Abstract
Introduction
About 10% of tumors derived from nongynecologic, noncoelomic tissues react with the OC125 antibody. Some patients with advanced prostate cancer were found to have elevated serum CA-125 level.
Materials and Methods
We examined the clinical history of 11 patients with castration-resistant prostate cancer and an elevated serum CA-125 level. Pathological review and immunohistochemical staining were performed on tumors from 8 of these patients.
Results
Patients with advanced prostate cancer and an elevated serum CA-125 level responded to androgen ablative therapy (median duration, 27 months). They were predisposed to develop persistent or recurrent urinary symptoms and visceral metastases. Eight of 11 patients had a low or undetectable serum prostate-specific antigen level (≤4 ng/ml) or an elevated serum carcinoembryonic antigen level (>6 ng/ml). In 3 of 7 patients whose specimens were available for further review, the tumors contained histologic features compatible with a diagnosis of ductal or endometrioid adenocarcinoma of the prostate.
Conclusions
Patients with prostate cancer and an elevated serum CA-125 level have unique clinical and pathologic characteristics. Some of these patients possess tumors compatible with a subtype of prostate cancer known as ductal adenocarcinoma. Additional studies need to be performed to elucidate the biologic basis of the various subtypes of prostate cancer.
Keywords: prostate cancer, CA-125, ductal adenocarcinoma
INTRODUCTION
It seems paradoxical that a masculine disease like prostate cancer may be associated with a feminine biomarker like CA-125. However, another male-exclusive malignancy, namely seminal vesicle carcinoma, has also been found to produce CA-125.1 In fact, about 28% of patients with nongynecologic tumors have elevated serum CA-125 levels and 10% of tumors derived from nongynecologic, noncoelomic tissues react with the OC125 antibody.2 To our knowledge, there is only one other report that described CA-125 expression in prostate cancer.3 Since prostate cancer normally metastasize to the pelvic-retroperitoneal lymph nodes and to the bones rather than to the coelomic structures, such as the pleura or peritoneum, it is presumed that the cancer cells themselves produce CA-125.
This study was prompted by a serendipitous observation that serum CA-125 level was elevated in some patients with castration-resistant prostate cancer. Further investigation revealed that several of these patients contained tumors with pathological features consistent with a diagnosis of ductal or endometrioid adenocarcinoma of the prostate.4–12 These patients had unique clinical presentations such as intractable urinary symptoms and atypical visceral metastases after hormonal ablative therapy. Since not all ductal adenocarcinomas produce an elevated CA-125 level, we postulate that there may be different subtypes of prostate ductal adenocarcinoma. A distinct subtype of ductal adenocarcinoma may be associated with increased serum CA-125 level. We report the clinical characteristics and pathological findings of 11 patients with advanced prostate carcinoma and an elevated serum CA-125 level (>35 ng/ml).
MATERIALS AND METHODS
Clinical Data
Between December 1, 1998 and April 1, 1999, we measured at least one serum CA-125 level in 55 non-consecutive patients with castration-resistant prostate cancer. These patients were either referred for evaluation of progression of disease or followed for evidence of progression of disease (i.e., increasing serum PSA, or worsening clinical symptoms) by one of the authors (ST) in the Genitourinary Medical Oncology Clinic at The University of Texas M. D. Anderson Cancer Center. Eleven patients were found to have an elevated serum Ca-125 level (>35 ng/ml) and were selected for further studies (Table 1). Patients with evidence of pleural, pericardial, or peritoneal metastases were excluded. The clinical history, cystoscopic findings, laboratory results, and treatment effects were obtained from patient charts and from the computer data management system of the M. D. Anderson Cancer Center. Survival of patients was measured from the time of diagnosis and androgen ablative therapy until death from any cause or last follow-up visit.
TABLE 1.
Prostate cancer and serum Ca-125 levels
| Patients* | Dates | Ca-125 (0–35 U/ml) | PSA (<4 ng/ml) | CEA (0–6 ng/ml) |
|---|---|---|---|---|
| #1 | 1/12/99 | 56.7 | 0.4 | 24.0 |
| #2 | 1/27/99 2/25/99 |
65.1 78.1 |
229 | 2.6 |
| #3 | 12/2/98 1/6/99 |
43.6 56.7 |
10.9 | 19.4 |
| #4 | 1/12/99 | 137 | 1727 | 2.8 |
| #5 | 1/13/99 | 105 | 3.9 | <1.0 |
| #6 | 2/16/99 | 69.6 | 46.3 | 145 |
| #7 | 2/9/99 | 384 | 0.2 | 10.2 |
| #8 | 2/25/99 | 334 | <0.1 | 2164 |
| #9 | 3/3/99 | 168 | 4.0 | 4.0 |
| #10 | 3/24/99 | 109 | 1169 | 21.4 |
| #11 | 3/19/99 | 51.6 | 293 | 1.4 |
Out of 55 patients checked during the period of 12/1/98 through 4/1/99
Tissue Analysis
Eight tissue blocks from 7 of the 11 patients were available for the study (Table 2). Two specimens were obtained from a transurethral resection of the bladder, 3 from biopsy of the prostate, 2 from biopsy of recurrent tumor at the prostate anastomotic site, and one from a cystoprostatectomy. Some specimens were procured from the same patients before and after androgen ablative therapy. Six samples of fine-needle biopsies of metastases to the liver, lung, adrenal, and pancreas from 5 of the 11 patients were also available for examination. We performed immunohistochemical studies (prostate-specific antigen [PSA], CA-125, and carcinoembryonic antigen [CEA]) on formalin-fixed, paraffin-embedded sections (4–5 μm thick) from each specimen. For each antibody, known tissue-positive controls and tumor sections were stained simultaneously.
TABLE 2.
Pathological and immunohistochemical characteristics
| Patients | Source of tissue | Gleason score | Pathology | CA-125 | PSA | CEA |
|---|---|---|---|---|---|---|
| #1 | Prostate biopsy (12/96) Prostate TURP (1/97) |
9 9 |
Ductal cribriform Ductal cribriform |
− − |
+ focal + |
NA + |
| #2 | Prostate TURP (6/90) Lymph node (8/90) Lung FNA (6/98) |
9 | Ductal papillary/cribriform Ductal papillary/cribriform |
− − NA |
+ + + |
++ focal + − |
| #3 | Prostate biopsy (4/89) Liver-FNA (4/96) |
6 | Acinar | NA NA |
NA + |
NA + |
| #4 | Anastomosis (7/95) Liver-FNA (7/98) |
8 | Poorly differentiated carcinoma | − − |
+ NA |
Focal + NA |
| #7 | Ureter biopsy (1/95) Bladder biopsy (2/98) |
8 | Ductal endometrioid | − ++ |
NA NA |
NA NA |
| #8 | Prostatectomy (7/85) Anastomosis (10/97) Liver-FNA (5/98) |
9 10 |
Poorly differentiated carcinoma Poorly differentiated carcinoma |
NA NA focal + |
NA NA NA |
NA NA NA |
| #9 | Adrenal-FNA (12/98) Pancreas-FNA (3/99) |
NA | NA focal + |
NA + |
NA NA |
Immunostaining
Commercially available antibody against OC 125 (Dako) (diluted 1:50 in 5% newborn calf serum in phosphate-buffered saline (NCS/PBS) was used to identify tumor cells highly expressive of the CA-125 antigen. Antibody against PSA (Biogenex, San Ramon CA) (dilution 1:100 in 5% NCS/PBS) and CEA (Dako, Carpinteria CA) (diluted 1:200 in 5% NCS/PBS) were used to evaluate PSA and CEA expression in the tumor specimens, respectively. The antibody 34βE12 (Dako) (diluted 1:50 in 5% NCS/PBS) was used as a marker for basal cells and to differentiate high-grade prostatic intraepithelial neoplasia from invasive carcinoma. All sections were deparaffinized and rehydrated. For immunostaining, sections were first washed in PBS, followed by quenching of endogenous peroxidases in the tissue by submerging the sections in 0.3% hydrogen peroxide in methanol. The antigens were then retrieved from all sections by consecutive microwave heatings of sections immersed in buffered citric acid, pH 6.0 (Citra Solution; Biogenex).
The streptavidin-biotin (ABC) method was used to detect each antibody probe. Materials from Vectastain immunodetection kits against either mouse or rabbit immunoglobulins were used for staining. Normal horse or goat serum was applied to the sections as a blocking agent and then removed before the appropriate primary antibody was applied. The sections were incubated in the primary antibody solution, then in a solution of biotinylated secondary antibodies against mouse or rabbit immunoglobulins, followed by a Vectastain avidin-peroxidase fusion protein solution. The sections were subsequently developed for stain formation in hydrogen peroxide-diaminobenzidine solution. Immunostaining was scored according to the intensity of staining (1+ weak or 2+ strong) and the percentage of positively stained tumor cells (focal or diffuse).
RESULTS
Clinical Studies
Eleven out of 55 patients (20%) were found to have at least one elevated serum Ca-125 level (>35 ng/ml) during the 4 months period. The clinical characteristics of the 11 patients who had an elevated serum Ca-125 level were described in Table 3. The median age was 64 years. The median survivals since diagnosis and androgen deprivation therapy were 113 and 51 months, respectively. The duration of effective androgen deprivation therapy for these patients was 27 months. Six of 8 patients who received cytotoxic chemotherapies responded to treatment based on palliative benefit, PSA or CEA decline, and/or radiographic improvement. Eight of 11 patients had a low or undetectable serum PSA level (≤4 ng/ml) or an elevated serum CEA level (>6 ng/ml). Eight of 11 patients developed liver or lung metastases. In addition, 3 patients developed bladder invasion, 2 patients had adrenal metastases, and one patient each had metastases to the brain, pancreas, and penis. Seven patients developed bone metastases, including one whose bone metastases were predominantly osteolytic and another whose only site of osseous metastasis was in the base of skull. Interestingly, the remaining four patients did not develop skeletal metastases.
TABLE 3.
Clinical characteristics
| Patients | Age | Duration of response to hormonal Rx (months) | Survival after hormonal Rx (months) | Survival since diagnosis (months) | Sites of metastasis | Response to chemotherapy (date) |
|---|---|---|---|---|---|---|
| #1 | 72 | 19 | 32 | 55 | Brain, liver, lungs, lytic bone, penis | No response to 5-fluorouracil, paclitaxel, doxorubicin, or cyclophosphamide |
| #2 | 61 | 38 | 62 | 120 | Lungs | No treatment |
| #3 | 62 | 53 | 94 | 127 | Base of skull, liver | Ketoconazole/doxorubicin/estramustine/vinblastine (12/98); no response to mitoxantrone, docetaxel |
| #4 | 62 | 11 | 40 | 84 | Bladder, bone, liver, LN | Ketoconazole/doxorubicin (7/98); no response to cyclophosphamide |
| #5 | 77 | 14 | 83 | 84 | Bone | Ketoconazole/doxorubicin/strontium-89 (9/95) |
| #6 | 52 | 64 | 97 | 184 | Adrenal, liver, LN | Ketoconazole/doxorubicin (8/97); estramustine/paclitaxel/etoposide (2/98); cyclophosphamide/vincristine/dexamethasone (2/99); no response to mitoxantrone, estrmustine/vinblastine, docetaxel |
| #7 | 64 | 30 | 51 | 170 | Bladder, lungs, LN | No treatment |
| #8 | 51 | 37 | 60 | 170 | Bladder, bone, liver, LN, lungs | Ketoconazole/doxorubicin/estramustine/vinblastine (11/97); Cyclophosphamide/cisplatin/etoposide (5/98); Cyclophosphamide/doxorubicin/vincristine (3/99); no response to docetaxel, etoposide, carboplatin |
| #9 | 66 | 12 | 18 | 113 | Adrenal, bladder, pancreas | No treatment |
| #10 | 69 | 27 | 47 | 48 | Bone | No response to mitoxantrone, doxorubicin, paclitaxel, vinblastine, etoposide |
| #11 | 64 | 12 | 30 | 75 | Bone, lungs | Ketoconazole/mitoxantrone/Sr-89 (6/98) Did not benefit from cytoxan or taxotere |
Pathologic and immunohistochemical Studies
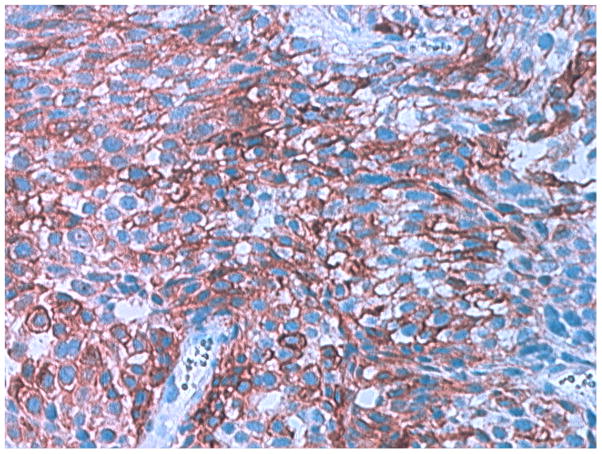
Eight specimens from 7 of the 11 patients were available for pathologic review (Table 2). Overall, 3 of 7 patients whose pathologic slides were available for review had a pathologic diagnosis consistent with ductal adenocarcinoma of the prostate. The tumors displayed a papillary and cribriform pattern. The papillary pattern showed papillary fronds with cells lining fibromuscular stalks. The tumor cells were pseudostratified with abundant cytoplasm and elongated nuclei. Prominent nucleoli were present. This papillary pattern also had areas of solid growth with numerous blood vessels. The cribriform areas were composed of back to back glands with classical cribriform architecture and the previously described cytology. Central comedo-necrosis was frequently associated with this pattern. Mitotic figures were easily identified. The Gleason score for 6 of the 7 specimens was 8 or greater. The Gleason score was not included in the report of 2 specimens. All analyzed specimens stained positive for PSA (Table 2). CA-125 was expressed in 1 tumor specimen (Figure 1) and in 1 of 5 cytological specimens. The two patients whose tumor or cytological specimens stained positively for CA-125 had higher serum CA-125 levels (>150 ng/ml).
Figure 1.
The immunohistochemical staining pattern of CA-125 expression in human prostate tumor from patient#7.
DISCUSSION
Results of this study suggest that CA-125 may be a useful biomarker to distinguish a specific subtype of prostate ductal adenocarcinoma. Ductal adenocarcinoma of the prostate appears to be a distinct pathologic entity with unique clinical and biologic features. Patients with prostate ductal adenocarcinoma and an elevated serum CA-125 level tend to pursue an indolent course in spite of harboring a high Gleason score tumor. They respond to androgen ablation and chemotherapy. They are predisposed to develop persistent local urinary symptoms and atypical visceral metastases after androgen ablative therapy. The finding that CA-125 is expressed in a particular subtype of prostate cancer may help to refine current diagnosis of prostate cancer. It supports the view that there is a unique origin for the different types of prostate cancer.13
OC 125 is a murine IgG1 monoclonal antibody raised against an ovarian carcinoma cell line derived from the ascites of a patient with serous cystadenocarcinoma.14 It recognizes an epitope on a molecule called Cancer Antigen 125 (CA-125). CA-125 is a differentiation antigen associated with the coelomic epithelium (i.e. mullerian epithelia, peritoneum, pleura, and pericardium) and almost all of its normal (i.e. ovary, fallopian tubes, endometrium, endocervix) and neoplastic derivatives (i.e. ovarian and endometrial carcinoma, mesothelioma). Sometimes, OC 125 reactivity is also noted in normal breast ducts, bronchiolar epithelium, and colonic mucosal cells adjacent to the tumors.2 In fact, CA-125 has been found expressed in the normal epithelium of the pancreas, colon, gall bladder, stomach, lung, and kidney.
Only 1% of apparently healthy individuals have elevated serum CA-125 levels. In contrast, 82% of patients with confined ovarian cancer and 28% of patients with nongynecological tumors have elevated serum CA-125 levels.2 Most, if not all, of the latter patients are presumed to have serosal metastases. About 10% of tumors derived from nongynecologic, noncoelomic tissues react with the OC125 antibody.2 In non-pregnant women, CA-125 is believed to arise from 3 possible sources: the ovary, the endometrium, and the peritoneum.15 In men, CA-125 could be produced from coelomic derivatives (e.g.. the peritoneum or the pleura) or a mullerian vestige. Since prostate cancer normally does not metastasize to the coelomic tissues, the prostate cancer cells that produce CA-125 may represent a mimicry of or is a derivative from a mullerian vestige.
Ductal adenocarcinoma of the prostate was first recognized as a variant of prostate carcinoma by Melicow in 1967.4 Pure ductal adenocarcinoma is rare and has an incidence of about 1% of all prostate carcinoma, whereas mixed ductal and acinar carcinoma comprises about 6% of all prostate carcinoma.5 Ductal adenocarcinoma has been described to be a more virulent form of prostate cancer by some reports7,10, but more indolent by others.11,12 We previously reported pure ductal prostate adenocarcinoma tends to pursue an indolent clinical course and poses an increased risk for local recurrence.16 Currently, it is unclear whether ductal adenocarcinoma is a distinct biological and clinical entity compared to acinar carcinoma of the prostate.17 It is also unknown whether the different histological types (i.e. endometrioid, primary ductal, and secondary ductal) and patterns (i.e. papillary, cribriform, and solid) of prostate ductal adenocarcinoma have any biological, clinical, or prognostic significance. Elucidation of the biology of prostate ductal adenocarcinoma will help to resolve some of these issues.
The diagnosis of prostate ductal adenocarcinoma can be elusive and problematic. Currently, ductal adenocarcinoma is diagnosed based on histological and immunohistochemical criteria. Ductal adenocarcinoma is characterized by the presence of tall pseudostratified epithelial cells arranged in a papillary pattern arising from the veramontanum or the primary ducts in the prostatic urethra. A cribriform pattern with slit-like lumens is found deeper within the secondary ducts. However, it is difficult to discern ductal adenocarcinoma with a solid pattern from a poorly differentiated carcinoma of the prostate.18 Furthermore, half of the ductal adenocarcinomas may stain only focally or weakly for PSA or PAP.19 It is likely that many poorly differentiated carcinomas with negative PSA or PAP staining will not be recognized or reported as ductal adenocarcinomas. The discovery of specific biomarkers will be invaluable for the diagnosis of prostate ductal adenocarcinoma.
There is controversy surrounding the biological origin of prostate ductal adenocarcinomas. The idea that certain ductal adenocarcinomas have a mullerian origin is based on the observation that they tend to arise in the middle lobe or the central zone of the prostate.20 It is hypothesized that the middle lobe has a distinct embryological origin compared to the rest of the prostate. Here, in the vicinity of the prostatic utricle, there is an intermingling of mesodermal cells derived from the mullerian-wolffian ducts with endodermal cells originated from the urogenital sinus.21 In contrast, acinar carcinoma of the prostate originates from the urogenital sinus. Perhaps, a CA-125 producing prostate cancer merely reflects its coelomic identity rather than its mullerian-wolffian origin. Unfortunately, the kinetics of Ca-125 during disease progression or treatment could not be assessed due to the retrospective nature of this study. And important questions about the underlying biology of its regulation by androgen receptor or other molecules, secretion of Ca-125 into the blood stream, remain unanswered. Nevertheless, CA-125 may be a useful biomarker that distinguishes ductal from acinar carcinomas of the prostate and alludes to the presence of a specific subtype of prostate ductal adenocarcinoma.
A closer examination of the clinical characteristics of the 11 patients in this study revealed some interesting observations. The patients responded adequately to androgen deprivation therapy (27 months). A majority also responded to some types of chemotherapy. They had a relatively prolonged survival of 57 months after androgen deprivation therapy and 113 months after diagnosis, considering that most of them (6 of 8) had high Gleason scores (8 – 10) at the time of diagnosis (3 patients) or during subsequent biopsies (3 patients). Eight patients had either a low or undetectable serum PSA or elevated CEA levels. There was an increased incidence of atypical visceral metastases in the liver, lungs, adrenals, pancreas, brain, and penis. Furthermore, a large proportion of the patients either did not develop bone metastases (4 patients) or had focal or late-onset osseous metastases (2 patients). The tumor tended to invade into the bladder causing gross hematuria and/or urinary obstructive symptoms requiring TURP and catherization (e.g. percutaneous nephrostomy, suprapubic). For this reason, several patients (6 of 11) underwent a salvage prostatectomy or cystoprostatectomy.
CONCLUSIONS
We described the pathologic features and clinical characteristics of patients with prostate cancer and elevated serum CA-125 level. The clinical characteristics indicated that these patients might pursue an indolent course in spite of an aggressive (i.e., high Gleason score) tumor. They responded to androgen ablation and chemotherapy. They appeared to be susceptible to persistent local urinary symptoms and atypical visceral metastases after androgen ablative therapy. The pathologic features of some of the tumors were compatible with a diagnosis of ductal or endometrioid adenocarcinoma of the prostate. Results of this study suggest that ductal adenocarcinoma is a distinct clinical and biologic entity. CA-125 may be a useful biomarker for refining the diagnosis of prostate cancer in general and of prostate ductal adenocarcinoma in particular. The results need to be confirmed in another database or in a prospective clinical trial.
Acknowledgments
The authors thank Cindy Soto, Gail Frazier, Ph.D., Bart Grossman, M.D., and Monica Liebert, Ph.D. for their help and advice in this work. They also thank Amy Gonzalez, R.N., and Rosaly General, R.N., M.A., for their excellent care of patients in the Genitourinary Medical Oncology Clinic. This study was supported in part by National Cancer Institute core grant CA16672.
References
- 1.Ohmori T, Okada K, Tabei R, et al. CA125-producing adenocarcinoma of the seminal vesicle. Pathol Int. 1994;44(4):333–337. doi: 10.1111/j.1440-1827.1994.tb03372.x. [DOI] [PubMed] [Google Scholar]
- 2.Kabawat SE, Bast RC, Jr, Bhan AK, et al. Tissue distribution of a coelomic-epithelium-related antigen recognized by the monoclonal antibody OC125. Int J Gynecol Pathol. 1983;2(3):275–285. doi: 10.1097/00004347-198303000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Torenbeek R, Lagendijk JH, Van Diest PJ, et al. Value of a panel of antibodies to identify the primary origin of adenocarcinomas presenting as bladder carcinoma. Histopathology. 1998;32(1):20–27. doi: 10.1046/j.1365-2559.1998.00328.x. [DOI] [PubMed] [Google Scholar]
- 4.Melicow MM, Pachter MR. Endometrial carcinoma of proxtatic utricle (uterus masculinus) Cancer. 1967;20(10):1715–1722. doi: 10.1002/1097-0142(196710)20:10<1715::aid-cncr2820201022>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 5.Dube VE, Farrow GM, Greene LF. Prostatic adenocarcinoma of ductal origin. Cancer. 1973;32(2):402–409. doi: 10.1002/1097-0142(197308)32:2<402::aid-cncr2820320218>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 6.Lemberger RJ, Bishop MC, Bates CP, et al. Carcinoma of the prostate of ductal origin. Br J Urol. 1984;56(6):706–709. doi: 10.1111/j.1464-410x.1984.tb06152.x. [DOI] [PubMed] [Google Scholar]
- 7.Bostwick DG, Kindrachuk RW, Rouse RV. Prostatic adenocarcinoma with endometrioid features. Clinical, pathologic, and ultrastructural findings. Am J Surg Pathol. 1985;9(8):595–609. doi: 10.1097/00000478-198508000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Epstein JI, Woodruff JM. Adenocarcinoma of the prostate with endometrioid features. A light microscopic and immunohistochemical study of ten cases. Cancer. 1986;57(1):111–119. doi: 10.1002/1097-0142(19860101)57:1<111::aid-cncr2820570123>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 9.Sufrin G, Gaeta J, Staubitz WJ, et al. Endometrial carcinoma of prostate. Urology. 1986;27(1):18–23. doi: 10.1016/0090-4295(86)90198-6. [DOI] [PubMed] [Google Scholar]
- 10.Ro JY, Ayala AG, Wishnow KI, et al. Prostatic duct adenocarcinoma with endometrioid features: immunohistochemical and electron microscopic study. Semin Diagn Pathol. 1988;5(3):301–311. [PubMed] [Google Scholar]
- 11.Lee SS. Endometrioid adenocarcinoma of the prostate: a clinicopathologic and immunohistochemical study. J Surg Oncol. 1994;55(4):235–238. doi: 10.1002/jso.2930550407. [DOI] [PubMed] [Google Scholar]
- 12.Millar EK, Sharma NK, Lessells AM. Ductal (endometrioid) adenocarcinoma of the prostate: a clinicopathological study of 16 cases. Histopathology. 1996;29(1):11–19. doi: 10.1046/j.1365-2559.1996.d01-483.x. [DOI] [PubMed] [Google Scholar]
- 13.Tu SM, Lin SH, Logothetis CJ. Stem-cell origin of metastasis and heterogeneity in solid tumours. Lancet Oncol. 2002;3(8):508–513. doi: 10.1016/s1470-2045(02)00820-3. [DOI] [PubMed] [Google Scholar]
- 14.Bast RC, Jr, Feeney M, Lazarus H, et al. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest. 1981;68(5):1331–1337. doi: 10.1172/JCI110380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bischof P. What do we know about the origin of CA 125? Eur J Obstet Gynecol Reprod Biol. 1993;49(1–2):93–98. doi: 10.1016/0028-2243(93)90131-u. [DOI] [PubMed] [Google Scholar]
- 16.Tu SM, Lopez A, Leibovici D, et al. Ductal adenocarcinoma of the prostate: clinical features and implications after local therapy. Cancer. 2009;115(13):2872–2880. doi: 10.1002/cncr.24326. [DOI] [PubMed] [Google Scholar]
- 17.Bock BJ, Bostwick DG. Does prostatic ductal adenocarcinoma exist? Am J Surg Pathol. 1999;23(7):781–785. doi: 10.1097/00000478-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Epstein J. Prostatic duct adenocarcinoma. Philadelphia, PA: Lippincott-Raven; 1995. pp. 169–177. [Google Scholar]
- 19.Epstein J. Transitional cell carcinoma. Philadelphia, PA: Lippincott-Raven; 1995. pp. 231–234. [Google Scholar]
- 20.Timms B. Anatomic perspectives of prostate development. Boca Raton, FL: CRC Press; 1997. pp. 29–31. [Google Scholar]
- 21.Glenister TW. The development of the utricle and of the so-called ‘middle’ or ‘median’ lobe of the human prostate. J Anat. 1962;96:443–455. [PMC free article] [PubMed] [Google Scholar]