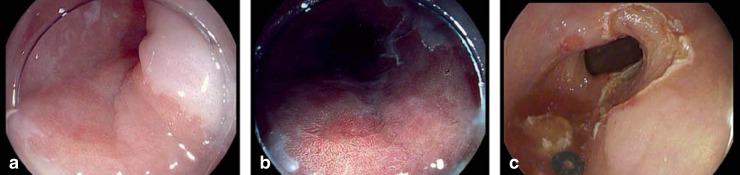
Figure 4.
Endoscopic appearance of an early-stage Barrett’s carcinoma
a) high-definition video endoscopy; b) clearer contours after spraying with acetic acid; c) site after endoscopic resection
The following histological criteria represent an indication for esophageal resection, as they indicate an increased risk of lymph node metastases (3):
Invasion of lymph (L1) or blood vessels (V1)
Invasion of the upper third of the submucosa (T1sm1) and the presence of either of the following risk factors: size >20 mm, poor differentiation grade (G3)
Deep invasion of the submucosa (≥500 µm)
Tumor residue at the basal resection margin (R1 basal).
Treatment schedule.
A year after Barrett’s esophagus is first diagnosed, an endoscopic follow-up with biopsy should be performed. Further surveillance is carried out on an individual basis depending on the patient’s risk profile.
In the West, tumor removal is usually performed as endoscopic mucosal resection. Various techniques are available for this (e.g., snare electrocautery after suction into a special cap or after rubber band ligation); there is no relevant difference between them in terms of safety and efficacy (3, (e20, (e21). In Asia in particular, endoscopic submucosal dissection is increasingly frequently being performed; this technique is more cumbersome but offers the advantage of en bloc resection of even quite large lesions, and thus allows reliable assessment of the lateral resection margins by the pathologist.
At 14.5%, the recurrence rate of Barrett’s carcinoma after endoscopic resection alone is quite high (24). For this reason, ablation of the non-dysplastic Barrett’s esophagus should be carried out after carcinoma resection, as a two-stage procedure (3). This significantly reduces the risk of recurrence (29). There are various options for removing the remaining Barrett’s esophagus. Endoscopic resection in cases where the Barrett’s epithelium extends circumferentially is associated with an unacceptably high rate of stenosis (88%) (e22). Ablation using argon plasma coagulation (APC) is possible, but often residual Barrett’s epithelium remains (sometimes beneath the squamous epithelium). Recently, radiofrequency ablation has proved to be a suitable procedure; used with a special application catheter, it can thermally destroy columnar epithelium in a circumferential or sectoral manner (30). A precondition for all ablative procedures is that they are followed by high-dose therapy with a proton pump inhibitor (PPI), in order to produce an environment in which squamous epithelium will grow rather than columnar epithelium (e23) (Figure 3).
Two-stage procedure.
The recurrence rate of Barrett’s carcinoma after endoscopic resection alone is high. For this reason, ablation of the non-dysplastic Barrett’s esophagus should be carried out after the tumor resection as a two-stage procedure.
Locally advanced carcinoma
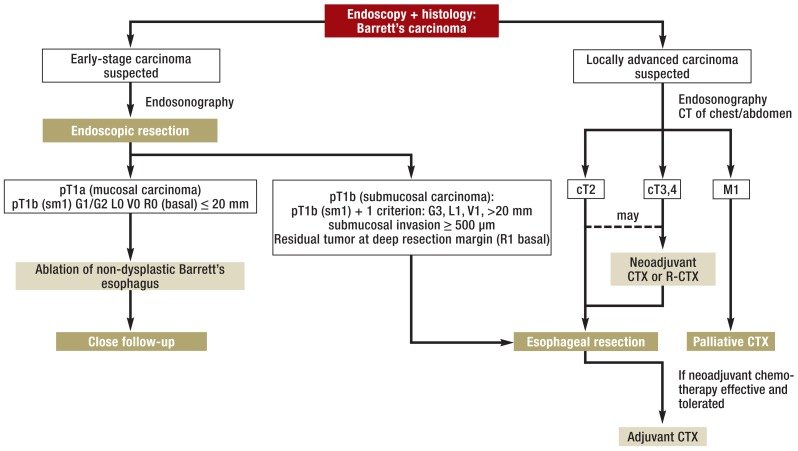
In patients with locally advanced disease without distant metastases (M0), esophageal resection with gastric pull-up is indicated as primary treatment for cT1 (sm1) sm2,3 or after neoadjuvant therapy for cT3–4 and possibly for cT2 tumors, since the long-term prognosis is better after this form of treatment than after esophageal resection alone (31, 32). Neoadjuvant therapy is in the form of chemotherapy. One interesting option is to allow the preoperative chemotherapy to be guided by the tumor response on PET (e24), although this treatment approach has not yet been adopted widely. Radiochemotherapy, too, which is standard for squamous cell carcinoma of the esophagus, improves patient survival (33, e25). However, it is not yet clear whether radiochemotherapy is superior to chemotherapy alone for Barrett’s carcinoma (23, 34, e26). In a randomized study, after a year a trend in favor of chemotherapy alone was visible (Nilsson, personal communication). One meta-analysis showed that neoadjuvant treatment had no negative effect on the postoperative morbidity and mortality of patients with esophageal adenocarcinoma (35). If preoperative chemotherapy has been adequately tolerated and if an R0 resection was performed, postoperative continuation of chemotherapy is to be recommended (perioperative treatment approach) (23). It has not yet been conclusively determined whether esophageal carcinoma should be operated on in the traditional open manner or using a minimally invasive or a hybrid technique. The minimally invasive procedure is probably associated with a lower complication rate compared to open surgery (rate of postoperative pneumonia in the only randomized study: 12% versus 34%, p = 0.005) for similar mortality and similar yield from lymph node resection (36, e27). No long-term data exist on the prognosis for patients. Up until 3 years after surgery no difference is seen. One factor relevant to prognosis is certainly the expertise of the surgical team, so esophageal resection should preferably be carried out in specialized centers. This requirement to some extent conforms to the idea underlying the minimum caseload requirements in German hospitals for certain invasive procedures. The concept of sentinel lymph node navigation is not applicable in esophageal cancer (e28). However, it is possible that lymph node metastases might be found preoperatively at PET-CT and removed along with the tumor at surgery. Figure 5 illustrates the algorithm for diagnosis and treatment of patients with Barrett’s carcinoma.
Figure 5.
Algorithm for diagnosis and treatment of Barrett’s carcinoma (follow-up after endoscopic resection after 3 months, then every 6 months for 2 years, then once a year). Carcinoma restricted to the mucosa is treated endoscopically. In some cases where there is superficial submucosal invasion (T1b–sm1), endoscopic resection may suffice. Stage cT2 carcinomas should, and stage cT3/cT4a carcinomas must, be referred for neoadjuvant therapy (chemo- or radiochemotherapy) followed by esophageal resection. CTX, chemotherapy; R-CTX, radiochemotherapy
Locally advanced carcinoma.
In these cases, esophageal resection with gastric pull-up is indicated as primary treatment for cT1 (sm1) sm2,3 and after neoadjuvant therapy for cT3–4 and possibly for cT2 tumors, since the long-term prognosis is better after this form of treatment than after esophageal resection alone.
Metastatic carcinoma
In patients with distant metastases, as a rule, only palliative and supportive treatment can be offered. However, in some individual cases where the metastasization is limited (e.g., resectable liver metastases) and the patient has a good performance score, a multi-mode individualized treatment program can be implemented with curative intent.
Sentinel lymph nodes.
The concept of sentinel lymph nodes is not applicable in esophageal cancer.
Palliative chemotherapy
At the metastatic stage, patients in good general condition should be offered chemotherapy, because many randomized controlled trials have shown that this prolongs survival and improves quality of life (evidence level 1a) (37). The procedure is similar to that for gastric cancer (37). Tumor response, toxicity, co-morbidity, and the patient’s wishes are the criteria that determine the duration of treatment. Platinum-containing combination chemotherapy is superior to monotherapy. In a few selected cases, intensive treatment regimes (e.g., DCF: docetaxel–cisplatin–5-fluorouracil) are used. In cases with overexpression of the human epidermal growth factor receptor HER2, which occurs in about one in five carcinomas, a survival advantage can be achieved by additional administration of the monoclonal antibody trastuzumab (38). After primary or secondary failure of the first-line chemotherapy, patients in good general condition should be offered a second-line therapy. The choice of drugs depends on the primary therapy (37).
Palliative chemotherapy.
Patients with metastatic disease but in acceptable general condition should be offered palliative chemotherapy with the aim of prolonging survival and improving quality of life.
Palliative endoscopic therapy
In patients with symptomatic obstruction of the esophagus or the esophagogastric junction by the primary tumor, recanalization by ablation (argon plasma coagulation, laser) or stent implantation can help to improve symptoms. Bougienage alone is usually insufficient for symptom control. Because patients with tumors in this region often suffer from severe loss of appetite, early placement of a percutaneous endoscopic gastrostomy to ensure adequate nutrition can be an important and helpful measure.
Palliative radiotherapy
Radiotherapy may be considered for the treatment of symptomatic stenoses or tumor hemorrhage that cannot be treated endoscopically (37). The effect starts to be felt much later than with stent implantation, but it is possible that it lasts longer.
Decision criteria.
Tumor response, toxicity, co-morbidity, and the patient’s wishes are the criteria for decisions about duration of treatment. Platinum-containing combination chemotherapy is superior to monotherapy.
Palliative medicine
Nutritional problems due to lack of appetite or dysphagia, severe nausea, weight loss, and general debility and fatigue are often the predominant clinical issues in palliative treatment of patients with Barrett’s carcinoma. A comprehensive program of palliative care by a trained team can stabilize the quality of life of both the patient and the patient’s relatives, and can adequately meet the needs that arise in this life situation (39).
Follow-up care
After endoscopic treatment of a Barrett’s carcinoma followed by ablation of the non-dysplastic Barrett’s esophagus, close endoscopic surveillance is essential, and this makes considerable demands on patient compliance. Follow-up examinations should be scheduled first at 3 months after the intervention, then 6-monthly for 2 years, and then at yearly intervals (3). This is because of the risk of local recurrence or a second cancer, which if discovered early can be cured by further interventions, and because it is not unusual to find residual or recurrent Barrett’s epithelium at follow-up (24, e29). It must also be borne in mind that in almost all cases of neoplastic Barrett’s esophagus, the intestinal metaplasia has already spread below the squamous epithelium at diagnosis, making it impossible for endoscopic diagnosis and treatment to be reliable (40). In all clinical situations where treatment is intended to be curative, regular endoscopic surveillance is routine. The value of this has not be formally proven, however.
Palliative radiotherapy.
Radiotherapy may be considered for the treatment of symptomatic stenoses or, occasionally, tumor hemorrhage that cannot be treated endoscopically.
Close follow-up surveillance.
After endoscopic treatment alone of a well-differentiated early carcinoma, close follow-up surveillance is required.
Figure 1.
From gastroesophageal reflux disease (GERD) to Barrett’s carcinoma: risk of progression (according to 7, 8). About 40% of carcinomas occur without clinical signs of pre-existing reflux disease.
LG-IEN, low-grade intraepithelial neoplasia; HG-IEN, high-grade intraepithelial neoplasia
Further Information On Cme. This article has been certified by the North Rhine Academy for Postgraduate and Continuing Medical Education.
Deutsches Ärzteblatt provides certified continuing medical education (CME) in accordance with the requirements of the Medical Associations of the German federal states (Länder). CME points of the Medical Associations can be acquired only through the Internet, not by mail or fax, by the use of the German version of the CME questionnaire within 6 weeks of publication of the article. See the following website: cme.aerzteblatt.de
Participants in the CME program can manage their CME points with their 15-digit –uniform CME number“ (einheitliche Fortbildungsnummer, EFN). The EFN must be entered in the appropriate field in the cme.aerzteblatt.de website under –meine Daten“ (–my data“), or upon registration. The EFN appears on each participant’s CME certificate.
This CME unit can be accessed until 21 June 2015, and earlier CME units until the dates indicated:
“Carpal Tunnel Syndrome” (issue 1–2/2015), until 29 March 2015;
“Dry Eye” (issue 5/2015), until 26 April 2015;
“Diving Medicine in Clinical Practice” (issue 9/2015), until 24 May 2015
Please answer the following questions to participate in our certified Continuing Medical Education program. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
According to Robert Koch Institute statistics, how high is the 1-year prevalence of esophageal carcinoma among men in the German population?
400–500
1000–2000
4000–6000
8000–10 000
10 000–12 000
Question 2
What is the recommended primary diagnostic investigation in a 60-year-old obese smoker with suspected Barrett’s esophagus?
Esophageal barium swallow
Esophagogastroduodenoscopy
pH–impedance testing
Esophageal manometry
Chest CT
Question 3
Which of the following is a risk factor for Barrett’s carcinoma?
Reflux disease
Hiatus hernia
Female sex
Underweight
Helicobacter pylori infection
Question 4
According to the treatment algorithm, when staging confirmed Barrett’s carcinoma, which further diagnostic study is recommended?
Chest and upper abdominal CT
Abdominal MRI
Chest X-ray
Echocardiography
Body plethysmography
Question 5
A patient has undergone endoscopic removal of early carcinoma in Barrett’s esophagus (pTNM stage: pT1a [m2] L0 V0 G1 R0). What do you recommend as the next step?
Follow-up in 1 year
PET-CT
Esophageal resection
Ablation of the non-dysplastic Barrett’s esophagus
Long-term PPI treatment
Question 6
A patient has undergone endoscopic removal of an early carcinoma in Barrett’s esophagus (pTNM stage: pT1b [sm1] L1 V0 G3 R0). What do you recommend as the next step?
Follow-up in 3 months
Adjuvant chemotherapy
Esophageal resection
Ablation of the Barrett’s mucosa
PPI plus ASA
Question 7
What benefits patients with metastatic Barrett’s carcinoma most in terms of their malignancy?
Statins
Bevacizumab
Cetuximab
Everolimus
Chemotherapy
Question 8
In a patient with Barrett’s esophagus, the pathologist diagnoses low-grade intraepithelial neoplasia. What do you recommend?
Endoscopic resection of the Barrett’s mucosa
Endoscopic ablation of the Barrett’s mucosa
Limited esophageal resection
Start high-dose PPI treatment
Obtaining a second opinion on the histology
Question 9
What is the operation of choice for non-metastatic Barrett’s carcinoma in the lower third of the esophagus, stage T2 M0?
Esophageal resection with gastric pull-up
Limited esophageal resection (Merendino)
Esophageal resection with small bowel interposition
Esophageal resection with colon interposition
Proximal gastric resection
Question 10
According to the treatment algorithm, for which diagnosis after primary therapy is close endoscopic surveillance sufficient?
Esophageal resection in a patient with locally limited carcinoma (pT1b)
Endoscopic R0 resection of a well-differentiated early carcinoma ≤ 20 mm
Endoscopic R0 resection of a poorly differentiated early carcinoma >20 mm
Esophageal resection in a patient with locally advanced carcinoma (pT2–4)
Esophageal resection in a patient with lymph node metastases
Acknowledgments
Translated from the original German by Kersti Wagstaff, MA.
Footnotes
Conflict of interest statement
Professor Labenz has received consultancy fees from Covidien.
Professors Hölscher, Koop, Tannapfel, and Kiesslich declare that no conflict of interest exists.
References
- 1.Edgren G, Adami HO, Weiderpass E, Nyrén O. A global assessment of the oesophageal adenocarcinoma epidemic. Gut. 2013;62:1406–1414. doi: 10.1136/gutjnl-2012-302412. [DOI] [PubMed] [Google Scholar]
- 2.Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R. Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900–1920. doi: 10.1111/j.1572-0241.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- 3.Koop H, Fuchs KH, Labenz J, et al. S2k Leitlinie: Gastroösophageale Refluxkrankheit. Z Gastroenterol. 2014;52:1299–1346. doi: 10.1055/s-0034-1385202. [DOI] [PubMed] [Google Scholar]
- 4.Masclee GMC, Coloma PM, de Wilde M, Kuipers EJ, Sturkenboom MC. The incidence of Barrett’s oesophagus and oesophageal adenocarcinoma in the United Kingdom and the Netherlands is levelling off. Aliment Pharmacol Ther. 2014;39:1321–1330. doi: 10.1111/apt.12759. [DOI] [PubMed] [Google Scholar]
- 5.Ronkainen J, Aro P, Storskrubb T, et al. Prevalence of Barrett’s esophagus in the general population: an endoscopic study. Gastroenterology. 2005;129:1825–1831. doi: 10.1053/j.gastro.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 6.De Jonge PJF, van Blankenstein M, Grady WM, Kuipers EJ. Barrett’s oesophagus: epidemiology, cancer risk and implications for management. Gut. 2014;63:191–202. doi: 10.1136/gutjnl-2013-305490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubenstein JH, Taylor JB. Meta-analysis: the association of oesophageal adenocarcinoma with symptoms of gastro-oesophageal reflux. Aliment Pharmacol Ther. 2010;32:1222–1227. doi: 10.1111/j.1365-2036.2010.04471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russo A, Bronte G, Cabibi D, et al. The molecular changes driving the carcinogenesis in Barrett’s esophagus: which came first, the chicken or the egg? Crit Rev Oncol Hematol. 2013;86:278–289. doi: 10.1016/j.critrevonc.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Jankowski JA, Satsangi J. Barrett’s esophagus: evolutionary insights from genomics. Gastroenterology. 2013;144:667–669. doi: 10.1053/j.gastro.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 10.Chak A, Ochs-Balcom H, Falk G, et al. Familiarity in Barrett’s esophagus, adenocarcinoma of the esophagus, and adenocarcinoma of the gastroesophageal junction. Cancer Epidemiol Biomarkers Prev. 2006;15:1668–1673. doi: 10.1158/1055-9965.EPI-06-0293. [DOI] [PubMed] [Google Scholar]
- 11.Lepage C, Drouillard A, Jouve J-L, Faivre J. Epidemiology and risk factors for oesophageal adenocarcinoma. Dig Liver Dis. 2013;45:625–629. doi: 10.1016/j.dld.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 12.Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400–412. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 13.Erichsen R, Robertson D, Farkas DK, et al. Erosive reflux disease increases risk for esophageal adenocarcinoma, compared with nonerosive reflux. Clin Gastroenterol Hepatol. 2012;10:475–480. doi: 10.1016/j.cgh.2011.12.038. [DOI] [PubMed] [Google Scholar]
- 14.Derakhshan MH, Liptrot S, Paul J, Brown IL, Morrison D, McColl KE. Oesophageal and gastric intestinal-type adenocarcinoma show the same male predominance due to a 17 year delayed development in females. Gut. 2009;58:16–23. doi: 10.1136/gut.2008.161331. [DOI] [PubMed] [Google Scholar]
- 15.Islami F, Kamangar F. Helicobacter pylori and esophageal cancer risk: a meta-analysis. Cancer Prev Res. 2008;1:329–338. doi: 10.1158/1940-6207.CAPR-08-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solaymani-Dodaran M, Logan RF, West J, Card T, Coupland C. Risk of oesophageal cancer in Barrett’s esophagus and gastro-oesophageal reflux. Gut. 2004;53:1070–1074. doi: 10.1136/gut.2003.028076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anaparthy R, Gaddam S, Kanakadandi V, et al. Association between length of Barrett’s esophagus and risk of high-grade dysplasia or adenocarcinoma in patients without dysplasia. Clin Gastroenterol Hepatol. 2013;11:1430–1436. doi: 10.1016/j.cgh.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Coleman HG, Bhat SK, Murray LJ, et al. Symptoms and endoscopic features at Barrett’s esophagus diagnosis: implications for neoplastic progression risk. Am J Gastroenterol. 2014;109:527–534. doi: 10.1038/ajg.2014.10. [DOI] [PubMed] [Google Scholar]
- 19.Hvid-Jensen F, Pedersen L, Drewes AM, Sørensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med. 2011;365:1375–1383. doi: 10.1056/NEJMoa1103042. [DOI] [PubMed] [Google Scholar]
- 20.Curvers WL, ten Kate FJ, Krishnadath KK, et al. Low-grade dysplasia in Barrett’s esophagus: overdiagnosed and underestimated. Am J Gastroenterol. 2010;105:1523–1530. doi: 10.1038/ajg.2010.171. [DOI] [PubMed] [Google Scholar]
- 21.Qumseya BJ, Wang H, Badie N, et al. Advanced imaging technologies increase detection of dysplasia and neoplasia in patients with Barrett’s esophagus: a meta-analysis and systematic review. Clin Gastroenterol Hepatol. 2013;11:1562–1570. doi: 10.1016/j.cgh.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abela JE, Going JJ, Mackenzie JF, McKernan M, O’Mahoney S, Stuart RC. Systematic four-quadrant biopsy detects Barrett’s dysplasia in more patients than nonsystematic biopsy. Am J Gastroenterol. 2008;103:850–855. doi: 10.1111/j.1572-0241.2007.01746.x. [DOI] [PubMed] [Google Scholar]
- 23.Stahl M, Ruhstaller T. Multimodale Therapie des Ösophaguskarzinoms. Internist. 2014;55:7–14. doi: 10.1007/s00108-013-3315-7. [DOI] [PubMed] [Google Scholar]
- 24.Pech O, May A, Manner H, et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology. 2014;146:652–660. doi: 10.1053/j.gastro.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Hölscher AH, Bollschweiler E, Schröder W, Metzger R, Gutschow C, Drebber U. Prognostic impact of upper, middle and lower third mucosal or submucosal infiltration in early esophageal cancer. Ann Surg. 2011;254:802–807. doi: 10.1097/SLA.0b013e3182369128. [DOI] [PubMed] [Google Scholar]
- 26.Bennett C, Green S, DeCaestecker J, et al. Surgery versus radical endotherapies for early cancer and high-grade dysplasia in Barrett’s oesophagus. Cochrane Database of Systematic Reviews. 2012;Issue 11 doi: 10.1002/14651858.CD007334.pub4. Art. No.: CD007334. DOI: 10.1002/ 14651858.CD007334,pub4 (last accessed on 1 December 2014) [DOI] [PubMed] [Google Scholar]
- 27.Pech O, Boschweiler E, Manner H, Leers J, Ell C, Hölscher AH. Comparison between endoscopic and surgical resection of mucosal esophageal adenocarcinoma in Barrett’s esophagus at two high-volume centers. Ann Surg. 2011;254:62–72. doi: 10.1097/SLA.0b013e31821d4bf6. [DOI] [PubMed] [Google Scholar]
- 28.Manner H, Pech O, Heldmann Y, et al. Efficacy, safety, and long-term results of endoscopic treatment for early stage adenocarcinoma of the esophagus with low-risk sm1 invasion. Clin Gastroenterol Hepatol. 2013;11:630–635. doi: 10.1016/j.cgh.2012.12.040. [DOI] [PubMed] [Google Scholar]
- 29.Manner H, Rabenstein T, Pech O, et al. Ablation of residual Barrett’s epithelium after endoscopic resection: a randomized long-term follow-up study of argon plasma coagulation vs. surveillance (APE study). Endoscopy. 2014;46:6–12. doi: 10.1055/s-0033-1358813. [DOI] [PubMed] [Google Scholar]
- 30.Phoa KN, Pouw RE, van Vilsteren FG, et al. Remission of Barrett’s esophagus with early neoplasia 5 years after radiofrequency ablation with endoscopic resection: a Netherlands cohort study. Gastroenterology. 2013;145:96–104. doi: 10.1053/j.gastro.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 31.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 32.Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681–692. doi: 10.1016/S1470-2045(11)70142-5. [DOI] [PubMed] [Google Scholar]
- 33.Van Hagen P, Hulshoff M, van Lanschot JJB, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 34.Stahl M, Walz MK, Stuschke M, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcoinoma of the esophagogastric junction. J Clin Oncol. 2009;27:851–856. doi: 10.1200/JCO.2008.17.0506. [DOI] [PubMed] [Google Scholar]
- 35.Kumagai K, Rouvelas I, Tsai JA, et al. Meta-analysis of postoperative morbidity and perioperative mortality in patients receiving neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal and gastro-oesophageal junctional cancers. Br J Surg. 2014;101:321–338. doi: 10.1002/bjs.9418. [DOI] [PubMed] [Google Scholar]
- 36.Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet. 2012;379:1887–1892. doi: 10.1016/S0140-6736(12)60516-9. [DOI] [PubMed] [Google Scholar]
- 37.Moehler M, Al-Batran SE, Andus T, et al. German S3-guideline Diagnosis and treatment of esophagogastric cancer. Z Gastroenterol. 2011;49:461–531. doi: 10.1055/s-0031-1273201. [DOI] [PubMed] [Google Scholar]
- 38.Kasper S, Schuler M. Targeted therapies in gastroesophageal cancer. Eur J Cancer. 2014;50:1247–1258. doi: 10.1016/j.ejca.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advance cancer: a cluster-randomized controlled trial. Lancet. 2014;383:1721–1730. doi: 10.1016/S0140-6736(13)62416-2. [DOI] [PubMed] [Google Scholar]
- 40.Anders M, Lucks Y, Abbas El-Masry M, et al. Subsquamous extension of intestinal metaplasia is detected in 98 % of cases of neoplastic Barrett’s esophagus. Clin Gastroenterol Hepatol. 2014;12:405–410. doi: 10.1016/j.cgh.2013.07.013. [DOI] [PubMed] [Google Scholar]
- e1.Bollschweiler E, Knoppe K, Wolfgarten E, Hölscher AH. Prävalenz von Refluxsymptomen in der allgemeinen Bevölkerung von Köln. Z Gastroenterol. 2007;45:177–181. doi: 10.1055/s-2006-927402. [DOI] [PubMed] [Google Scholar]
- e2.Robert Koch-Institut. Krebs in Deutschland. www.rki.de/Krebs/DE/Content/Publikationen/Krebs_in_Deutschland/kid_. 2013/kid_2013_c15_speiseroehrepdf?__blob=publicationFile. (last accessed on 9 February 2015)
- e3.Nocon M, Labenz J, Willich SN. Lifestyle factors and symptoms of gastro-oesophageal reflux - a population-based study. Aliment Pharmacol Ther. 2006;23:169–174. doi: 10.1111/j.1365-2036.2006.02727.x. [DOI] [PubMed] [Google Scholar]
- e4.Kastelein F, van Olphen S, Spaander M, Steyerberg E, Bruno M. Impact of surveillance for Barrett’s esophagus on survival of patients with neoplastic progression: results of a large multicenter prospective cohort study. United European Gastroenterol J 2014. (Suppl 1) [Google Scholar]
- e5.Khandwalla HE, Graham DY, Kramer JR, et al. Barrett’s esophagus suspected at endoscopy but no specialized intestinal metaplasia on biopsy, what’s next? Am J Gastroenterol. 2014;109:178–182. doi: 10.1038/ajg.2013.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e6.Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg. 1998;85:1457–1459. doi: 10.1046/j.1365-2168.1998.00940.x. [DOI] [PubMed] [Google Scholar]
- e7.Pohl H, Arash H, Ell C, et al. Length of Barrett’s esophagus and cancer risk—implications from a population based study. Gastroenterology. 2014;146 [Google Scholar]
- e8.Van Soest EM, Siersema PD, Dieleman JP, Sturkenboom MC, Kuipers EJ. Age and sex distribution of the incidence of Barrett’s esophagus found in a Dutch primary care population. Am J Gastroenterol. 2005;100:2599–2600. doi: 10.1111/j.1572-0241.2005.00305_6.x. [DOI] [PubMed] [Google Scholar]
- e9.Fischbach LA, Graham DY, Kramer JR, et al. Association between Helicobacter pylori and Barrett’s esophagus: a case-control study. Am J Gastroenterol. 2014;109:357–368. doi: 10.1038/ajg.2013.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e10.Varghese S, Lao-Sirieix P, Fitzgerald RC. Identification and clinical implementation of biomarkers for Barrett’s esophagus. Gastroenterology. 2012;142:435–441. doi: 10.1053/j.gastro.2012.01.013. [DOI] [PubMed] [Google Scholar]
- e11.Navarro Silvera SA, Mayne ST, Gammon MD, et al. Diet and lifestyle factors and risk of subtypes of esophageal and gastric cancers: classification tree analysis. Ann Epidemiol. 2014;24:50–57. doi: 10.1016/j.annepidem.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e12.Pohl H, Wrobel K, Bojarski C, et al. Risk factors in the development of esophageal adenocarcinoma. Am J Gastroenterol. 2013;108:200–207. doi: 10.1038/ajg.2012.387. [DOI] [PubMed] [Google Scholar]
- e13.Thrift AP, Garcia JM, El-Serag HB. A multibiomarker risk score helps predict risk for Barrett’s esophagus. Clin Gastroenterol Hepatol. 2014;12:1267–1271. doi: 10.1016/j.cgh.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e14.Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA. 2014;311:1209–1217. doi: 10.1001/jama.2014.2511. [DOI] [PubMed] [Google Scholar]
- e15.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med. 2009;360:2277–2288. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- e16.Boerwinkel DF, Swager A-F, Curvers WL, Bergman JJGHM. The clinical consequences of advanced imaging techniques in Barrett’s esophagus. Gastroenterology. 2014;146:622–629. doi: 10.1053/j.gastro.2014.01.007. [DOI] [PubMed] [Google Scholar]
- e17.Neuhaus H, Terheggen G, Rutz EM, Vieth M, Schuhmacher B. Endoscopic submucosal dissection plus radiofrequency ablation of neoplastic Barrett’s esophagus. Endoscopy. 2012;44:1105–1113. doi: 10.1055/s-0032-1310155. [DOI] [PubMed] [Google Scholar]
- e18.Participants in the Paris Workshop. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon. Gastrointest Endosc. 2003;58:3–43. doi: 10.1016/s0016-5107(03)02159-x. [DOI] [PubMed] [Google Scholar]
- e19.Wittekind Ch, Meyer HJ., editors. UICC. Neue TNM-Klassifikation. 7th edition. Weinheim: Wiley-Blackwell; 2010. TNM Klassifikation maligner Tumoren; pp. 69–73. [Google Scholar]
- e20.Konda VJ, Waxman I. Endotherapy for Barrett’s esophagus. Am J Gastroenterol. 2012;107:827–833. doi: 10.1038/ajg.2012.70. [DOI] [PubMed] [Google Scholar]
- e21.Watson TJ. Endoscopic therapies for Barrett’s neoplasia. J Thorac Dis. 2014;6:S298–S308. doi: 10.3978/j.issn.2072-1439.2014.03.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e22.Van Vilsteren FG, Pouw RE, Seewald S, et al. Stepwise radical endoscopic resection versus radiofrequency ablation for Barrett’s oesophagus with high-grade dysplasia or early cancer: a multicentre randomised trial. Gut. 2011;60:765–773. doi: 10.1136/gut.2010.229310. [DOI] [PubMed] [Google Scholar]
- e23.Overholt BF. Acid suppression and reepithelialization after ablation of Barrett’s esophagus. Dig Dis. 2000-2001;18:232–239. doi: 10.1159/000051404. [DOI] [PubMed] [Google Scholar]
- e24.Lordick F, Ott K, Krause BJ, et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol. 2007;8:797–805. doi: 10.1016/S1470-2045(07)70244-9. [DOI] [PubMed] [Google Scholar]
- e25.Van Hagen P, Wijnhoven BP, Nafteux P, et al. Recurrence pattern in patients with a pathologically complete response after neoadjuvant chemoradiotherapy and surgery for oesophageal cancer. Br J Surg. 2013;100:267–273. doi: 10.1002/bjs.8968. [DOI] [PubMed] [Google Scholar]
- e26.Burmeister BH, Thomas JM, Burmeister EA, et al. Is concurrent therapy required in patients receiving preoperative chemotherapy for adenocarcinoma of the oesophagus? A randomised phase II trial. Eur J Cancer. 2011;47:354–360. doi: 10.1016/j.ejca.2010.09.009. [DOI] [PubMed] [Google Scholar]
- e27.Uttley L, Campbell F, Rhodes M, Cantrell A, Stegenga H, Lloyd-Jones M. Minimally invasive oesophagectomy versus open surgery: is there an advantage? Surg Endosc. 2013;27:724–731. doi: 10.1007/s00464-012-2546-3. [DOI] [PubMed] [Google Scholar]
- e28.Takeuchi H, Fujii H, Ando N, et al. Validation study of radio-guided sentinel lymph node navigation in esophageal cancer. Ann Surg. 2009;249:757–763. doi: 10.1097/SLA.0b013e3181a38e89. [DOI] [PubMed] [Google Scholar]
- e29.Gupta M, Iyer PG, Lutzke L, et al. Recurrence of esophageal intestinal metaplasia after endoscopic mucosal resection and radiofrequency ablation of Barrett’s esophagus: results from a US multicenter consortium. Gastroenterology. 2013;145:79–86. doi: 10.1053/j.gastro.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]