Abstract
Signals that convey related information may impose selection on each other, creating evolutionary links between different components of the communicative repertoire. Here, we ask about the consequences of the evolutionary loss of one signal (a colour patch) on another (a motion display) in Sceloporus lizards. We present data on male lizards of four species: two pairs of sister taxa representing two independent evolutionary losses of the static colour patch (Sceloporus cozumelae and Sceloporus parvus; Sceloporus siniferus and Sceloporus merriami). Males of the two species that have undergone an evolutionary loss of blue-belly patches (S. cozumelae, S. siniferus) were less active than their blue-bellied sister taxa (S. parvus, S. merriami), consistent with the suggestion that the belly patches were lost to reduce conspicuousness of species with high predation pressure. In contrast, the headbob display appears to have become more, rather than less, conspicuous over evolutionary time. The colour patch is exhibited primarily during aggressive encounters, whereas headbob displays are multifunction signals used in several different contexts, including aggressive encounters. Males of species that have lost the colour patch produced more motion displays, and the structure of those motion displays were more similar to those produced during combat. In both evolutionary episodes, a static colour signal appears to have been replaced by dynamic motion displays that can be turned off in the presence of predators and other unwanted receivers. The predominant pattern is one of evolutionary compensation and interactions between multiple signals that convey related information.
Keywords: communicative display, conspicuous visual signal, dynamic signal, evolutionary compensation, multimodal signal, Sceloporus, static signal
Whenever animals use multiple signals, these signals can also be evolutionarily linked by the common demands of communication, such that shifts in one signal can influence the evolution of others (inter-signal interactions, sensu Hebets & Papaj, 2005). Many animals maintain repertoires of multiple communicative displays (e.g. Bro-Jørgensen, 2010; Higham & Hebets, 2013; Uetz, Roberts, Clark, Gibson, & Gordon, 2013). Multiple signals allow animals to convey information to different receivers or across different environments (e.g. Lyons, Goedert, & Morris, 2014; Uetz et al., 2013), to separate attention getting from information content (Endler, Gaburro, & Kelley, 2014; Preininger et al., 2013) or to provide the security of a backup signal for particularly important messages. Multiple signals with similar or related content may also be evolutionarily maintained in the context of fluctuating frequency-dependent selection (Bro-Jørgensen, 2010), because of selection acting to increase the complexity and diversity of the repertoire (e.g. Akre & Ryan, 2011), or because of differential responses to natural and sexual selection (e.g. Chen, Symonds, Melville, & Stuart-Fox, 2013; Ord & Martins, 2006). Here, we make use of a replicated natural experiment in which male Sceloporus lizards have twice lost a conspicuous colour signal to ask whether evolution of a second signal, the headbob display, has been shifted via compensatory change.
Evolutionary interactions between signals, both trade-offs and positive relationships, have been reported in several comparative studies. In a now-classic example, birdsongs with broad frequency ranges tend to have lower trill rates, suggesting that there is a mechanical constraint imposed on the evolution of one aspect of the signal by the other (Podos, 1997). In contrast, there is a positive evolutionary relationship between the complexity of visual and seismic signals in wolf spiders, suggesting selection for increasing elaboration (Hebets, Vink, Sullivan-Beckers, & Rosenthal, 2013). Across Sceloporus lizards, there is evidence of evolutionary tradeoffs between headbob display duration and complexity (Martins, 1993) and between visual and chemical markers of territory boundaries (Ossip-Klein, Fuentes, Hews, & Martins, 2013). Whereas loss of an aggressive colour signal in males of one species of Sceloporus lizards was accompanied by a compensatory increase in chemical behaviour (Hews & Benard, 2001; Hews, Date, Hara, & Castellano, 2011), there was no evidence of a compensatory shift in chemical cues for male crickets that have lost an acoustic signal (Gray, Bailey, Poon, & Zuk, 2014).
Evolutionary relationships between signals may be particularly important for signals that are functionally intertwined, such as signals that convey similar information (Ossip-Klein et al., 2013) or that modify the receiver's response to a second signal (e.g. Hebets, 2005; Rowland, Ruxton, & Skelhorn, 2013; Thompson, Bissell, & Martins, 2008). In many animals, selection has pushed signals to become more complex, carrying more information to more receivers (content-based hypotheses; Hebets & Papaj, 2005). In wood warblers, for example, sexual selection has shaped the evolution of more elaborate trills in some species and more complex songs in others (Cardoso & Hu, 2011). Male—male competition has similarly shaped an abundance of conspicuous colour, motion and chemical signals in lizards (e.g. Chen, Stuart-Fox, Hugall, & Symonds, 2012; Ord, Blumstein, & Evans, 2001; Pérez i de Lanuza, Font, & Monterde, 2013). Other forces shape signals that are easier or harder to detect in contrast to a particular environment (efficacy-based hypotheses; Hebets & Papaj, 2005). For example, male bowerbirds increase mating success by using multiple colour elements in their bowers to alter female perception (Endler et al., 2014).
Here, we ask whether the evolutionary loss of a colour signal (a male belly patch) in Sceloporus lizards has been accompanied by compensatory shifts in a motion signal (headbob display), particularly in aspects of the motion signal that are functionally similar to the colour signal. Sceloporus males actively show their colourful belly patches primarily during aggressive male—male combat (Carpenter & Ferguson, 1977), whereas headbob displays are a multifunction signal used primarily in long-distance broadcasts of individual, sex and species identity (Martins, 1991, 1994; Martins, Bissell, & Morgan, 1998). When headbob displays are produced during combat, they tend to be longer and are accompanied by postures that exaggerate male body size (Martins, 1993); these structural differences also elicit stronger responses from display receivers (Martins, Ord, & Davenport, 2005) than do broadcast headbob displays. In the current study, we focus on two evolutionary episodes in which the colour patch was lost, perhaps due to increased selective pressure from visual predators or to relaxed sexual selection from decreased male—male combat. For each episode, we ask whether male lizards of species that have lost the conspicuous colour patch also produce less conspicuous headbob displays, or whether the plain-bellied Sceloporus species have compensated for the loss of the colour signal by producing more aggressive headbob displays (i.e. more similar to those used during combat). We report comprehensive behavioural data from unmanipulated animals in the field, using the natural experiment of two independent evolutionary events to retain some generality while asking whether animals with multiple signals can compensate for signal loss.
Methods
Subject Species
We collected data on two species pairs that represent independent evolutionary losses of the colourful belly patches typical of male Sceloporus lizards. Clade A includes Sceloporus parvus and Sceloporus cozumelae; Clade B includes Sceloporus merriami and Sceloporus siniferus. Males of most of the 90+ species of Sceloporus lizards have colourful belly patches, and it is likely that males of the root ancestor of this genus also exhibited colourful belly patches (Wiens, 2001). In each of our two target clades, we chose one species (S. parvus, S. merriami) from among those in which males retain the colourful bellies typical of the genus, and a second species (S. cozumelae, S. siniferus) in which males have plain bellies that lack the colour patch (Fig. 1). The two species within each clade diverged and the colourful belly patch was probably lost a very long time ago: ∼15 million years ago if we estimate times from a recent phylogeny (Wiens, Kuczynski, Arif, & Reeder, 2010) and place the root of the genus at the time of the oldest known fossil Sceloporus (Lawing, 2012; Yatkola, 1976). There are other extant and extinct Sceloporus species within each clade that are not represented in the current study; these four were chosen primarily for logistical reasons.
Figure 1.
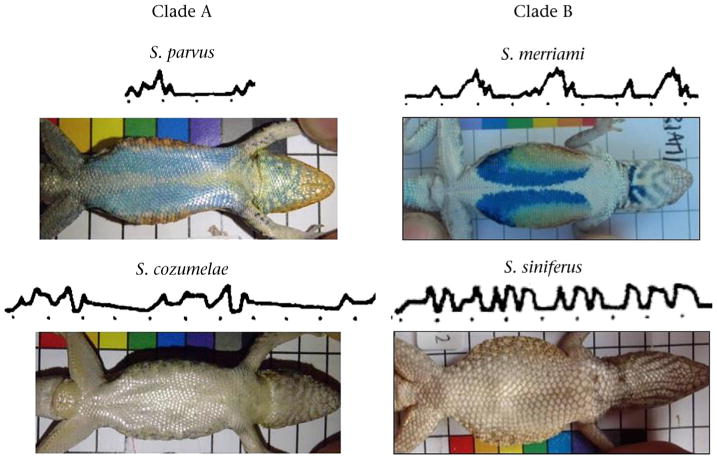
Display action pattern graphs (DAPgraphs) and colour photos of the ventral surface (belly) of a typical adult male of each Sceloporus species. DAPgraphs are schematic depictions of the species-typical displays for each clade, modified from Carpenter (1978). In each DAPgraph, the Y axis represents the height of the head as it is raised and lowered during the display, and dots on the X axis mark time in seconds. Images from Carpenter (1978) were cut to reflect the total duration and average number of headbobs/display reported in our study.
We sampled each species during the peak mating season (peak of male gonadal activity). In Clade A, Male S. parvus have blue belly patches (in the population we sampled, they are a pale blue), whereas female S. parvus and both male and female S. cozumelae have plain bellies (Fig. 1). We sampled male S. parvus in June 2013 in rocky outcrops of the desert scrub habitat near Querétaro, México. We collected behavioural data from male S. cozumelae on sandy beaches near Cancún, México in May 2013. For Clade B, we recorded behaviour of male S. merriami on the reddish-grey walls of a slot canyon in the Chihuahua desert Big Bend TX in May 2011 and 2012. We recorded male S. siniferus in the thick vegetation of a semideciduous tropical rainforest at the Huatulco National Park, MX in June and July 2012. Sceloporus merriami males have colourful belly patches that include blue and green elements, whereas the females have plain bellies (Fig. 1). Both male and female S. siniferus have plain bellies.
Procedure
We measured 21—51 males for each species using a Canon Elura 100 camcorder to record the undisturbed behaviour of each male from a distance of 2—10 m. The number of individuals we sampled from each species and viewing distances depended on local abundance and habitat conditions. Although we strived for 10 min focal animal samples, trials often ended sooner when the subject moved out of sight. For Clade A, we collected data from 21 male S. parvus, with trials ranging from 6 to 18 min in duration and summing to 4.9 h of observation time. We collected data from 23 male S. cozumelae, with trials ranging from 1 to 21 min in duration and summing to 3.9 h of observation time. We were able to collect more and longer behavioural samples from males of Clade B. We collected data from 51 male S. merriami, with trials ranging from 6 to 18 min in duration and summing to 10.1 h of observation time. We collected data from 34 male S. siniferus, with trials ranging from 2 to 23 min in duration and summing to 6.3 h of observation time.
Scoring
Afterwards, one observer (E.P.M.) scored each recording using an ethogram that categorized all behaviour that occurred during our samples while focusing on headbob displays. We scored ‘activity’ as the total number of behavioural acts, including chemical behaviour, visual displays and three types of movement. ‘Motion displays’ consisted primarily of headbob displays, but also included occasional shudder displays (typical of male courtship), full-shows (static postural displays including arched back, lateral flattening and gular extension) and tail wags. For each headbob display, we also recorded the total duration of the display, the number of individual up-and-down motions (i.e. headbobs) and whether the display was accompanied by full-show postural elements (e.g. arched back, lateral flattening, gular extension). We combined counts of tongue flicks, gapes and jaw rubs into a single measure of ‘chemical behaviour’. Finally, we used three measures of movement, counting small motions of the head or limbs as ‘adjustments’, scoring displacementsof less than 10 cm as ‘moves’ and locomotion across longer distances as ‘travel’.
Hypothesis Tests and Statistics
We began by testing (1) whether the presence of the colour patch predicted overall activity level. If the colour patch was lost due to increased selective pressure from visual predators or to relaxed sexual selection (i.e. less male—male combat), plain-bellied species are also likely to have become less visually conspicuous by decreasing their overall activity. Second, we focused on the amount of information transferred via headbob displays and tested (2) whether the loss of the colour signal was accompanied by a decrease in the frequency or duration of headbob displays. Third, we asked whether there was a shift in the specific type of information transferred by examining the detailed structure of the headbob displays used. Headbob displays used during combat tend to have more up-and-down motions per display and include fullshow body postures (Martins et al., 2005). We thus tested (3) whether the evolutionary loss of colour patches was accompanied by an evolutionary increase in the structural aggression of the headbob displays (i.e. number of headbobs per display and use of full-show body postures).
In each case, we used two-way ANOVAs to test the importance of colour in explaining behavioural differences, while taking into account also the phylogenetic differences between the two clades. One factor (clade) compared lizards in Clade A (S. parvus and S. cozumelae) with those in Clade B (S. merriami and S. siniferus). The second factor (colour) reflected the difference between species with colourful belly patches (S. parvus and S. merriami) and those with plain bellies (S. cozumelae and S. siniferus). We fitted all models using the ‘aov’ command in R (R Development Core Team, 2006), and applying type I SS to explain variation using the clade factor first. For each model, we also examined the residuals to confirm that the data conformed to the usual assumptions (homoscedasticity and normality) of ANOVA. When the assumptions were violated, we used nonparametric tests instead.
Results
Plain-bellied Males Were Less Active
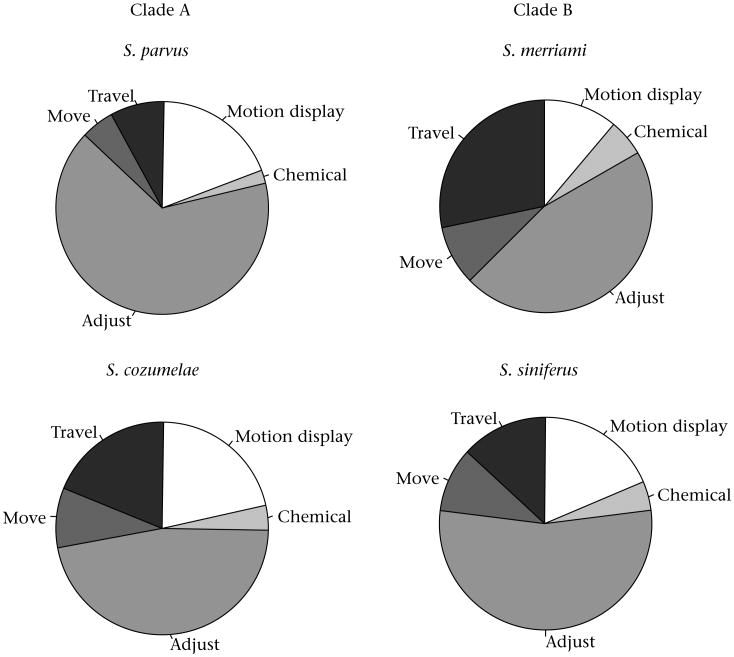
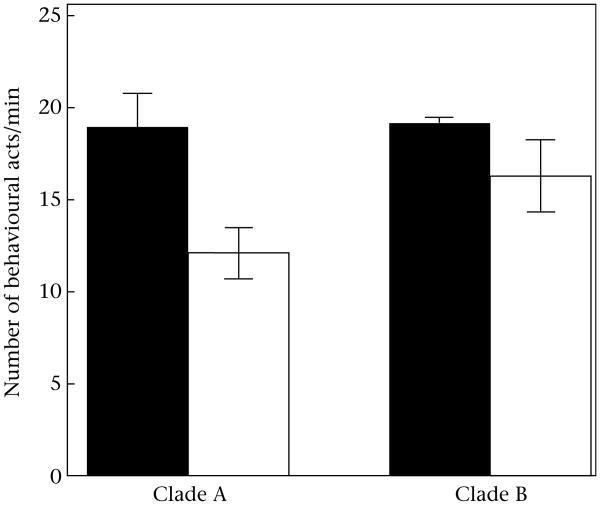
Even during the peak of the mating season, Sceloporus males spend most of their time basking, such that the most common behaviour contributing to our general measure of activity was a slight movement of head or limbs (‘adjust’, Fig. 2). Plain-bellied male lizards in both clades were less active than were males of the species with colourful belly patches (Fig. 3), such that there was a significant effect of colour (F1,125 = 5.03, P = 0.03) in the two-way ANOVA. In Clade A, the difference in activity was primarily due to a difference in slight motions of head and limbs (‘adjust’, Fig. 2), whereas in Clade B, the activity difference was mostly a difference in longer bouts of locomotion (‘travel’, Fig. 2). There was no evidence of a difference in the proportion of social signals (motion displays or chemical behaviour), as might be expected if relaxed sexual selection were responsible for the difference in activity. The two clades did not differ substantively in activity (i.e. the main effect of clade was not significant: F1,125 = 1.96, P = 0.16), and there was no detectable colour*clade interaction effect (F1,125 = 0.96, P = 0.33).
Figure 2.
Relative proportion of behavioural acts underlying our measure of activity in male Sceloporus. ‘Motion display’ consists primarily of headbob displays, and ‘chemical’ consists mostly of tongue-flicks. ‘Adjust’, ‘move’ and ‘travel’ are body motions that involve increasingly more distance. See Methods for more detailed descriptions. Clade A and B are as in Fig. 1. Male S. parvus and S. merriami have colourful belly patches, whereas male S. cozumelae and S. siniferus do not.
Figure 3.
Mean ± SE activity of male Sceloporus lizards in each clade, as measured by the number of behavioural acts per minute (i.e. summing bouts of locomotion, motion displays and chemical behaviour; Fig. 2). Black bars: males with colourful belly patches (Clade A: S. parvus; Clade B: S. merriami); white bars: males lacking colourful belly patches (Clade A: S. cozumelae; Clade B: S. siniferus).
Plain-bellied Males Spent More Time in Headbob Displays, but Details Depend on the Clade
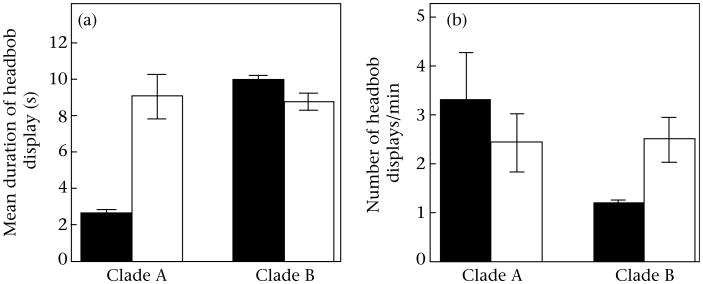
The evolutionary loss of the colourful belly patch was associated with longer and more frequent headbob displays, but the two clades differed in exactly which types of changes occurred, leading to significant interaction effects in the ANOVAs. In Clade A, the main difference was in the average duration of headbob displays (Fig. 4a; colour*clade interaction: F1,71 = 19.31, P < 0.0001). On average, S. cozumelae (plain belly) displays were more than four times longer than S. parvus displays (Tukey post hoc: P < 0.0001), whereas there was no difference in the average duration of headbob displays in Clade B (Tukey post hoc: P = 0.65). In Clade B, the difference was in terms of the frequency of headbob displays (Fig. 4b; colour*clade interaction: F1,124 = 4.61, P < 0.04). Sceloporus siniferus (plain belly) males produced headbob displays about twice as often as S. merriami males (Tukey post hoc: P < 0.05). There was no difference in the frequency of headbob displays in Clade A (Tukey post hoc: P = 0.70). Intriguingly, males of the two species with colourful belly patches also differed from each other in both measures, raising the possibility that headbob displays have evolved differently in the two clades (or at least in the two species with colourful belly patches).
Figure 4.
Mean ± SE (a) duration of headbob displays and (b) number of headbob displays/min for male Sceloporus lizards in each clade. Black bars: males with colourful belly patches (Clade A: S. parvus; Clade B: S. merriami); white bars: males lacking colourful belly patches (Clade A: S. cozumelae; Clade B: S. siniferus).
Plain-bellied Males Used More Aggressive Motion Displays
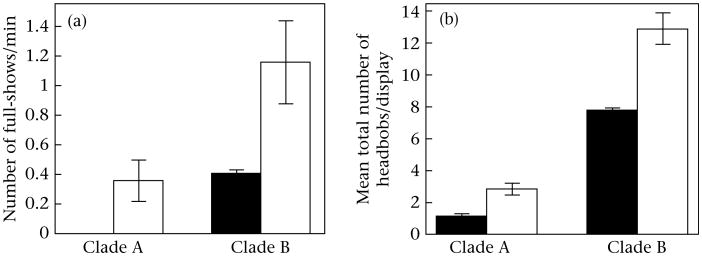
Male lizards from species with plain bellies used headbob displays that were more aggressive in structure (i.e. more like headbob displays typically used in combat) than did lizards from species with colourful belly patches (Fig. 5). In Clade A, S. parvus (blue belly) males did not use the full-show postures that emphasize the belly patches (i.e. arched back, gular extension, lateral flattening) at all during these unmanipulated observations. A chi-square test confirmed that S. parvus and S. cozumelae males differed in the frequency of trials in which headbob displays with full-show postures were observed (χ21 = 4.6, P = 0.03). In Clade B, males of both species used full-show postures during headbob displays, but S. siniferus (plain belly) males were nearly twice as likely to include full-show postures as S. merriami males (t27 = 2.3, P = 0.013; means were calculated using only those displays in which full-show postures were used).
Figure 5.
Mean ± SE (a) number of full-shows (up-and-down motions) and (b) total number of headbobs/display for male Sceloporus lizards in each clade. Black bars: males with colourful belly patches (Clade A: S. parvus; Clade B: S. merriami); white bars: males lacking colourful belly patches (Clade A: S. cozumelae; Clade B: S. siniferus).
In both clades, males of the plain-bellied species used nearly twice as many up-and-down motions per display as did males of the species with colourful belly patches (Fig. 4b), leading to a strong, significant effect of belly colour (F1,71 = 36.7, P < 0.0001) in the two-way ANOVA. This difference was more pronounced in Clade B (S. siniferus and S. merriami) than in Clade A (S. parvus and S. cozumelae), leading also to a significant clade*colour interaction effect (F1,71 = 37.4, P < 0.003). In part, this difference in variation was due to male lizards in Clade B using significantly more up-and-down motions in their headbob displays than males in Clade A, leading also to a significant clade effect (F1,72 = 208.5, P < 0. 0001).
Discussion
In two independent evolutionary episodes, we found evidence of evolutionary compensation between communicative signals. Male Sceloporus lizards from species without the colourful belly patches typical of the genus spent more time engaged in headbob displays and produced headbob displays that were more aggressive in structure (i.e. more similar to those used in combat than in broadcast) than did males from species with colourful belly patches. Male lizards of plain-bellied species were also less active, engaging in fewer bouts of locomotion and small body adjustments, consistent with the suggestion that an increase in visual predators are responsible for the loss of conspicuousness. Although relaxed sexual selection may also explain a loss of conspicuousness, males of both plain-bellied species relied more heavily on headbob displays than did their colourful-bellied counterparts, using longer or more frequent displays despite the decrease in overall activity levels. Plain-bellied lizards also used more aggressive headbob displays, with more headbobs per display and more frequent use of full-show body postures, effectively compensating for the loss of the aggressive signal of the colour patch by transferring the aggressive information content to the motion display. Our study does not address whether selection acted first on colour patches or headbob displays, and it seems likely that changes in both signal types were gradual and tightly intertwined.
Although producing headbob displays makes the lizards more conspicuous, use of a dynamic signal gives the displayer more immediate control and better ability to avoid the attention of predators and other unwanted receivers. Sceloporus belly patches, like most colour patches, are conspicuous signals that are difficult to turn off or hide, even when unwanted receivers are present. Even though the belly patches are partially obscured by their ventral position, they are often readily visible on basking lizards as they raise their chests or limbs off a hot substrate, and on running animals as they move between perches. Bro-Jørgensen (2010) suggested that we should expect dynamic signals to evolve when the information changes on short time frames, and we should expect multiple fixed signals to evolve when messages with roughly comparable content are needed in different contexts. Here, our results are consistent with the hypothesis that a second benefit of dynamic signals is to give receivers tighter control over unintended information flow to predators and other unwanted receivers. Additional studies are needed to confirm that predators are an important selective factor. Most communication happens in a rich and complex sensory environment (Baugh & Ryan, 2010; Schmidt, Römer, & Riede, 2013) with a multitude of potential eavesdroppers (e.g. Dzieweczynski, Gill, & Perazio, 2012; Sprau, Roth, Amrhein, & Naguib, 2012; Webster & Laland, 2013). Unintended receivers can have powerful evolutionary effects on signals (Lichtenberg, Zivin, Hrncir, & Nieh, 2014), and by shifting from static to dynamic signals species may gain evolutionary flexibility.
As suggested in Ossip-Klein et al. (2013), shared information content seems to be an important feature of evolutionary compensation. Ossip-Klein et al. (2013) found a relationship only between evolutionary changes in two signals that are both used in broadcasts of territorial defence (motion and chemical), and not between signals that shared the same sensory modality or spatial distance in a study of 39 Sceloporus species. The current study finds a complementary relationship between signals that communicate aggression (colour and motion) that went undetected in Ossip-Klein et al. (2013), probably because the earlier study used coarse measures of the headbob display from the published literature (one data point for each species), and searched for patterns that were consistent across all taxa. In the current study, we found that the most striking patterns depended on rich behavioural detail, and that there were important differences in the process by which the two clades achieved the same result. Time frame may also be important. Gray et al. (2014) found no evidence that the chemical secretions of male crickets have evolved to compensate for the recent loss of an acoustic signal. Female crickets could not distinguish between the chemical signals of male crickets from populations that did or did not have song. Perhaps the cricket populations in this study have not been separated for sufficient time for evolutionary compensation to occur, or perhaps the chemical signals are conveying different information from the acoustic signals.
Our results reflect a broader evolutionary pattern of signal loss and specialization, similar to that described by Wiens (2001), and in contrast to those that found evolutionary increases in signal complexity (e.g. Hebets et al., 2013; Ord et al., 2001). Most recent frameworks focus on the many possible benefits of using multiple signals (e.g. Rowe & Halpin, 2013), and provide less context for understanding the consequences of signal loss. Mathematical models based on game theory (e.g. Ruxton & Schaefer, 2013; Wilson, Dean, & Higham, 2013) may provide a more balanced approach that incorporates both costs and benefits. It may also be useful to draw parallels between a communication system consisting of multiple signals used in different contexts and a reaction norm that offers a plastic response to variable environments (e.g. Foster, 2013; Lande, 2014).
Evolutionary interactions between multiple signals that convey related information can both constrain and speed evolution. Use of multiple signals allows animals to respond flexibly to different physical and social contexts, evolving repertoires of signals that are separately tuned to different aspects of the environment (Higham & Hebets, 2013). As reviewed by Bro-Jørgensen (2010), subgroups of animals in different environments may rely on different combinations of signals, leading quickly to reproductive isolation, divergence and speciation (e.g. Seddon et al., 2013; Uy & Safran, 2013). However, interactions between signals can also slow future evolution by requiring animals to respond simultaneously to multiple, sometimes conflicting, selective pressures. For example, if rapid environmental shifts create selective pressure for longer or shorter headbob displays, Sceloporus lizards may not be able to respond quickly if there are competing selective pressures imposed by colour or chemical signals. Detailed information on the evolutionary interactions among multiple signals will be crucial to making predictions about behavioural response to climate change and other rapid environmental shifts (Partan, 2013).
In conclusion, our study finds evidence for two episodes of evolutionary compensation between two elements of a multiple signal system in which aggression shifted from a static colour signal to a dynamic motion signal. Shifting from static to dynamic signals gives the sender more immediate control and finer-tuned ability to avoid the attention of unwanted receivers. Additional studies are needed to test whether predation pressure or social density are important differences between species in this system. It also appears that compensation occurs more readily between signals with shared information content. Although the shift from colour to motion signals occurred in slightly different ways in the two evolutionary episodes, both shifts made the motion signals more aggressive, translating the aggressive information content of the colour signal into motion. Additional studies are needed to determine whether very long periods of time (15 million years in this study) are needed for compensation to evolve, whether compensation is limited to displays that share very similar information content and whether indirect selection between multiple signals are important in other systems.
Acknowledgments
We thank Molly Morris, two anonymous referees, Jesualdo Fuentes, Delia Shelton, Delawrence Sykes, José Oyola-Morales, Patrick Cain and Jake Pruett for many discussions and help in the field. We also thank the staff and volunteers at the Parque Nacional Huatulco for logistical support, and SEMARNAT for permits to work with Mexican species (S. siniferus: 09/O1-0557/12/13; S. parvus and S. cozumelae: 09/k5-0904/01/13). This material is based on work supported by the National Science Foundation under grant numbers IOS-1050274 to E.P.M. and IOS-1052247 to D.K.H. A.G.O.-K. was supported by a Common Themes in Reproductive Diversity training grant (NIH-NICD 5T32HD049336-10). Data from this publication have been archived in the Dryad Digital Repository.
References
- Akre KL, Ryan MJ. Female túngara frogs elicit more complex mating signals from males. Behavioral Ecology. 2011;22(4):846–853. http://dx.doi.org/10.1093/beheco/arr065. [Google Scholar]
- Baugh AT, Ryan MJ. Mate choice in response to dynamic presentation of male advertisement signals in túngara frogs. Animal Behaviour. 2010;79(1):145–152. http://dx.doi.org/10.1016/j.anbehav.2009.10.015. [Google Scholar]
- Bro-Jørgensen J. Dynamics of multiple signalling systems: animal communication in a world in flux. Trends in Ecology & Evolution. 2010;25(5):292–300. doi: 10.1016/j.tree.2009.11.003. http://dx.doi.org/10.1016/j.tree.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Cardoso GC, Hu Y. Birdsong performance and the evolution of simple (rather than elaborate) sexual signals. American Naturalist. 2011;178(5):679–686. doi: 10.1086/662160. http://dx.doi.org/10.1086/662160. [DOI] [PubMed] [Google Scholar]
- Carpenter CC. Comparative display behavior in the genus Sceloporus (Iguanidae) Contributions in biology and geology to the Milwaukee Public Museum. 1978;18:1–71. [Google Scholar]
- Carpenter CC, Ferguson GW. Variation and evolution of stereotyped behaviour in reptiles. In: Gans C, TInkle DW, editors. Biology of reptilia: Ecology and behaviour. New York, NY: Academic Press; 1977. pp. 335–554. [Google Scholar]
- Chen IP, Stuart-Fox D, Hugall AF, Symonds MR. Sexual selection and the evolution of complex color patterns in dragon lizards. Evolution. 2012;66(11):3605–3614. doi: 10.1111/j.1558-5646.2012.01698.x. [DOI] [PubMed] [Google Scholar]
- Chen IP, Symonds MRE, Melville J, Stuart-Fox D. Factors shaping the evolution of colour patterns in Australian agamid lizards (Agamidae): a comparative study. Biological Journal of the Linnean Society. 2013;109(1):101–112. http://dx.doi.org/10.1111/bij.12030. [Google Scholar]
- Dzieweczynski TL, Gill CE, Perazio CE. Opponent familiarity influences the audience effect in male—male interactions in Siamese fighting fish. Animal Behaviour. 2012;83(5):1219–1224. http://dx.doi.org/10.1016/j.anbehav.2012.02.013. [Google Scholar]
- Endler JA, Gaburro J, Kelley LA. Visual effects in great bowerbird sexual displays and their implications for signal design. Proceedings of the Royal Society B: Biological Sciences. 2014;281(1783) doi: 10.1098/rspb.2014.0235. http://dx.doi.org/10.1098/rspb.2014.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster SA. Evolution of behavioural phenotypes: influences of ancestry and expression. Animal Behaviour. 2013;85(5):1061–1075. http://dx.doi.org/10.1016/j.anbehav.2013.02.008. [Google Scholar]
- Gray B, Bailey NW, Poon M, Zuk M. Multimodal signal compensation: do field crickets shift sexual signal modality after the loss of acoustic communication? Animal Behaviour. 2014;93(0):243–248. http://dx.doi.org/10.1016/j.anbehav.2014.04.033. [Google Scholar]
- Hebets EA. Attention-altering signal interactions in the multimodal courtship display of the wolf spider Schizocosa uetzi. Behavioral Ecology. 2005;16(1):75–82. doi: 10.1093/beheco/arn080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebets EA, Papaj DR. Complex signal function: developing a framework of testable hypotheses. Behavioral Ecology and Sociobiology. 2005;57(3):197–214. http://dx.doi.org/10.1007/s00265-004-0865-7. [Google Scholar]
- Hebets EA, Vink CJ, Sullivan-Beckers L, Rosenthal MF. The dominance of seismic signaling and selection for signal complexity in Schizocosa multimodal courtship displays. Behavioral Ecology and Sociobiology. 2013;67(9):1483–1498. http://dx.doi.org/10.1007/s00265-013-1519-4. [Google Scholar]
- Hews DK, Benard MF. Negative association between conspicuous visual display and chemosensory behavior in two phrynosomatid lizards. Ethology. 2001;107(9):839–850. http://dx.doi.org/10.1046/j.1439-0310.2001.00712.x. [Google Scholar]
- Hews DK, Date P, Hara E, Castellano MJ. Field presentation of male secretions alters social display in Sceloporus virgatus but not S. undulatus lizards. Behavioral Ecology and Sociobiology. 2011;65(7):1403–1410. [Google Scholar]
- Higham J, Hebets E. An introduction to multimodal communication. Behavioral Ecology and Sociobiology. 2013;67(9):1381–1388. doi: 10.1007/s00265-013-1565-y. http://dx.doi.org/10.1007/s00265-013-1590-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande R. Evolution of phenotypic plasticity and environmental tolerance of a labile quantitative character in a fluctuating environment. Journal of Evolutionary Biology. 2014;27(5):866–875. doi: 10.1111/jeb.12360. http://dx.doi.org/10.1111/jeb.12360. [DOI] [PubMed] [Google Scholar]
- Lawing AM. Doctoral thesis. Bloomington, IN: Indiana University; 2012. Geographic and morphological response of species and communities to their climate and environment. Dissertations & Theses @ CIC Institutions; ProQuest Dissertations & Theses A&I database. [Google Scholar]
- Lichtenberg EM, Zivin JG, Hrncir M, Nieh JC. Eavesdropping selects for conspicuous signals. Current Biology. 2014;24(13):R598–R599. doi: 10.1016/j.cub.2014.05.062. http://dx.doi.org/10.1016/j.cub.2014.05.062. [DOI] [PubMed] [Google Scholar]
- Lyons SM, Goedert D, Morris MR. Male-trait-specific variation in female mate preferences. Animal Behaviour. 2014;87(0):39–44. http://dx.doi.org/10.1016/j.anbehav.2013.10.001. [Google Scholar]
- Martins EP. Individual and sex differences in the use of the push-up display by the sagebrush lizard, Sceloporus graciosus. Animal Behaviour. 1991;41(3):403–416. http://dx.doi.org/10.1016/s0003-3472(05)80841-3. [Google Scholar]
- Martins EP. Contextual use of the push-up display by the sagebrush lizard, Sceloporus graciosus. Animal Behaviour. 1993;45(1):25–36. [Google Scholar]
- Martins EP. Structural complexity in a lizard communication system: the Sceloporus graciosus “push-up” display. Copeia. 1994;1994:944–955. [Google Scholar]
- Martins EP, Bissell AN, Morgan KK. Population differences in a lizard communicative display: evidence for rapid change in structure and function. Animal Behaviour. 1998;56(5):1113–1119. doi: 10.1006/anbe.1998.0872. http://dx.doi.org/10.1006/anbe.1998.0872. [DOI] [PubMed] [Google Scholar]
- Martins EP, Ord TJ, Davenport S. Combining motions into complex displays: playbacks with a robotic lizard. Behavioral Ecology and Sociobiology. 2005;58(4):351–360. http://dx.doi.org/10.1007/s00265-005-0954-2. [Google Scholar]
- Ord TJ, Blumstein DT, Evans CS. Intrasexual selection predicts the evolution of signal complexity in lizards. Proceedings of the Royal Society B: Biological Sciences. 2001;268(1468):737–744. doi: 10.1098/rspb.2000.1417. http://dx.doi.org/10.1098/rspb.2000.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ord TJ, Martins EP. Tracing the origins of signal diversity in anole lizards: phylogenetic approaches to inferring the evolution of complex behaviour. Animal Behaviour. 2006;71(6):1411–1429. http://dx.doi.org/10.1016/j.anbehav.2005.12.003. [Google Scholar]
- Ossip-Klein AG, Fuentes JA, Hews DK, Martins EP. Information content is more important than sensory system or physical distance in guiding the long-term evolutionary relationships between signaling modalities in Sceloporus lizards. Behavioral Ecology and Sociobiology. 2013;67:1513–1522. http://dx.doi.org/10.1007/s00265-013-1535-4. [Google Scholar]
- Partan S. Ten unanswered questions in multimodal communication. Behavioral Ecology and Sociobiology. 2013;67(9):1523–1539. doi: 10.1007/s00265-013-1565-y. http://dx.doi.org/10.1007/s00265-013-1565-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez i de Lanuza G, Font E, Monterde JL. Using visual modelling to study the evolution of lizard coloration: sexual selection drives the evolution of sexual dichromatism in lacertids. Journal of Evolutionary Biology. 2013;26(8):1826–1835. doi: 10.1111/jeb.12185. http://dx.doi.org/10.1111/jeb.12185. [DOI] [PubMed] [Google Scholar]
- Podos J. A performance constraint on the evolution of trilled vocalizations in a songbird family (Passeriformes: Emberizidae) Evolution. 1997:537–551. doi: 10.1111/j.1558-5646.1997.tb02441.x. [DOI] [PubMed] [Google Scholar]
- Preininger D, Boeckle M, Freudmann A, Starnberger I, Sztatecsny M, Hödl W. Multimodal signaling in the small torrent frog (Micrixalus saxicola) in a complex acoustic environment. Behavioral Ecology and Sociobiology. 2013;67(9):1449–1456. doi: 10.1007/s00265-013-1489-6. http://dx.doi.org/10.1007/s00265-013-1489-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. A language and environment for statistical computing Retrieved from. Vienna, Austria: R Foundation for Statistical Computing; 2006. http://www.R-project.org. [Google Scholar]
- Rowe C, Halpin C. Why are warning displays multimodal? Behavioral Ecology and Sociobiology. 2013;67(9):1425–1439. http://dx.doi.org/10.1007/s00265-013-1515-8. [Google Scholar]
- Rowland HM, Ruxton GD, Skelhorn J. Bitter taste enhances predatory biases against aggregations of prey with warning coloration. Behavioral Ecology. 2013;24(4):942–948. http://dx.doi.org/10.1093/beheco/art013. [Google Scholar]
- Ruxton G, Schaefer HM. Game theory, multi-modal signalling and the evolution of communication. Behavioral Ecology and Sociobiology. 2013;67(9):1417–1423. http://dx.doi.org/10.1007/s00265-013-1596-4. [Google Scholar]
- Schmidt AKD, Römer H, Riede K. Spectral niche segregation and community organization in a tropical cricket assemblage. Behavioral Ecology. 2013;24(2):470–480. http://dx.doi.org/10.1093/beheco/ars187. [Google Scholar]
- Seddon N, Botero CA, Tobias JA, Dunn PO, MacGregor HEA, Rubenstein DR, et al. Sexual selection accelerates signal evolution during speciation in birds. Proceedings of the Royal Society B: Biological Sciences. 2013;280(1766):20131065. doi: 10.1098/rspb.2013.1065. http://dx.doi.org/10.1098/rspb.2013.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprau P, Roth T, Amrhein V, Naguib M. Distance-dependent responses by eavesdroppers on neighbour—stranger interactions in nightingales. Animal Behaviour. 2012;83(4):961–968. http://dx.doi.org/10.1016/j.anbehav.2012.01.015. [Google Scholar]
- Thompson JT, Bissell AN, Martins EP. Inhibitory interactions between multimodal behavioural responses may influence the evolution of complex signals. Animal Behaviour. 2008;76(1):113–121. http://dx.doi.org/10.1016/j.anbehav.2007.12.015. [Google Scholar]
- Uetz GW, Roberts JA, Clark DL, Gibson JS, Gordon SD. Multimodal signals increase active space of communication by wolf spiders in a complex litter environment. Behavioral Ecology and Sociobiology. 2013;67(9):1471–1482. http://dx.doi.org/10.1007/s00265-013-1557-y. [Google Scholar]
- Uy JA, Safran R. Variation in the temporal and spatial use of signals and its implications for multimodal communication. Behavioral Ecology and Sociobiology. 2013;67(9):1499–1511. http://dx.doi.org/10.1007/s00265-013-1492-y. [Google Scholar]
- Webster MM, Laland KN. Local enhancement via eavesdropping on courtship displays in male guppies, Poecilia reticulata. Animal Behaviour. 2013;86(1):75–83. http://dx.doi.org/10.1016/j.anbehav.2013.04.014. [Google Scholar]
- Wiens JJ. Widespread loss of sexually selected traits: how the peacock lost its spots. Trends in Ecology & Evolution. 2001;16(9):517–523. http://dx.doi.org/10.1016/s0169-5347(01)02217-0. [Google Scholar]
- Wiens JJ, Kuczynski CA, Arif S, Reeder TW. Phylogenetic relationships of phrynosomatid lizards based on nuclear and mitochondrial data, and a revised phylogeny for Sceloporus. Molecular Phylogenetics and Evolution. 2010;54(1):150–161. doi: 10.1016/j.ympev.2009.09.008. http://dx.doi.org/10.1016/j.ympev.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Wilson A, Dean M, Higham J. A game theoretic approach to multimodal communication. Behavioral Ecology and Sociobiology. 2013;67(9):1399–1415. http://dx.doi.org/10.1007/s00265-013-1589-3. [Google Scholar]
- Yatkola DA. Mid-Miocene lizards from western Nebraska. Copeia. 1976;1976:645–654. [Google Scholar]