Introduction
Multiple sclerosis (MS) is an autoimmune, neurodegenerative disorder marked by chronic inflammatory demyelination in the central nervous system (CNS). MS has a complex etiology involving both genetic susceptibility and exposure to environmental factors (Margaret H, Nancy S et al. 2011); there is, for example, a strong link between low sunlight exposure and high MS risk (McFarlin and McFarland 1982, 1998, Garcion, Wion-Barbot et al. 2002, Correale, Ysrraelit et al. 2009). In recent years our understanding of the role of vitamin D3 in CNS functioning has increased rapidly, with low vitamin D level being identified as a potential risk factor for MS this implies that abnormal functioning of CNS causes MS. While vitamin D3 is best known as a calcium homeostasis modulator, both experimental and clinical observations provide evidence that vitamin D3 is also one of several environmental factors that can affect MS prevalence; these observations are supported by the fact that there is a reduced risk of the illness associated with low sunlight exposure and a benefit from use of vitamin D supplements (van der Mei, Ponsonby et al. 2003, Munger, Zhang et al. 2004). Because sunlight exposure produces vitamin D3, the high MS risk associated with low sunlight exposure has been attributed to insufficient sunlight for catalyzing synthesis of the vitamin D3 hormone, 1,25-dihydroxyvitamin D3 (1,25-(OH)2D3) (Haines, Terwedow et al. 1998). Consistent with this hypothesis, 1,25(OH)2D3 has been shown to strongly inhibit experimental autoimmune encephalomyelitis (EAE), an animal model of MS, and this effect is mainly through an immunomodulatory mechanism (Lemire and Archer 1991, Spach, Nashold et al. 2006, Constantinescu, Farooqi et al. 2011). However, whether 1,25(OH)2D3 can directly promote endogenous neuroregeneration/remyelination is not known.
Neural stem cells (NSCs) are precursor cells that have the capacity to undergo self-renewal and to differentiate into multiple neural cell types, including neurons, astrocytes and oligodendrocytes (Li, Li et al. 2013). The majority of NSCs come from two areas: the subventricular zone (SVZ) and the subgranular zone of the hippocampus (Doetsch, Caille et al. 1999). NSC proliferation and differentiation plays an important role in neural development and repair; however, this function is dramatically reduced in CNS inflammatory disorders such as MS, resulting in a failure in spontaneous remyelination and neural recovery (Franklin and Ffrench-Constant 2008). Although NSC-based transplantation has become a potential therapeutic approach in the treatment of neurological disorders (Martino and Pluchino 2007, Yang, Xing et al. 2009, Kovacs, Wagner et al. 2012), a treatment that can promote endogenous NSC proliferation and neural cell differentiation would hold great promise for neural repair and cellular repopulation in the CNS. In the present study, we investigated the possibility that, in addition to its known immunomodulatory effect, 1,25(OH)2D3 may have a direct beneficial effect on NSCs, thus promoting neural cell differentiation and neuroprotection and reducing neurological deficits in EAE/MS.
Materials and Methods
NSC Culture
NSCs were generated from C57BL/6 female adult mice (8–10 weeks old). Whole brain was harvested (Neural Tissue Dissociation kit, Miltenyi Biotec, San Diego, CA) and cell suspensions plated onto a tissue culture petri dish (100×15 mm) in proliferation media (DMEM-F12 medium, B27 supplement, Gibco Life Technologies, Grand Island, NY) along with penicillin 10,000 IU/ml and streptomycin 10,000 µg/ml (Corning Cellgro, Mediatech, Inc., Manassas, VA) in the presence of 20 ng/ml human recombinant epidermal growth factor (EGF, Peprotech, Rocky Hill, NJ) and 10 ng/ml basic fibroblast growth factor (bFGF, Peprotech, Rocky Hill, NJ). Culture medium was changed every 3 days. Under these conditions, cells grew rapidly, forming small clusters gradually enlarged by cell division, and the early phase of neurosphere formation was observed after 3–5 days. Passaging of free-floating neurospheres was performed by dissociation with Accutase cell detachment solution (Innovative Cell Technologies, San Diego, CA) into small neurospheres or single cells in the same medium. NSCs at the passage 5, which had been identified by neurosphere and nestin+, were used in this study(Pluchino, Quattrini et al. 2003). All animal protocols were approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University, following NIH guidelines.
Proliferation of NSCs
NSC proliferation at different concentrations of 1,25(OH)2D3 in culture was determined by Bromodeoxyuridine (BrdU) assay. BrdU can be incorporated into DNA during the synthesis-phase of the cell cycle as a substitute for thymidine, thereby serving as a marker for proliferation. Evaluation of cell proliferation could thus be obtained from culture and incorporation of BrdU DNA synthesis assays. NSCs at passage 5 were plated at 1 × 105 cells /cm2 in microtiter plate and cultured with proliferation induction media for five days. 1, 25(OH)2D3 at concentrations of 10−12–10−6 M was added the following day. BrdU (BrdU Cell Proliferation ELISA Kit, Abcam, Cambridge, MA) was added to the cells and incubated at 37°C for 4–6 hrs. The cell cycle length was determined as the time required for incorporation of BrdU into all proliferative cells by ELISA analysis (Multiskan FC Microplate Photometer, Thermo Fisher Scientific Inc., Waltham, MA).
NSC proliferation was also evaluated by counting their absolute numbers. Neurosphere dissociated cells were transferred to poly-D lysine and laminin (Sigma-Aldrich Co., St. Louis, MO) coated 96-well plates at a density of 5 × 104 cells/ml and maintained in proliferation medium at 37°C. The following day, 1,25(OH)2D3, at a concentration of 10−6 M, was added to the culture medium. neurospheres were dissociated into single cells and counted by hemocytometer at days 1, 3, 5, 9, and 14 of culture.
NSC differentiation
To evaluate NSC differentiation, single cell suspensions of NSCs (1.25×105 cells/ml) at passage 5 were cultured in 24-well plates and incubated with stem cell differentiation induction medium, i.e., NSC basal medium plus 10% NSC differentiation supplements (Stem Cell Technologies, Vancouver, BC, V5Z 1B3, Canada) at 37°C. Various concentrations of 1,25(OH)2D3 (10−12–10−6 M) were added to the cells on the following day and NSC differentiation was evaluated after 14 days of culture.
Immunocytochemistry
Cells were grown (1.25 × 105 cells /ml) on coated coverslips (cover glasses, circles, 12 mm, thickness 0.13–0.17 mm; Carolina Biological Supply Company, Burlington, CA) with poly-D lysine and laminin (Sigma-Aldrich Co. St. Louis, MO) in 24-well plates. Differentiation media with or without 1,25(OH)2D3 at 10−6 M was added to the cells on the following day. After 14 days, cells were fixed with 4% paraformaldehyde (Mediatech, Inc., Manassas, VA) in PBS and processed for immunocytochemistry staining. Blocking was performed in 3% BSA, 8% horse serum and 0.3% Triton X-100 for 2 hours at room temperature. Cells were then incubated with primary antibodies: anti-GalC (1:50) as an intracellular oligodendrocyte marker; anti-GFAP (1:150), an intracellular astrocytic marker; anti-NeuN (1:500), a diagnostic neuronal marker and anti-nestin (1:50), an undifferentiated NSC marker, at 4°C overnight. Secondary antibodies were applied for 1 h at room temperature after rinsing coverslips twice with PBS. Cells that lacked the primary antibody served as negative control.
Cells were mounted with vectashields mounting media (Vector Laboratories, Inc., Burlingame, CA) and visualized by fluorescence microscope (Nikon Eclipse E600, Optical Apparatus Co. DeWitt, Iowa). Images were acquired with a Retiga 1300 (Q Imaging Co., Surrey BC, Canada) high performance CCD camera.
Real-time quantitative PCR
Experiments were performed by adding 10−12–10−6 M 1,25(OH)2D3 next day after the cells have been dissociated from primary neurospheres. After 5 days, total RNA was extracted from the cells and cDNA prepared from 1 µg of total RNA (RNeasy Kit, QIAGEN) according to the manufacturer’s instructions (QuantiTect Reverse Transcription Kit, QIAGEN,Valencia,CA). PCR reactions (QuantiFast SYBR Green PCR Kit, QIAGEN, Valencia, CA) were initiated with denaturation at 95°C for 10 s, annealing at 60°C for 30 s, polymerization at 72°C for 30 s and with 40 cycles. The experiment was carried out in triplicate for expression of VDR and neurotrophic factors, with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) housekeeping gene primer serving as an endogenous control and internal standard. Sequencing primers are shown in Table 1. Real-Time quantitative PCR was performed with ABI Prism 7000 sequence detection system (Applied Bio-Systems, Foster City, CA).
Table 1.
Sequences of primers used in real time RT-PCR
| Gene Name | Primer sequence |
|---|---|
| VDR | Forward : AGA TGA CCC TTC TGT GAC CC Reverse : AGC TTC TTC AGT CCC ACC TG |
| NT-3 | Forward : CCA TTG ACA TTC GGG GAC ACC Reverse : GCC TGG CTT CTT TAC ATC TCG TTTC |
| BDNF | Forward : GAG CGT GTG TGA CAG TAT TAG CG Reverse : CGA TTG GGT AGT TCG GCA TT |
| GDNF | Forward : GTC TGG CAG CCA ACA AAC AG Reverse : TAG CAG CCA CAA AGG GAG TG |
| CNTF | Forward : GCA GAG CGA CTC CAA GAG AAC CT Reverse : GCT GGT AGG CGA AGG CAG AAA |
| GAPDH | Forward : ACC ACA GTC CAT GCC ATC AC Reverse : TCC ACC ACC CTG TTG CTG TA |
Statistical analysis
Data are presented as the mean ± SEM of 3 independent experiments, each carried out in triplicate. Comparisons were analyzed by using unpaired, two-tailed, t-tests. P < 0.05 was considered to be statistically significant. The graphs of the original data were produced using GraphPad Prism software version 6.00 for Windows GraphPad, USA).
Results
NSCs express VDR, which can, in turn, be upregulated by 1,25(OH)2D3
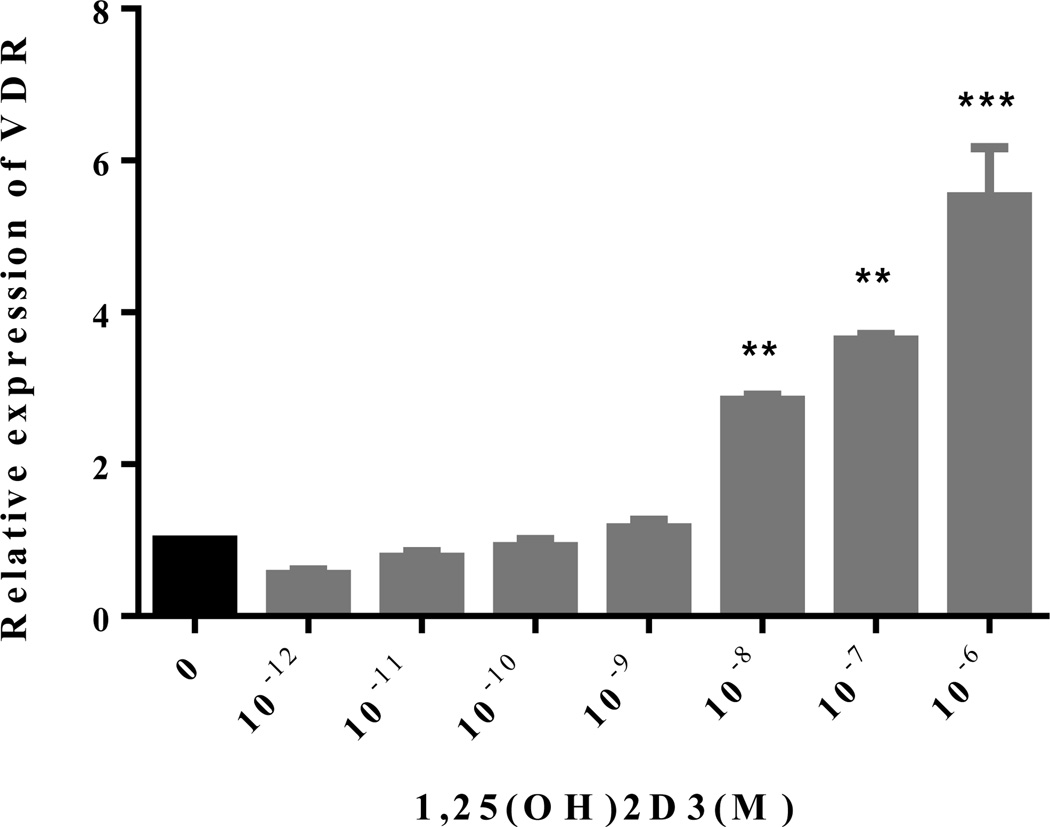
To study the direct effect of 1,25(OH)2D3 on NSCs, we first determined whether VDR is expressed on these cells, and whether the vitamin has, in turn, a regulatory effect on this receptor. To this end, NSCs in the presence or absence of 1,25(OH)2D3 (10−12–10−6 M) were cultured for 5 days, and VDR expression was determined with quantitative RT-PCR. As shown in Figure 1, VDR is constitutively expressed on NSCs and adding 1,25(OH)2D3 upregulated its expression in a concentration-dependent manner. These results provided a basis for the direct effect of the vitamin on NSCs, and indicated a positive feedback loop whereby 1,25(OH)2D3 upregulates expression of its receptor in NSCs (Figure 1).
Figure 1. 1,25(OH)2D3 upregulates VDR expression in NSCs.
NSCs at the 5th passage were cultured in the presence or absence of 1,25(OH)2D3 (10−12–10−6 M) for 5 days, and VDR mRNA expression was determined with quantitative RT-PCR. Experiments were performed in triplicate using VDR and GAPDH primers. Data represent the mean ± SEM of triplicate samples analyzed. **P < 0.01, and ***P < 0.001, compared with cultures without 1,25(OH)2D3. One representative of three experiments is shown.
1,25(OH)2D3 enhanced NSC proliferation
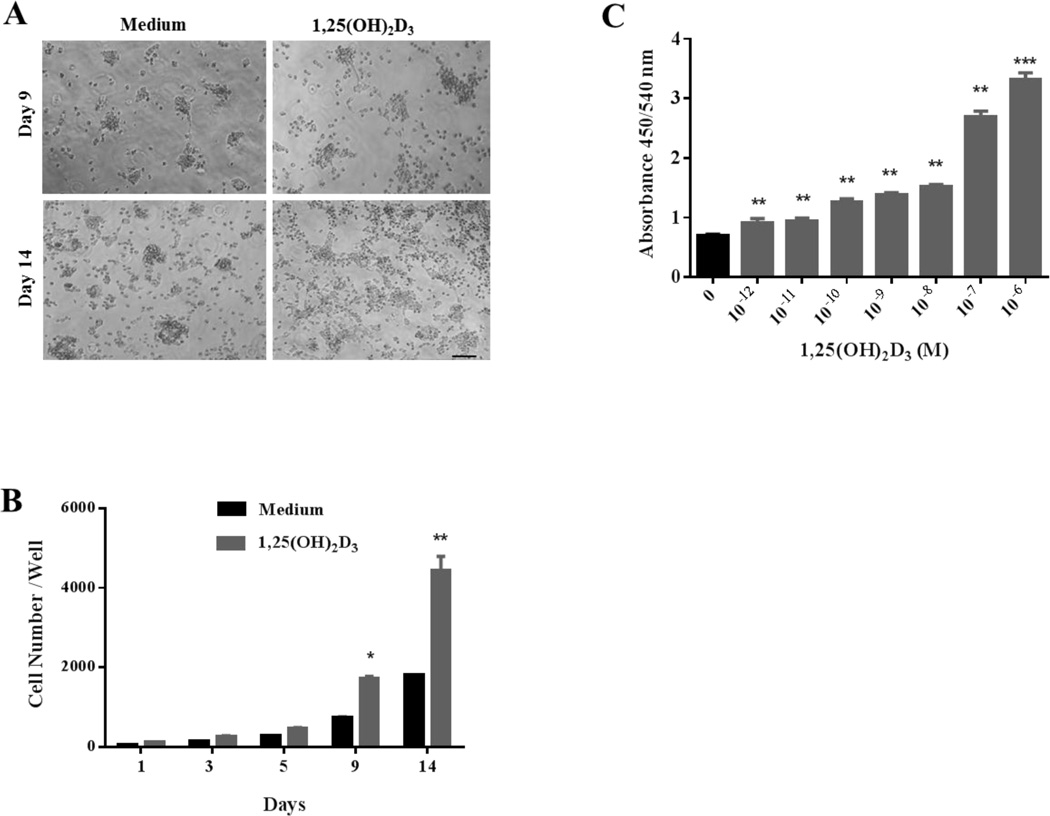
We then determined the effect of 1,25(OH)2D3 on NSC proliferation. Cells were cultured in stem cell proliferation medium, with different concentrations of 1,25(OH)2D3. Cell numbers were counted by hemocytometer at days 1, 3, 5, 9 and 14 of culture. As shown in Figure 2A, NSCs treated with 10−6 M of 1,25(OH)2D3 exhibited more neurosphere formation and larger cell size in comparison to those cultured with medium only. The absolute numbers of NSCs cultured with 1,25(OH)2D3 at days 9 and 14 were significantly increased (Figure 2B).
Figure 2. 1,25(OH)2D3 enhances NSC proliferation.
NSCs at the 5th passage were cultured in the presence or absence of 1,25(OH)2D3. (A) Phase-contrast images of NSCs were taken at days 9 and 14 after starting culture with 1,25(OH)2D3 at 10−6 M (scale bar = 100 µm). (B) At days 1, 3, 5, 9, and 14 after starting culture with 1,25(OH)2D3 at 10−6 M, neurospheres in each well were dissociated into single cells and cell numbers per well were counted by hemocytometer. At least five wells were assessed at each time point. (C) Analysis of NSC growth by BrdU incorporation assay. At day 14 of culture in the presence or absence of 1,25(OH)2D3(10−12–10−6 M), culture medium was supplemented with BrdU for 4–6 hrs and incorporation of BrdU was analyzed by ELISA (absorbance wavelength). Data represent the mean ± SEM of triplicate samples. *P < 0.05, **P < 0.01, ***P < 0.001, comparison between 1,25(OH)2D3-treated and untreated cells. One representative of three experiments is shown.
The effect of 1,25(OH)2D3 on NSC growth was further measured with the amount of BrdU incorporation into newly synthesized DNA strands of actively proliferating NSCs. Consistent with the results shown in Figure 2A and B, NSC proliferation was enhanced in a concentration-dependent manner when cells were cultured with 1,25(OH)2D3 (Figure 2C). Together, these results indicate that 1,25(OH)2D3 has a potent effect on NSC proliferation.
1,25(OH)2D3 enhanced NSC differentiation into neurons and oligodendrocytes
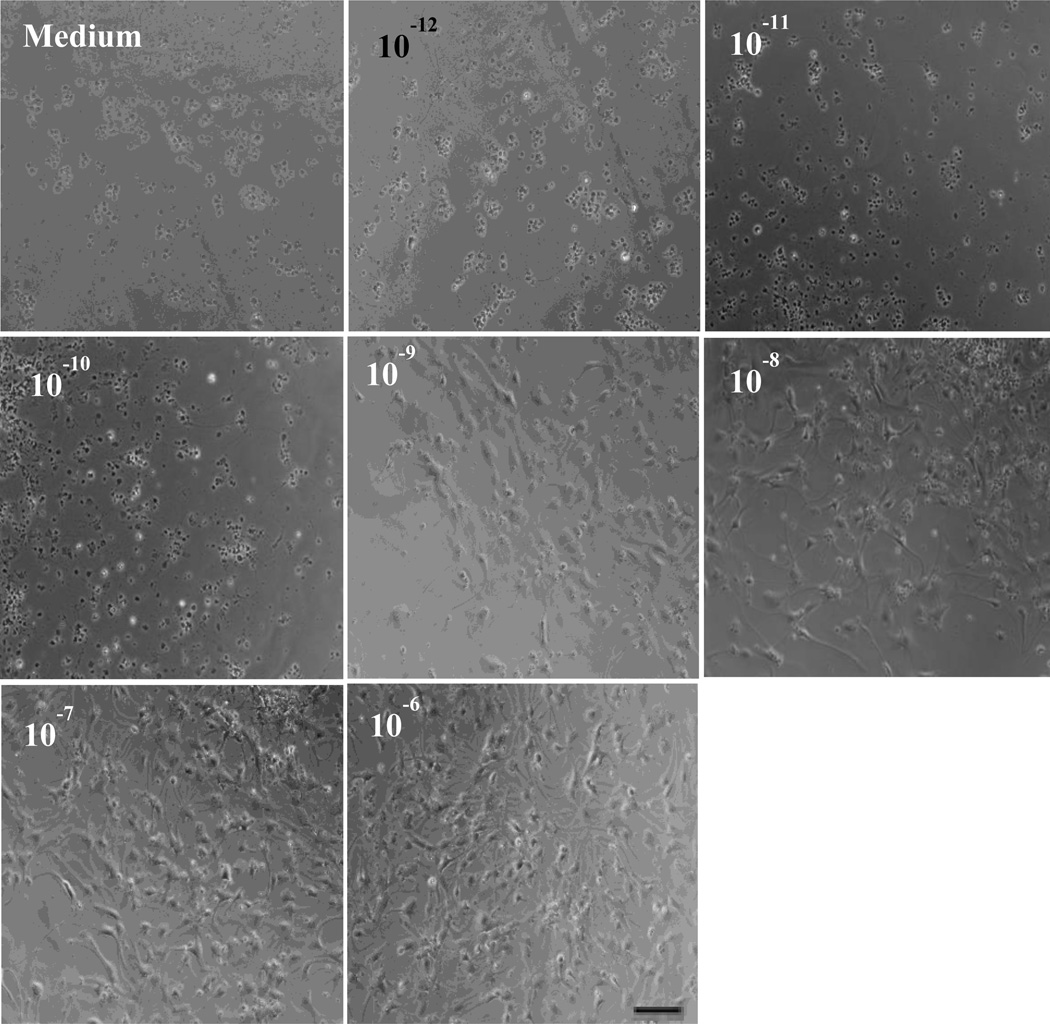
To study the possible effect of 1,25(OH)2D3 in NSC differentiation, NSC suspension was cultured in differentiation medium with or without adding 1,25(OH)2D3 at concentrations of 10−12–106 M. As shown in Figure 3, the morphology of NSCs was altered in 1,25(OH)2D3 concentration-dependent manner, i.e., cells with branches were more frequently observed in cultures with 10−6 M of 1,25(OH)2D3, indicating a more mature phenotype of neural cells.
Figure 3. 1,25(OH)2D3 drives NSCs to a more mature/differentiated morphology.
Dissociated single NSCs were cultured in differentiation medium in 24-well plates (1.25 × 105 cells /ml) in the presence or absence of various concentrations of 1,25(OH)2D3 (10−12–10−6 M) for 14 days, and cell morphology was monitored. Scale bars =20 µm. One representative of three experiments is shown.
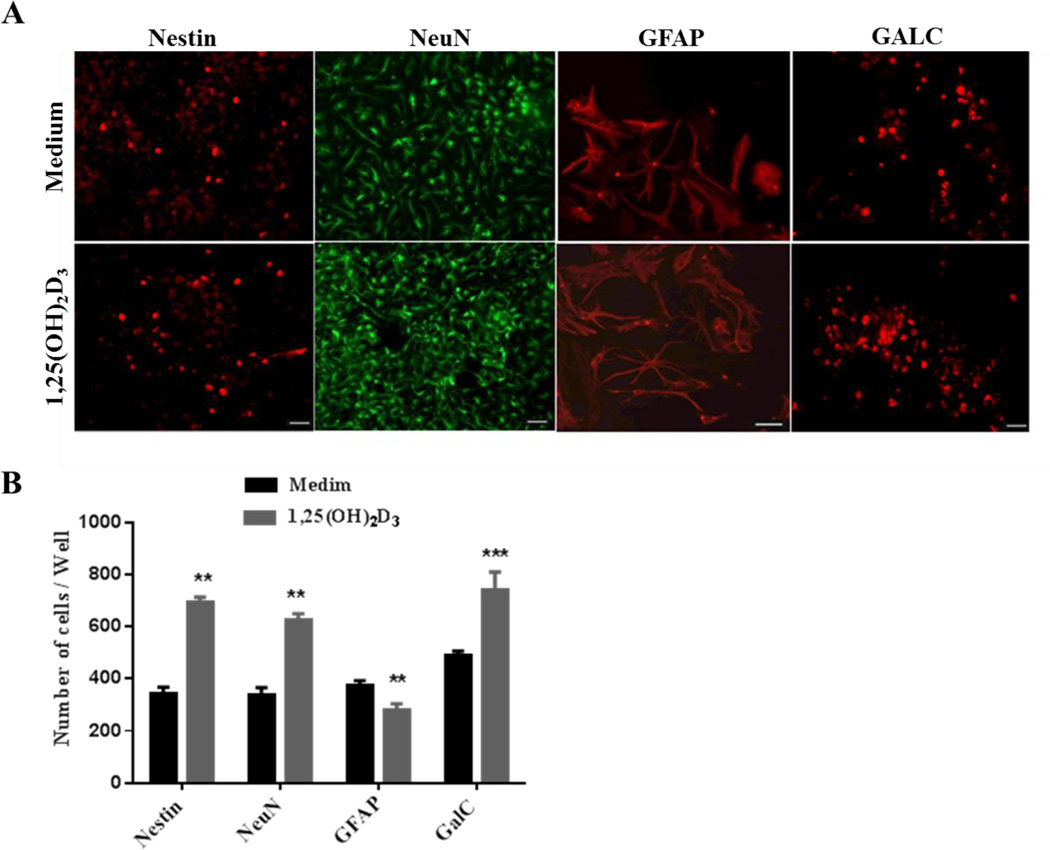
NSC differentiation into mature neural cells was further verified by immunostaining, using antibodies for neural cell lineage markers, including NeuN (neurons), GFAP (astrocytes), GalC (oligodendrocytes) and Nestin (undifferentiated NSCs). Our results showed that significantly higher numbers of 1,25(OH)2D3–treated NSCs differentiated into NeuN+ neurons, and GalC+ oligodendrocytes, while a smaller number of these NSCs differentiated into GFAP+ astrocytes. Furthermore, a higher number of NSCs cultured with only medium remained undifferentiated (nestin+) (Fig. 4A, B). These results are consistent with the morphological changes shown in Figure 3, and indicate a promoting effect of 1,25(OH)2D3 in neuron/oligodendrocyte differentiation of NSCs.
Figure 4. 1,25(OH)2D3 drives NSCs toward neuron/oligodendrocyte differentiation.
Dissociated single cells were cultured in differentiation medium in coated 24-well plates (1.25 × 105 cells/ml) for 14 days. (A) Cells were stained with neural markers including nestin (undifferentiated NSCs), NeuN (neurons), GalC (oligodendrocytes) (scale bars = 20, 40 µm) and GFAP (astrocytes; scale bars = 40 µm). One of three representative experiments is shown. C: Quantitative analysis of cells positive for different neural markers. Data represent the mean ± SEM of triplicate samples analyzed. **P < 0.01, ***P < 0.001, comparison between 1,25(OH)2D3-treated and untreated cells. One representative of three experiments is shown.
1,25(OH)2D3 upregulated expression of neurotrophic factors
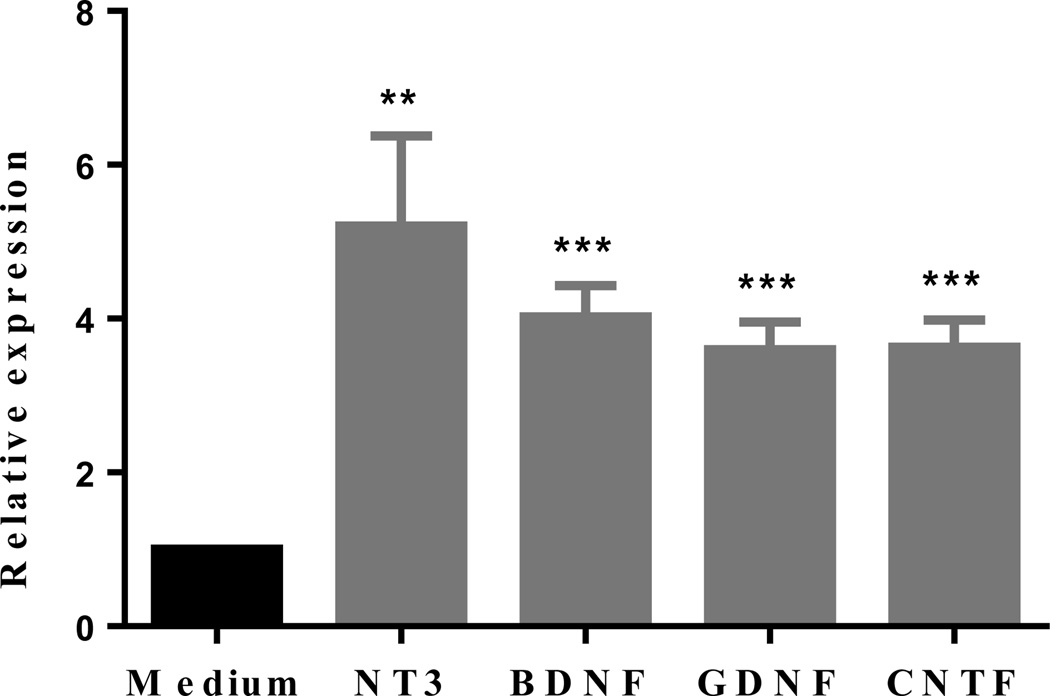
Given the important role of neurotrophic factors in the development and maintenance of nervous system, we have determined expression of neurotrophic factors by NSCs after culture with 1,25(OH)2D3. We have found that NSCs expressed significantly more NT-3, BDNF, CNTF and GDNF in the presence of 1,25(OH)2D3 (Figure 5). Thus, upregulation of these factors may provide a mechanistic explanation for the enhanced NSC proliferation and neuron/oligodendrocyte differentiation in the presence of 1,25(OH)2D3.
Figure 5. 1,25(OH)2D3 induces expression of neurotrophic factors of NSCs.
NSCs were cultured in the presence or absence of 1,25(OH)2D3 (10−6 M) for 5 days and mRNA expression of neurotrophic factors was determined by quantitative RT-PCR. The experiments were performed in triplicate, and the relative expression level of each neurotrophic factor was calculated by the ratio of values in wells with and without added 1,25(OH)2D3. Data represent the mean ± SEM of triplicate samples analyzed. **P < 0.01, ***P < 0.001, comparison between 1,25(OH)2D3-treated and untreated cells. One representative of three experiments is shown.
Discussion
In the present study we demonstrate that NSCs express VDR and that 1,25(OH)2D3 significantly upregulates this expression. Further, 1,25(OH)2D3 enhanced NSC proliferation and enhanced their differentiation into neurons and oligodendrocytes, with reduced astryogliosis. These results provide a novel mechanism for the therapeutic effect of 1,25(OH)2D3 in EAE.
The therapeutic effect of 1,25(OH)2D3 in EAE has been attributed to immunomodulation. For example, 1,25(OH)2D3 inhibited the IL-12/IFN-γ axis (Muthian, Raikwar et al. 2006) and Th17 cell differentiation (Zhang, Jin et al. 2009), suppressed inflammatory cell trafficking into the CNS, and induced apoptosis of these cells (Hayes 2000). It has recently been shown that 1,25(OH)2D3 induced IDO tolerogenic dendritic cells and enhanced Treg differentiation. However, whether this vitamin also directly affects remyelination of neural cells as a mechanism for EAE recovery is not known. Given that 1,25(OH)2D3 can pass through the blood-brain barrier, and that the VDR is present in the brain cells, 1,25(OH)2D3 may also exert a direct effect in the CNS (DeLuca, Kimball et al. 2013). Indeed, 1,25(OH)2D3 induced various neurotrophic factors that are involved in neuroprotection and immunomodulation (Correale, Ysrraelit et al. 2010). It has been shown that oligodendrocytes express VDR, and 1,25(OH)2D3 depletion leads to a slower rate of oligodendrocyte differentiation with an increased apoptosis, axonal injury and demyelination (Huang, Ferrari et al. 2012). These results indicate that 1,25(OH)2D3 maintains the equilibrium between oligodendroglial differentiation and axonal adhesion during normal brain development. In addition, 1,25(OH)2D3 plays a supportive role in neuronal differentiation and neuroprotection (Newmark and Newmark 2007). However, whether VDR is also expressed in NSCs is not known. In the present study we have shown that NSCs constitutively express this receptor, which can be, in turn, significantly upregulated upon 1,25(OH)2D3 treatment. The function of VDR on NSCs was further confirmed by our results showing an increase in NSC proliferation and differentiation after 1,25(OH)2D3 was added to the culture of these cells. Consistent with our findings, it has been recently shown that 1,25(OH)2D3 effectively blocked the toxic effect of L-DOPA on NSCs, and that this effect may be through promoting prosurvival signaling, including activation of the PI3K pathway, and reducing oxidative stress (Jang, Park et al. 2014). Taken together, these results indicate a direct effect of 1,25(OH)2D3 on NSCs.
NSCs are a unique population of cells that exhibit stem cell properties, including self-renewal, i.e., production of a large number of progeny, and multipotency, i.e., differentiation of the progeny into the three primary CNS phenotypes (Louis and Reynolds 2005). Because NSCs have the ability to support neurogenesis within restricted areas throughout adulthood and can undergo extensive in vitro expansion, they have been viewed as a renewable source of neural precursors for regenerative transplantation in various CNS diseases (Horner and Gage 2000, Teng, Lavik et al. 2002). Increasing evidence suggests that NSCs go through a process of self-renewal, proliferating and differentiating into the appropriate lineage when inflammatory damage or injury occurs in the nervous system (Pluchino and Martino 2008). In the present study we demonstrate that 1,25(OH)2D3 promoted NSC proliferation, as shown by a dose-dependent increase in BrdU incorporation, providing evidence for the effect of 1,25(OH)2D3 in the NSC growth.
The differentiation capacity of NSCs is one of the main reasons that they are being considered as a potential therapeutic tool in nervous system disorders. When inflammatory damage or injury occurs in the nervous system, NSCs proliferate, migrate into the disease foci, and differentiate into the appropriate cell lineage for neurorepair (Nait-Oumesmar, Picard-Riera et al. 2007). Our study shows that 1,25(OH)2D3 treatment drove NSCs to differentiate into a greater number of oligodendrocyte and neurons, thus promoting their capacity to support remyelination and axonal growth. The reduced astrocyte differentiation of 1,25(OH)2D3-treated NSCs would be beneficial in reducing astrogliosis, a main cause of the formation of MS plaques (Cristofanilli, Rosenthal et al. 2014). The mechanism underlying 1,25(OH)2D3-induced neural cell differentiation is not clear, but it is possibly that induction of neurotrophins plays an important role. Indeed, it has been found that 1,25(OH)2D3 upregulated neural cell expression of NT-3 and nerve growth factor (NGF) (Brown, Bianco et al. 2003) as well as GDNF (Naveilhan, Neveu et al. 1996), all of which promote neuron and oligodendrocyte precursor proliferation, survival, and differentiation (Numakawa 2014, Yang, Yan et al. 2014). In the present study we show that 1,25(OH)2D3 induced NSC expression of CTNF, which is primarily expressed in glial cells within the central and peripheral nervous systems and plays an important role in neural cell survival and neuron/oligodendrocyte differentiation (Selvaraj and Sendtner 2013). Increase in BDNF by 1,25(OH)2D3 can enhance its effects upon the oligodendroglia lineage, including proliferation, differentiation, maturation and myelination (Junhua 2012). Thus, upregulation of neurotrophic factors may be a mechanism underlying the effect of 1,25(OH)2D3 on NSC differentiation, survival and neuron/oligodendrocyte differentiation.
In summary, our study demonstrates that, in addition to its known role in the immune system functioning, 1,25(OH)2D3 directly promotes proliferation and neuron/oligodendrocyte differentiation of NSCs, thus representing a novel mechanism underlying its beneficial effects in MS/EAE therapy. These results, together with the capacity of 1,25(OH)2D3 to pass through the blood brain barrier (DeLuca, Kimball et al. 2013), indicate a direct effect of 1,25(OH)2D3 on NSC development and differentiation in vivo and, consequently, neuroregeneration and repair.
Acknowledgments
This study was supported by the National Institutes of Health (NIH) and the Multiple Sclerosis International Federation (MSIF). We thank Katherine Regan for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Burden of illness of multiple sclerosis: Part II: Quality of life. The Canadian Burden of Illness Study Group. Can J Neurol Sci. 1998;25(1):31–38. [PubMed] [Google Scholar]
- Brown J, Bianco JI, McGrath JJ, Eyles DW. 1,25-dihydroxyvitamin D3 induces nerve growth factor, promotes neurite outgrowth and inhibits mitosis in embryonic rat hippocampal neurons. Neurosci Lett. 2003;343(2):139–143. doi: 10.1016/s0304-3940(03)00303-3. [DOI] [PubMed] [Google Scholar]
- Constantinescu CS, Farooqi N, O'Brien K, Gran B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS) Br J Pharmacol. 2011;164(4):1079–1106. doi: 10.1111/j.1476-5381.2011.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correale J, Ysrraelit MC, Gaitan MI. Immunomodulatory effects of Vitamin D in multiple sclerosis. Brain. 2009;132(Pt 5):1146–1160. doi: 10.1093/brain/awp033. [DOI] [PubMed] [Google Scholar]
- Correale J, Ysrraelit MC, Gaitan MI. Gender differences in 1,25 dihydroxyvitamin D3 immunomodulatory effects in multiple sclerosis patients and healthy subjects. J Immunol. 2010;185(8):4948–4958. doi: 10.4049/jimmunol.1000588. [DOI] [PubMed] [Google Scholar]
- Cristofanilli M, Rosenthal H, Cymring B, Gratch D, Pagano B, Xie B, Sadiq SA. Progressive multiple sclerosis cerebrospinal fluid induces inflammatory demyelination, axonal loss, and astrogliosis in mice. Exp Neurol. 2014;261:620–632. doi: 10.1016/j.expneurol.2014.07.020. [DOI] [PubMed] [Google Scholar]
- DeLuca GC, Kimball SM, Kolasinski J, Ramagopalan SV, Ebers GC. Review: the role of vitamin D in nervous system health and disease. Neuropathol Appl Neurobiol. 2013;39(5):458–484. doi: 10.1111/nan.12020. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97(6):703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9(11):839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13(3):100–105. doi: 10.1016/s1043-2760(01)00547-1. [DOI] [PubMed] [Google Scholar]
- Haines JL, Terwedow HA, Burgess K, Pericak-Vance MA, Rimmler JB, Martin ER, Oksenberg JR, Lincoln R, Zhang DY, Banatao DR, Gatto N, Goodkin DE, Hauser SL. Linkage of the MHC to familial multiple sclerosis suggests genetic heterogeneity. The Multiple Sclerosis Genetics Group. Hum Mol Genet. 1998;7(8):1229–1234. doi: 10.1093/hmg/7.8.1229. [DOI] [PubMed] [Google Scholar]
- Hayes CE. Vitamin D: a natural inhibitor of multiple sclerosis. Proc Nutr Soc. 2000;59(4):531–535. doi: 10.1017/s0029665100000768. [DOI] [PubMed] [Google Scholar]
- Horner PJ, Gage FH. Regenerating the damaged central nervous system. Nature. 2000;407(6807):963–970. doi: 10.1038/35039559. [DOI] [PubMed] [Google Scholar]
- Huang JK, Ferrari CC, Monteiro de Castro G, Lafont D, Zhao C, Zaratin P, Pouly S, Greco B, Franklin RJ. Accelerated axonal loss following acute CNS demyelination in mice lacking protein tyrosine phosphatase receptor type Z. Am J Pathol. 2012;181(5):1518–1523. doi: 10.1016/j.ajpath.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang W, Park HH, Lee KY, Lee YJ, Kim HT, Koh SH. 1,25-dyhydroxyvitamin D Attenuates L-DOPA-Induced Neurotoxicity in Neural Stem Cells. Mol Neurobiol. 2014 doi: 10.1007/s12035-014-8835-1. [DOI] [PubMed] [Google Scholar]
- Junhua X. Neuroprotection on Multiple Sclerosis: A BDNF Perspective. Journal of Neurology & Neurophysiology. 2012 [Google Scholar]
- Kovacs GG, Wagner U, Dumont B, Pikkarainen M, Osman AA, Streichenberger N, Leisser I, Verchere J, Baron T, Alafuzoff I, Budka H, Perret-Liaudet A, Lachmann I. An antibody with high reactivity for disease-associated alpha-synuclein reveals extensive brain pathology. Acta Neuropathol. 2012;124(1):37–50. doi: 10.1007/s00401-012-0964-x. [DOI] [PubMed] [Google Scholar]
- Lemire JM, Archer DC. 1,25-dihydroxyvitamin D3 prevents the in vivo induction of murine experimental autoimmune encephalomyelitis. J Clin Invest. 1991;87(3):1103–1107. doi: 10.1172/JCI115072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Li K, Zhu L, Kan Q, Yan Y, Kumar P, Xu H, Rostami A, Zhang GX. Inhibitory effect of IL-17 on neural stem cell proliferation and neural cell differentiation. BMC Immunol. 2013;14:20. doi: 10.1186/1471-2172-14-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis SA, Reynolds BA. Generation and differentiation of neurospheres from murine embryonic day 14 central nervous system tissue. Methods Mol Biol. 2005;290:265–280. doi: 10.1385/1-59259-838-2:265. [DOI] [PubMed] [Google Scholar]
- Margaret HC, Nancy S K, Mitchell K R. Neuroprotective effects of vitamin D in multiple sclerosis. Neuroscience & Medicine. 2011 2011. [Google Scholar]
- Martino G, Pluchino S. Neural stem cells: guardians of the brain. Nat Cell Biol. 2007;9(9):1031–1034. doi: 10.1038/ncb0907-1031. [DOI] [PubMed] [Google Scholar]
- McFarlin DE, McFarland HF. Multiple sclerosis (first of two parts) N Engl J Med. 1982;307(19):1183–1188. doi: 10.1056/NEJM198211043071905. [DOI] [PubMed] [Google Scholar]
- Munger KL, Zhang SM, O'Reilly E, Hernan MA, Olek MJ, Willett WC, Ascherio A. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62(1):60–65. doi: 10.1212/01.wnl.0000101723.79681.38. [DOI] [PubMed] [Google Scholar]
- Muthian G, Raikwar HP, Rajasingh J, Bright JJ. 1,25 Dihydroxyvitamin-D3 modulates JAK-STAT pathway in IL-12/IFNgamma axis leading to Th1 response in experimental allergic encephalomyelitis. J Neurosci Res. 2006;83(7):1299–1309. doi: 10.1002/jnr.20826. [DOI] [PubMed] [Google Scholar]
- Nait-Oumesmar B, Picard-Riera N, Kerninon C, Decker L, Seilhean D, Hoglinger GU, Hirsch EC, Reynolds R, Baron-Van Evercooren A. Activation of the subventricular zone in multiple sclerosis: evidence for early glial progenitors. Proc Natl Acad Sci U S A. 2007;104(11):4694–4699. doi: 10.1073/pnas.0606835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveilhan P, Neveu I, Wion D, Brachet P. 1,25-Dihydroxyvitamin D3, an inducer of glial cell line-derived neurotrophic factor. Neuroreport. 1996;7(13):2171–2175. doi: 10.1097/00001756-199609020-00023. [DOI] [PubMed] [Google Scholar]
- Newmark HL, Newmark J. Vitamin D and Parkinson's disease--a hypothesis. Mov Disord. 2007;22(4):461–468. doi: 10.1002/mds.21317. [DOI] [PubMed] [Google Scholar]
- Numakawa T. Possible protective action of neurotrophic factors and natural compounds against common neurodegenerative diseases. Neural Regen Res. 2014;9(16):1506–1508. doi: 10.4103/1673-5374.139474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluchino S, Martino G. The therapeutic plasticity of neural stem/precursor cells in multiple sclerosis. J Neurol Sci. 2008;265(1–2):105–110. doi: 10.1016/j.jns.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, Galli R, Del Carro U, Amadio S, Bergami A, Furlan R, Comi G, Vescovi AL, Martino G. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422(6933):688–694. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- Selvaraj BT, Sendtner M. CNTF, STAT3 and new therapies for axonal degeneration: what are they and what can they do? Expert Rev Neurother. 2013;13(3):239–241. doi: 10.1586/ern.13.9. [DOI] [PubMed] [Google Scholar]
- Spach KM, Nashold FE, Dittel BN, Hayes CE. IL-10 signaling is essential for 1,25-dihydroxyvitamin D3-mediated inhibition of experimental autoimmune encephalomyelitis. J Immunol. 2006;177(9):6030–6037. doi: 10.4049/jimmunol.177.9.6030. [DOI] [PubMed] [Google Scholar]
- Teng YD, Lavik EB, Qu X, Park KI, Ourednik J, Zurakowski D, Langer R, Snyder EY. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Natl Acad Sci U S A. 2002;99(5):3024–3029. doi: 10.1073/pnas.052678899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Mei IA, Ponsonby AL, Dwyer T, Blizzard L, Simmons R, Taylor BV, Butzkueven H, Kilpatrick T. Past exposure to sun, skin phenotype, and risk of multiple sclerosis: case-control study. BMJ. 2003;327(7410):316. doi: 10.1136/bmj.327.7410.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HC, Xing S, Shan L, O'Connell K, Dinoso J, Shen A, Zhou Y, Shrum CK, Han Y, Liu JO, Zhang H, Margolick JB, Siliciano RF. Small-molecule screening using a human primary cell model of HIV latency identifies compounds that reverse latency without cellular activation. J Clin Invest. 2009;119(11):3473–3486. doi: 10.1172/JCI39199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Yan Y, Xia Y, Kang T, Li X, Ciric B, Xu H, Rostami A, Zhang GX. Neurotrophin 3 transduction augments remyelinating and immunomodulatory capacity of neural stem cells. Mol Ther. 2014;22(2):440–450. doi: 10.1038/mt.2013.241. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang X, Jin J, Tang Y, Speer D, Sujkowska D, Markovic-Plese S. IFN-beta1a inhibits the secretion of Th17-polarizing cytokines in human dendritic cells via TLR7 up-regulation. J Immunol. 2009;182(6):3928–3936. doi: 10.4049/jimmunol.0802226. [DOI] [PubMed] [Google Scholar]