Abstract
Thylakoid protein vitamin K epoxide reductase (AtVKOR/LTO1) is involved in oxidoreduction. The deficiency of this compound causes pleiotropic defects in Arabidopsis thaliana, such as severely stunted growth, smaller sized leaves, and delay of flowering. Transgenic complementation of wild-type AtVKOR (VKORWT) to vkor mutant lines ultimately demonstrates that the phenotype changes are due to this gene. However, whether AtVKOR functions in Arabidopsis through its protein oxidoreduction is unknown. To further study the redox-active sites of AtVKOR in vivo, a series of plasmids containing cysteine-mutant VKORs were constructed and transformed into vkor deficient lines. Compared with transgenic AtVKORWT plants, the size of the transgenic plants with a single conservative cysteine mutation (VKORC109A, VKORC116A, VKORC195A, and VKORC198A) were smaller, and two double-cysteine mutations (VKORC109AC116A and VKORC195AC198A) showed significantly stunted growth, similar with the vkor mutant line. However, mutations of two non-conservative cysteines (VKORC46A and VKORC230A) displayed little obvious changes in the phenotypes of Arabidopsis. Consistently, the maximum and actual efficiency of photosystem II (PSII) in double-cysteine mutation plants decreased significantly to the level similar to that of the vkor mutant line both under normal growth light and high light. A significantly decreased amount of D1 protein and increased accumulation of reactive oxygen species were observed in two double-cysteine mutations under high light. All of the results above indicated that the conservative cysteines in transmembrane domains were the functional sites of AtVKOR in Arabidopsis and that the oxidoreductase activities of AtVKOR were directly related to the autotrophic photosynthetic growth and PSII activity of Arabidopsis thaliana.
Keywords: AtVKOR, cysteine, disulfide bond, photosystem II, D1 protein
Introduction
In chloroplasts, disulfide bond formation, a covalent bond between two cysteines, is crucial for the maturation and function of proteins (Jocelyn, 1967; Buchanan and Luan, 2005; Onda, 2013). The majority of protein disulfides in chloroplasts are considered to be one important inert covalent linkage for structure stabilizing modifications (Nagahara, 2011; Romero et al., 2014). However, some regulatory disulfide bonds serve as signaling elements by interchanging their reduced or oxidized states, which plays important roles in photosynthesis, gene expression, signal transduction, and stress resistance (Buchanan and Luan, 2005; Wouters et al., 2010; Cremers and Jakob, 2013; Karamoko et al., 2013; Kieselbach, 2013). Intensive studies regarding the enzymatic reduction of disulfide bonds in redox-regulated proteins in chloroplasts have been conducted, while the reversible process, the formation of a disulfide bond, is yet to be elucidated (Aro and Ohad, 2003; Buchanan and Luan, 2005; Hall et al., 2010; Cremers and Jakob, 2013; Karamoko et al., 2013; Kieselbach, 2013).
A novel thylakoid protein, VKOR, from Cyanobacteria and Arabidopsis thaliana has been identified to promote disulfide bond formation (Furt et al., 2010; Li et al., 2010; Feng et al., 2011; Karamoko et al., 2011). Compared with Cyanobacteria VKOR, plant VKOR has an additional transit peptide at the N-terminus that targets the protein to chloroplasts (Furt et al., 2010; Feng et al., 2011; Wan et al., 2014). Different from VKOR in mammals, both thylakoid VKORs are fusion proteins comprising two domains, a transmembrane/VKOR domain and a soluble thioredoxin-like/Trx-like domain (Oldenburg et al., 2006; Li et al., 2010; Feng et al., 2011). In Arabidopsis, the transmembrane domain/VKOR domain of AtVKOR, which is homologous to VKORs from mammalian, Synechococcus sp. and Mycobacterium tuberculosis, contains two non-conservative cysteines (Cys46, Cys230) and four conservative cysteines forming two pairs (Feng et al., 2011). One pair is in a separated form (Cys109, Cys116), and the other pair is in a canonical Cys-X-X-Cys motif (Cys195, Cys198). Functional analyses reveal that these two pairs of conservative cysteines are indispensable for the oxidoreductase activities of AtVKOR in the process of catalyzing disulfide bond formation in Escherichia coli (Feng et al., 2011; Karamoko et al., 2011). In M. tuberculosis, two pairs of conservative cysteines of MtbVKOR are also found to play critical roles in the formation of disulfide bonds (Wang et al., 2011).
Arabidopsis VKOR is also called LTO1, and mutant lines of vkor (also called lto1) display severely deficient photosynthetic growth and low activities of PSII (Karamoko et al., 2011; Lu et al., 2013). Interestingly, the underlying mechanism of defects in the vkor mutant line has not been determined, although transgenic VKORWT to vkor mutant lines demonstrates that the phenotype changes are due to this gene (Karamoko et al., 2011; Lu et al., 2013). In vitro, the recombinant Trx-like domain of AtVKOR can promote the disulfide bond formation of targets in chloroplasts, such as proteins PsbO and FKBP13 (Karamoko et al., 2011; Lu et al., 2013). PsbO, a luminal subunit of PSII, carries a single intramolecular disulfide bond and is essential for the stability of the oxygen-evolving complex (Roose et al., 2010; Roberts et al., 2012). FKBP13, a peptidyl-prolyl cis–trans isomerase in the thylakoid lumen also contains an essential disulfide bond and its sulfhydryl oxidation plays a vital role in the photosynthetic electron transport chain (Gopalan et al., 2004; Kang et al., 2008). Experiments reveal that another luminal protein regulated by AtVKOR may be VDE, a luminal enzyme involved in thermal dissipation through the xanthophyll cycle, the activity of which is also dependent on its sulfhydryl oxidation (Bugos and Yamamoto, 1996; Latowski et al., 2004; Yu et al., 2014). Since the redox regulation mechanism of chloroplasts in high plants is complex and vague, we wondered whether AtVKOR regulated their growth and development through its oxidoreductase activity.
In this investigation, plasmids containing cysteine-mutant VKORs were constructed and transformed into vkor mutant lines. Based on the stunted growth phenotype and decreased PSII activity of vkor homozygosities containing mutations of single/double conservative cysteines in the VKOR domain, the important role of the oxidoreductase activities of AtVKOR in photosynthetic growth and PSII activity was confirmed.
Materials and Methods
Plant Materials and Growth Conditions
Wild-type Arabidopsis ecotype Columbia and the T-DNA insertion vkor mutant line have been described in a previous work (Lu et al., 2013). For growth on Murashige and Skoog (MS) medium, the seeds of wild-type and transgenic plants were surface-sterilized with 70% ethanol and 2.6% bleach for 5 and 10 min, respectively. Then, seeds were washed more than five times with sterilized water containing detergent Tween-20. The washed seeds of transgenic or wild-type plants (WTs) were allowed to germinate on MS medium with or without 50 μg ml-1 kanamycin. The seeds on plates were stratified for 48 h at 4°C in the dark for synchronized germination. After 2 weeks, the plantlets were transplanted into vermiculite under 120 μmol m-2 s-1 with short-day conditions (8-h-light/16-h-dark) or long-day conditions (16-h-light/8-h-dark) at a constant temperature of 22°C.
Plasmids for Plant Transformation
The verified sequences of single/double cysteine-mutant AtVKORs were fused to plant transformation plasmid pBI121, following the previous operations of the plasmid pBI121 of wild-type AtVKOR with full-length cDNA (Lu et al., 2013). The heterozygotic Arabidopsis of wild-type Columbia and the vkor mutant line were determined by T-DNA specific primers (Forward primer of AtVKOR, 5′-CTTACCTGCAATGCAATGTTG-3′; reverse primer of AtVKOR, 5′-ACCAGTTTCCAATTCGTGATG-3′; T-DNA specific primer, 5′-GCGTGGACCGCTTGCTGCAACT-3′) and were used for floral dip transformation. Transgenic plants were selected for kanamycin resistance and verified by genomic PCR with specific primers (forward primer for genomic PCR, 5′-GGCCATGGAGTCAAAGATTC-3′; reverse primer for genomic PCR, 5′-CATTGCAGTCGTGATCCC-3′). In the next generation, the homozygotes of cysteine-mutant VKORs to vkor mutant background were screened by T-DNA specific primers as described above.
RNA Extraction and Semiquantitative RT-PCR
The leaves of WT, the vkor mutant line, and transgenic cysteine-mutant VKORs and AtVKORWT plants were used for total RNA isolation using the method described by Lu et al. (2014). The cDNA synthesis was performed according to standard procedures of RevertAid Fist Strand cDNA Synthesis Kit (Fermentas, Canada). In a semiquantitative RT-PCR assay, elongation factor 1-alpha (EF1-α) was used as a control for normalization. The PCR cycles were as follows: one cycle of 5 min at 95°C, followed by 28 cycles each of 30 s at 95°C, 30 s at 57°C, and 30 s at 72°C, final cycle of 8 min at 72°C. Forward primer of EF1-α, 5′-GAGGCTGGTATCTCTAAGGA-3′, reverse primer of EF1-α, 5′-GGAAGTGCCTCAAGAAGAGA-3′; forward primer of AtVKOR, 5′-GTCGGTAACTTCTTATCCTAGACG-3′, reverse primer of AtVKOR, 5′- CTGAGAGTTTTGTGCTAAGG-3′. Each reaction was carried out in three biological replicates.
Measurements of Chlorophyll Fluorescence under Different Light
Fully expanded leaves from 8-weeks-old plants were detached and incubated in sterilized water under normal growth light (120 μmol m-2 s-1) and high light stress (600 μmol m-2 s-1) for 2 h, respectively. Chlorophyll fluorescence was measured using a pulsemodulated fluorometer (FMS-2, Hansatech, UK) as previously described (Yu et al., 2014). One part of the treated leaves were shielded for dark-adaption for more than 15 min, and then, the dark adapted leaves were used for the measurement of maximum quantum yield of PSII (Fv/Fm; Fv, the variable chlorophyll fluorescence yield, defined as Fm-Fo). The other part of treated leaves were directly used for the measurement of the Φ PSII. The parameters were then calculated as previously described (Krause and Weis, 1991): Fv/Fm = (Fm – Fo)/Fm, ΦPSII = (Fm’ – Fs)/Fm’, and NPQ = (Fm–Fm’)/Fm’. In every experiment, at least six leaves were measured, and three independent experiments were conducted.
Thylakoid Membrane Protein Preparation and Western Blot Detection
According to the previous description, the thylakoid membranes were prepared from the leaves of the vkor mutant line, cysteine-mutant AtVKORs and AtVKORWT transgenic plants under normal growth light or 2 h high light treatment (Lu et al., 2013; Yu et al., 2014). The chlorophyll content was determined in 80% (v/v) acetone according to previous operations (Yu et al., 2014). Protein samples corresponding to equal amounts of chlorophyll were separated through 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE). Then, the bands of proteins were transferred onto immobilon-P membranes (Millipore, USA) and blotted with specific D1-antibody. The immune-decorated signals were detected by sensitive fluorography with enhanced chemiluminescence (Amersham, Japan). More than three independent experiments were conducted. The D1 amount was quantified by assaying the intensity in the western blot using Image J software.
H2O2 and O2⋅- Determination
The contents of H2O2 and O2⋅- were determined according to the previous method (Jiang and Zhang, 2002; Clore et al., 2008). Leaves of the vkor mutant line, cysteine-mutant, VKORs and VKORWT transgenic plants under normal growth light and after 2 h high light treatment were ground to a fine power in liquid nitrogen and extracted using 50 mM PBS (pH 7.8). The absorbance was determined at 436 and 530 nm, respectively, and the contents of H2O2 and O2⋅- were calculated according to the standard curve of H2O2 reagent and NaNO2 reagent. Each experiment was carried out in three biological replicates.
Results
Replacement of the Conservative Cysteines in AtVKOR Caused the Pleiotropic Growth Defects in Arabidopsis
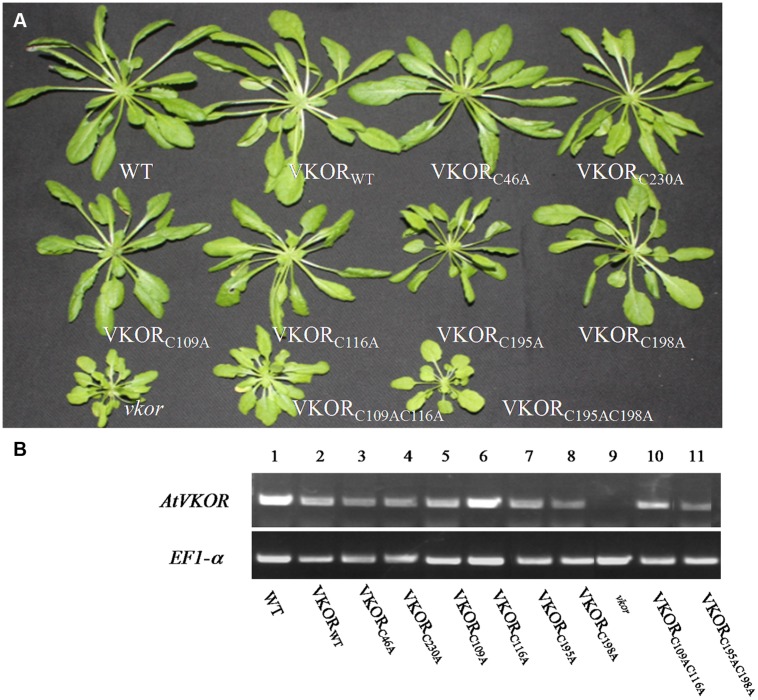
Six cysteines exist in the transmembrane domain of AtVKOR, including two non-conservative cysteines (Cys46, Cys230) and four conservative cysteines forming two pairs (Cys109/Cys116 and Cys195/Cys198) (Feng et al., 2011). Single/double-cysteine(s) mutant VKORs and wild-type AtVKOR (VKORWT) were successfully expressed in vkor mutant lines, respectively (Figure 1B). The representative phenotypes of the vkor homozygosities with the insertion of cysteine-mutant VKORs are shown in Figure 1A. While the AtVKORWT transgenic plants can completely recover the phenotype defects in the vkor line, transgenic plants with a single conservative cysteine mutation (AtVKORC109A, AtVKORC116A, AtVKORC195A, AtVKORC198A) did only partly recover the phenotype defects in the vkor mutant lines (Figure 1A). When double conservative cysteines were mutated to alanines, the AtVKORC109AC116A and AtVKORC195AC198A transgenic plants completely lost the ability to compensate for these defects in the vkor mutant, displaying significantly stunted growth, smaller sized leaves, and delayed flowering, quite similar to the defects of the vkor mutant line. However, the transgenic plants with non-conservative cysteine mutations (AtVKORC46A, AtVKORC230A) showed no obvious difference, compared with WT plants and AtVKORWT plants.
FIGURE 1.
Characteristics of cysteine-mutant AtVKORs plants. (A) The phenotypes of cysteine-mutant AtVKORs plants; plants were grown on soil for 8 weeks under normal growth conditions. (B) Transcriptional level analysis of AtVKOR in plants of cysteine-mutant AtVKORs.
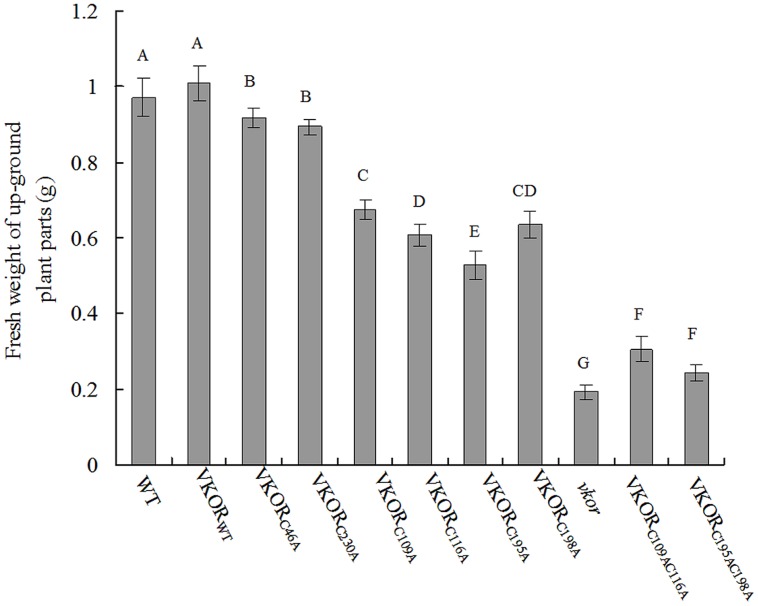
The changes of biomass were further detected in the transgenic plants. The fresh weight (FW) of transgenic plants with double-cysteine mutations (AtVKORC109AC116A and AtVKORC195AC198A) decreased significantly, only about 30% of that of WT and a little more than that of vkor deficient lines (Figure 2). As to transgenic plants with the non-conservative cysteine mutations (AtVKORC46A and AtVKORC230A), the FW decreased a little, about 95% of that of WTs (Figure 2). The changes of biomass were consistent with the phenotypes observed above, further confirming indispensability of the conservative cysteines of AtVKOR in photosynthetic growth of plants.
FIGURE 2.
The biomass of cysteine-mutant AtVKORs plants. The fresh weights (FW) of up-ground parts of WT, vkor mutant, AtVKORWT, and cysteine-mutant VKORs plants were measured. The experiments were repeated at least three times and more than three plants were used each time. The error bars indicated the SD. Significant differences are determined using one-way ANOVA and Ducan’s Multiple Range Test, as indicated with different letters at P < 0.05 significance level.
The effects of the non-conservative and conservative cysteines of AtVKOR to Arabidopsis phenotypes were consistent with their effects to the formation of a disulfide bond in E. coli, although a slight difference exists. The mutations of two non-conservative cysteines do not affect the function of AtVKOR in catalyzing the formation of a disulfide bond, but each single or double mutation of conservative cysteines absolutely leads to the loss of the catalytic ability by checking the motility and β-galactosidase activity in E. coli (Feng et al., 2011). Unlike the phenotype changes of bacteria, in Arabidopsis, transgenic plants with a single conservative cysteine VKORs mutation partly recovered the deficient phenotypes, which revealed that a single cysteine mutation did not break down the electron transferring in plants (Figure 1). Among the four single mutations, the growth of AtVKORC195A was the worst. Combined with the results of double mutations, we supposed the cysteine 195 was directly involved in electron transferring. Characterizations of plant phenotypes demonstrated that all four conservative cysteines could form in pairs when AtVKOR played functions in photosynthetic growth in Arabidopsis.
PSII Activities Were Inhibited in the Transgenic Plants With Conservative Cysteine VKORs Mutation Both Under Normal Growth Light and High Light
Arabidopsis VKOR has been proven to be required for the assembly of PSII; Karamoko et al., 2011), we wondered whether the cysteines in AtVKOR also affected the activities of PSII. The maximal quantum yield of PSII (Fv/Fm), an indicator for the efficiency of PSII photochemistry (Lu et al., 2011), was determined by a chlorophyll fluorescence measurement in the WT plants, vkor mutant line, cysteine-mutant VKORs, and AtVKORWT transgenic plants under growth light (120 μmol m-2 s-1). As shown in Table 1, no significant difference in the Fv/Fm ratio was observed among the AtVKORC46A, AtVKORC230A, AtVKORWT transgenic plants, and WT plants (Table 1), indicating that wild-type VKOR as well as the mutation of non-conservative cysteines in the VKOR domain were all sufficient to restore photosynthetic efficiency under normal growth conditions. However, the ratios of Fv/Fm in AtVKORC109AC116A and AtVKORC195AC198A plants (respectively, 0.662 and 0.673) were dramatically lower than that in the AtVKORWT plant (averagely 0.866), indicating the decreased activity of the reaction centers of PSII due to the redox-inactive mutation of conservative cysteines in the VKOR domain. Moreover, a single mutation at Cys195 could also arouse a significant decrease in Fv/Fm (averagely 0.699). A similar trend of actual PSII photochemical efficiency (ΦPSII) was observed in the investigated plants (Table 1). These low chlorophyll fluorescence parameters in transgenic plants with mutations of conservative cysteine VKORs were correlated with the impaired photosynthesis and reduced activities of PSII.
Table 1.
Photosynthetic characterization of wild-type Arabidopsis, vkor mutant lines, and transgenic cysteine-mutant VKORs and VKORWT plants under normal growth light vs. high light.
| Plants | Normal growth light (120 μmol m-2 s-1) |
2 h High light(600 μmol m-2 s-1) |
||||
|---|---|---|---|---|---|---|
| Fv/Fm | ΦPSII | NPQ | Fv/Fm | ΦPSII | NPQ | |
| WT | 0.865 ± 0.005 A | 0.769 ± 0.008 A | 1.204 ± 0.063 A | 0.718 ± 0.008 A | 0.436 ± 0.034 A | 1.604 ± 0.013 A |
| VKORWT | 0.866 ± 0.004 A | 0.766 ± 0.013 A | 1.201 ± 0.091 A | 0.725 ± 0.005 A | 0.445 ± 0.008 A | 1.612 ± 0.008 A |
| VKORC46A | 0.861 ± 0.004 A | 0.732 ± 0.013 AB | 1.151 ± 0.064 A | 0.712 ± 0.01 AB | 0.421 ± 0.02 AB | 1.524 ± 0.036 A |
| VKORC230A | 0.86 ± 0.002 A | 0.729 ± 0.02 AB | 1.166 ± 0.061 A | 0.702 ± 0.014 AB | 0.418 ± 0.017 AB | 1.516 ± 0.038 A |
| VKORC109A | 0.817 ± 0.013 B | 0.709 ± 0.02 B | 1.026 ± 0.035 B | 0.618 ± 0.026 BC | 0.353 ± 0.018 B | 1.23 ± 0.102 B |
| VKORC116A | 0.806 ± 0.026 B | 0.713 ± 0.046 B | 1.012 ± 0.013 B | 0.624 ± 0.022 C | 0.354 ± 0.022 B | 1.225 ± 0.102 B |
| VKORC195A | 0.699 ± 0.051 B | 0.653 ± 0.02 B | 0.998 ± 0.011 B | 0.605 ± 0.027 DE | 0.345 ± 0.016 BC | 1.219 ± 0.094 B |
| VKORC198A | 0.746 ± 0.047 B | 0.696 ± 0.037 B | 1.008 ± 0.014 B | 0.617 ± 0.024 D | 0.35 ± 0.011 BC | 1.221 ± 0.095 B |
| vkor | 0.565 ± 0.017 D | 0.396 ± 0.071 D | 0.905 ± 0.012 C | 0.441 ± 0.019 F | 0.219 ± 0.017 D | 1.043 ± 0.068 C |
| VKORC109AC116A | 0.662 ± 0.025 C | 0.514 ± 0.019 C | 0.945 ± 0.01 BC | 0.489 ± 0.026 DE | 0.278 ± 0.017 C | 1.112 ± 0.093 BC |
| VKORC195AC198A | 0.673 ± 0.025 C | 0.525 ± 0.015 C | 0.949 ± 0.14 BC | 0.493 ± 0.018 E | 0.289 ± 0.014 C | 1.116 ± 0.089 BC |
The number is reported as the mean ± SD of three independent measurements for each plant. Significant differences among wild-type Arabidopsis (WT), vkor mutant lines, and transgenic cysteine-mutant VKORs and VKORWT plants are determined using one-way ANOVA and Ducan’s Multiple Range Test, indicated with different letters at P < 0.05 significance level.
Excess light has negative impacts on plant photosynthesis, and previous research shows that the mutant line of vkor is sensitive to high light (Yu et al., 2014). Our results demonstrated that high irradiance (HL, 600 μmol m-2 s-1 for 2 h) increased the differences on Fv/Fm and ΦPSII among double conservative cysteine-mutant VKORs and AtVKORWT plants, compared with normal growth light (GL, 120 μmol m-2 s-1), as shown in Table 1, suggesting that the photoinhibition in AtVKORC109AC116A and AtVKORC195AC198A plants was further aggravated when exposed to high light.
To avoid photodamage, photosynthetic organisms have developed various photoprotective mechanisms to resist photooxidative damage and to repair damaged protein components (Nishiyama et al., 2001; Murata et al., 2007). One important pathway is to minimize excitation pressure on PSII by thermal dissipation, which can be reflected by the value of NPQ. In this investigation, we found that the NPQ values were lower in the AtVKORC109AC116A (averagely 0.945, GL; averagely 1.112, HL) and AtVKORC195AC198A (averagely 0.949, GL; averagely 1.116, HL) plants compared with AtVKORWT (averagely 1.201, GL; averagely 1.612, HL) plants under different illumination (Table 1), suggesting a low capability in the dissipation of excess light. Previous investigations have proved that the npq1 mutant exhibits greatly reduced NPQ with deficient VDE, a vital enzyme in xanthophyll cycle (Niyogi et al., 1998; Han et al., 2010). AtVKOR/LTO1 has been proven to be related with the xanthophyll cycle, one important mechanism to dissipate excess thermal energy (Yu et al., 2014). We supposed that mutations of conservative cysteines of AtVKOR affected the cycle of xanthophyll and resulted in the impaired photoprotection for thermal dissipation.
Levels of D1 Protein Decreased in the Transgenic Plants with a Conservative Cysteine VKORs Mutation
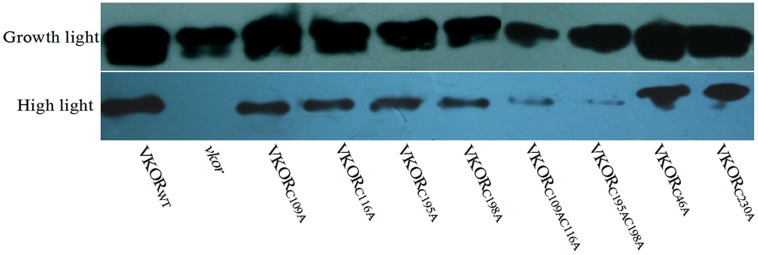
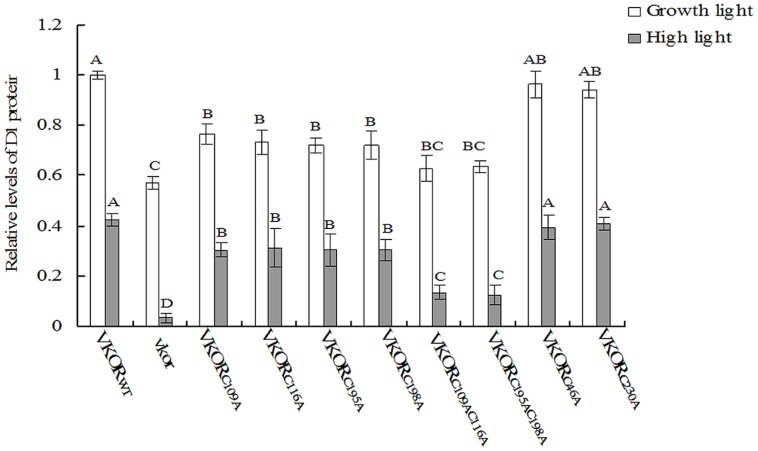
The turnover of D1 protein is one of photoprotective processes in PSII under high light stress (Nishiyama et al., 2001; Huesgen et al., 2006). Under normal growth light, the levels of D1 protein in transgenic plants with mutant AtVKORs of conservative cysteine were decreased, especially in double-cysteine mutants AtVKORC109AC116A and AtVKORC195AC198A (Figures 3 and 4). High irradiance increased the decrease extent of D1 protein, and only trace amount of D1 accumulation could be detected in the vkor mutant line and double-cysteine mutant plants, which is consistent with the previous result that the deficiency of AtVKOR accelerates the degradation of D1 protein (Yu et al., 2014). Little difference was observed in the level of D1 protein among the transgenic plants with mutant AtVKORs of non-conservative cysteine and AtVKORWT (Figures 3 and 4). The results above suggested that the turnover of D1 protein in the repair of photodamaged were impaired due to the mutations of conservative cysteine in the AtVKOR domain, which are directly related to its oxidoreductase activity.
FIGURE 3.
Immunoblot analysis of D1 accumulation in cysteine-mutant VKORs transgenic plants under growth light or in high light. Thylakoid membrane proteins were extracted from the leaves of AtVKORWT, vkor mutant and cysteine-mutant VKORs plants. The immunoblot was detected by D1-antibody. Growth light: 120 μmol m-2 s-1; High light: 600 μmol m-2 s-1 for 2 h.
FIGURE 4.
Quantified analysis of D1 immunoblot. Signals of immunoblot were quantified using the ImageJ program. The value of D1 accumulation in AtVKORWT transgenic plants under growth light was adjusted to one. The relative levels of D1 accumulations in cysteine-mutant VKORs plants compared to VKORWT plants were, respectively, calculated. The error bars indicated the SD. Significant differences are determined using one-way ANOVA and Ducan’s Multiple Range Test, indicated with different letters at P < 0.05 significance level. Growth light: 120 μmol m-2 s-1; High light: 600 μmol m-2 s-1 for 2 h.
Accumulation of ROS Increased in the Transgenic Plants with Conservative Cysteine VKORs Mutation
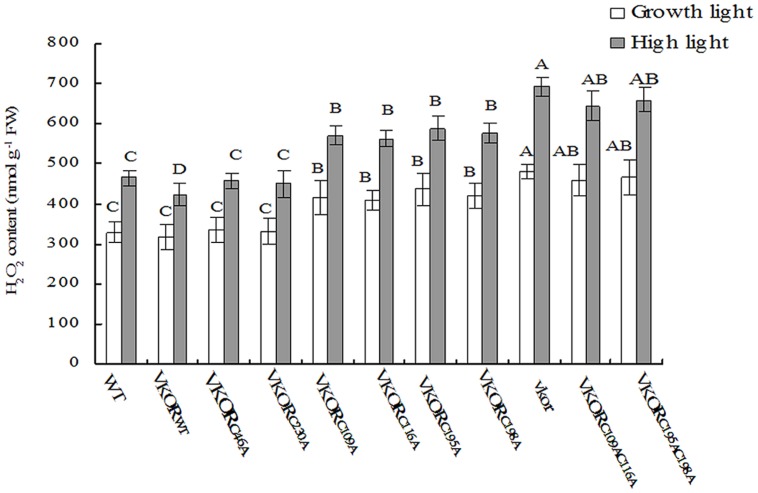
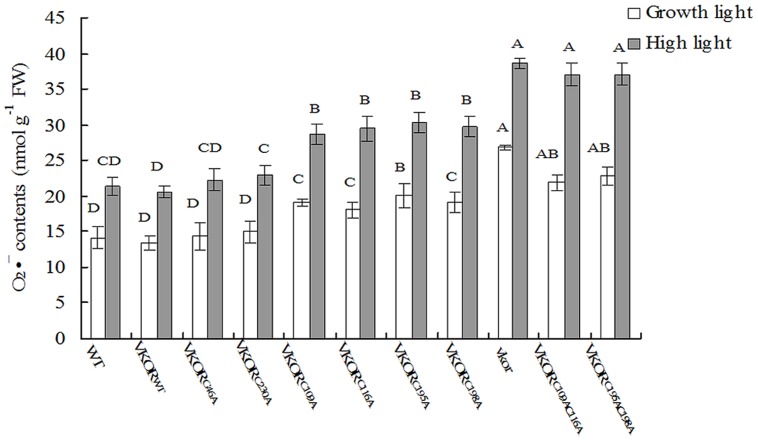
In chloroplasts, the damage of PSII assembly is usually associated with the harmful production of ROS, such as H2O2, O2⋅-, and singlet oxygen (Cejkova et al., 1998; Murata et al., 2007). Previous studies show that much more H2O2 and O2⋅- are accumulated in the vkor mutant than in wild-type plants (Lu et al., 2013). By checking the levels of H2O2 and O2⋅- in transgenic plants, we found that the amounts of ROS in plants with mutations of conservative cysteine VKORs were higher than that of AtVKORWT plants under growth light (Figures 5 and 6). Under high irradiance, the elevated accumulations of H2O2 and O2⋅- in AtVKORC109AC116A and AtVKORC195AC198A plants were detected, similar to that of the vkor mutant line (Figures 5 and 6). As to the transgenic plants of AtVKORC46A, AtVKORC230A, the levels of H2O2 and O2⋅- were quite closed to that in AtVKORWT plants (Figures 5 and 6). The results further indicated that the photoprotection mechanism was damaged in the transgenic plants with mutant AtVKORs of conservative cysteine.
FIGURE 5.
Levels of H2O2 in cysteine-mutant VKORs transgenic plants under growth light or in high light. Quantitative accumulations of H2O2 were, respectively, detected in 6-weeks-old leaves from WT, vkor mutant, AtVKORWT, and cysteine-mutant VKORs plants under growth light or in high light. The error bars indicated the SD. Significant differences are determined using one-way ANOVA and Ducan’s Multiple Range Test, indicated with different letters at P < 0.05 significance level. Growth light: 120 μmol m-2 s-1; High light: 600 μmol m-2 s-1 for 2 h.
FIGURE 6.
Levels of O2⋅– in cysteine-mutant VKORs transgenic plants under growth light or in high light. Quantitative accumulation of O2⋅- was, respectively, detected in 6-weeks-old leaves from WT, vkor mutant, AtVKORWT, and cysteine-mutant VKORs plants under growth light or in high light. The error bars indicated the SD. Significant differences are determined using one-way ANOVA and Ducan’s Multiple Range Test, indicated with different letters at P < 0.05 significance level. Growth light: 120 μmol m-2 s-1; High light: 600 μmol m-2 s-1 for 2 h.
Discussion
Thylakoid protein AtVKOR/LTO1, containing a conserved VKOR domain with four conservative cysteines, has been reported to participate in the transmembrane thiol-oxidation as an oxidoreductase in vitro and in E. coli (Feng et al., 2011; Karamoko et al., 2011). The necessity of the conservative cysteines of the AtVKOR domain is inferred from the fact that single or double cysteine mutations lead AtVKOR to losing its function of promoting disulfide bond formation in E. coli (Feng et al., 2011). Whether conservative cysteines of AtVKOR play essential roles in the complicated plant cells needs to be determined.
In this investigation, our results demonstrated that the cysteine-dependent oxidoreductase activity of AtVKOR was directly related to photosynthetic growth in plant. The AtVKORWT transgenic plants can completely recover the phenotype defects in the vkor deficient line. Similar to the AtVKORWT, the mutations of non-conservative cysteines in AtVKOR domain almost rescued the defects of vkor mutant, though there was a decrease of biomass at about 5% of WT. Little effects of non-conservative cysteines on the function of AtVKOR were further shown by checking photosynthetic parameters, for example Fv/Fm, ΦPSII, and D1 quantity. On the contrary, the double-mutations of each pair of conservative cysteines in AtVKOR domain did not compensate the defects of vkor mutant, displaying a similar phenotype of vkor mutant lines. The essentiality of the conservative cysteine residues to activity of VKOR has also been observed in the VKORs from other species (Li et al., 2010; Wang et al., 2011). Mutations of each conservative cysteines of MtbVKOR lead mycobacteria to losing the growing ability in minimal medium (Wang et al., 2011). The structural analysis shows that four conservative cysteines are spatially proximate in the active site of VKOR from Synechococcus sp. (Li et al., 2010). Attentively, it has been proven that AtVKOR has oxidation, reduction, and isomerization activity in vitro (Lu et al., 2013). The conservative cysteine of AtVKOR directly affects electron transferring and is related to the activity of oxidoreductase (Yang et al., 2015). Based on all the investigations, it is quite possible that oxidoreductase activity of AtVKOR affects the thiol-redox metabolism in chloroplasts and regulates the growth and development of plants.
AtVKOR is required for the assembly of PSII under growth light, and the deficient vkor mutant line is more susceptible to high light stress, compared with WT (Yu et al., 2014). Previous study proves that these defects could be abolished when AtVKORWT is transformed into the vkor mutant line, suggesting the phenotype changes are due to the gene (Lu et al., 2013). Our investigation further demonstrated that the stunted growth, the reduced PSII activity, and the aggravated photodamage were related to the damage of AtVKOR oxidoreductase activity due to the mutation of conservative cysteines in the AtVKOR domain. Transforming conservative cysteine mutant VKORs to vkor mutant line, especially double-cysteine mutant AtVKORs (AtVKORC109AC116A and AtVKORC195AC198A), could not eliminate the damage, based on the declined fluorescence parameters (Fv/Fm, ΦPSII and NPQ; Figure 1; Table 1). The high accumulation of ROS in transgenic plants of AtVKORC109AC116A and AtVKORC195AC198A further verified the severe photodamage.
The damage of PSII is a primary target of photodamage in the photosynthetic apparatus (Nishiyama et al., 2001; Murchie and Niyogi, 2011). To effectively repair the photodamage, diversified photoprotection processes emerge during the evolution of plants (Kirilovsky and Etienne, 1991; Cai et al., 2010; Lunch et al., 2013; Nath et al., 2013). The rapid D1 turnover, a cycle of degradation and re-synthesis of D1 protein, is one of effective photoprotection methods (Ali et al., 2006; Murata et al., 2007). The accumulation of D1 protein depends on the balance of the synthesis and degradation. As to the synthesis of D1 protein, no difference has been found at the transcription level of PsbA, the encoding gene of D1, between vkor deficient line and WT plants (Lu et al., 2013). However, the effects of AtVKOR on the translation steps of D1 remain to be elucidated. Recent investigations reveal that the de novo synthesis of proteins, particularly D1 protein, can be inhibited by excess ROS at translation level (Nishiyama et al., 2001, 2004, 2006, 2011; Murata et al., 2007; Takahashi et al., 2009). The ROS-induced suppression of protein synthesis is associated with the specific inactivation of elongation factor G via the formation of an intramolecular disulfide bond (Nishiyama et al., 2006, 2011; Murata et al., 2007). Much more ROS was accumulated in plants of mutant AtVKORs of conservative cysteines in our investigation. So it is quite possible that the synthesis of D1 protein in translation steps would be affected. As to the degradation of D1, when the synthesis of D1 is blocked, the degradation rate of D1 is accelerated in the vkor deficient line (Yu et al., 2014). The accelerated degradation of D1 in the vkor line may be related to instability of PSII, since AtVKOR is required for PSII assembly (Karamoko et al., 2011). In this investigation, the low accumulation of D1 in the transgenic plants suggested that the cysteine-dependent activity of AtVKOR was involved in the D1 turnover-dependent photoprotection mechanism.
One fast response to high irradiance is to dissipate excessive thermal energy by NPQ, the core component of which requires sufficient zeaxanthin produced by the xanthophyll cycle (Lunch et al., 2013). In the xanthophyll cycle, epoxide xanthophyll violaxanthin is rapidly converted via the antheraxanthin to the de-epoxide zeaxanthin, and zeaxanthin can directly participate in the dissipation of excess energy (Murchie and Niyogi, 2011). The key enzyme catalyzing the conversion from antheraxanthin to zeaxanthin is VDE, a thylakoid luminal protein containing essential disulfide bonds (Latowski et al., 2004). Previous investigation has showed that the xanthophyll cycle in the vkor mutant line is also impaired under high light, base on the ratio of xanthophyll pigments (Yu et al., 2014). In this investigation, a decline of NPQ both under growth light and high light was observed in transgenic plants of VKORC109AC116A and VKORC195AC198A, reflecting a low capability to dissipate excessive irradiance. Presumably, this is closely related to the declined activity of VDE, whose disulfide bonds in active site are not correctly formed due to the deficient oxidoreductase activity of AtVKOR in these transgenic plants. Altogether, our results suggested that conservative cysteines in AtVKOR domain were related to the oxidoreductase activity of AtVKOR, and the function was directly involved in photosynthetic growth and PSII activity in vivo.
Author Contributions
Designed the experiments: X-YW, J-JD. Performed the experiments: J-JD, C-YZ, YL, and H-RC. Analyzed the data: J-JD, C-YZ, YL, H-RC, and X-YW. Wrote the paper: J-JD and X-YW.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The present study was supported by the Special Research Fund of Public Welfare of China Agricultural Ministry (201303093).
Abbreviations
- AtVKORCXXA/AXXC/AXXA plants
complemented cysteine-mutant VKORs/vkor plants
- AtVKORWT plants
complemented wild-type AtVKOR/vkor plants
- H2O2
hydrogen peroxide
- LTO1
Lumen thiol oxidoreductase 1
- NPQ
non-photochemical quenching
- O2⋅-
superoxide anion radical
- ΦPSII
actual PSII efficiency
- PSII
photosystem II
- qRT-PCR
quantitative reverse transcription-polymerase chain reaction
- ROS
reactive oxygen species
- VDE
violaxanthin de-epoxidase
- VKOR
vitamin K epoxide reductase
- WT
wild-type Arabidopsis
References
- Ali N. A., Dewez D., Didur O., Popovic R. (2006). Inhibition of photosystem II photochemistry by Cr is caused by the alteration of both D1 protein and oxygen evolving complex. Photosynth. Res. 89 81–87 10.1007/s11120-006-9085-5 [DOI] [PubMed] [Google Scholar]
- Aro E. M., Ohad I. (2003). Redox regulation of thylakoid protein phosphorylation. Antioxid. Redox. Signal. 5 55–67 10.1089/152308603321223540 [DOI] [PubMed] [Google Scholar]
- Buchanan B. B., Luan S. (2005). Redox regulation in the chloroplast thylakoid lumen: a new frontier in photosynthesis research. J. Exp. Bot. 56 1439–1447 10.1093/jxb/eri158 [DOI] [PubMed] [Google Scholar]
- Bugos R. C., Yamamoto H. Y. (1996). Molecular cloning of violaxanthin de-epoxidase from romaine lettuce and expression in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 93 6320–6325 10.1073/pnas.93.13.6320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai B., Zhang A., Yang Z., Lu Q., Wen X., Lu C. (2010). Characterization of photosystem II photochemistry in transgenic tobacco plants with lowered Rubisco activase content. J. Plant Physiol. 167 1457–1465 10.1016/j.jplph.2010.05.004 [DOI] [PubMed] [Google Scholar]
- Cejkova J., Labsky J., Vacik J. (1998). Reactive oxygen species (ROS) generated by xanthine oxidase in the corneal epithelium and their potential participation in the damage of the corneal epithelium after prolonged use of contact lenses in rabbits. Acta Histochem. 100 171–184 10.1016/S0065-1281(98)80025-1 [DOI] [PubMed] [Google Scholar]
- Clore A. M., Doore S. M., Tinnirello S. M. (2008). Increased levels of reactive oxygen species and expression of a cytoplasmic aconitase/iron regulatory protein 1 homolog during the early response of maize pulvini to gravistimulation. Plant Cell Environ. 31 144–158 10.1111/j.1365-3040.2007.01744.x [DOI] [PubMed] [Google Scholar]
- Cremers C. M., Jakob U. (2013). Oxidant sensing by reversible disulfide bond formation. J. Biol. Chem. 288 26489–26496 10.1074/jbc.R113.462929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W. K., Wang L., Lu Y., Wang X. Y. (2011). A protein oxidase catalysing disulfide bond formation is localized to the chloroplast thylakoids. FEBS J. 278 3419–3430 10.1111/j.1742-4658.2011.08265.x [DOI] [PubMed] [Google Scholar]
- Furt F., Oostende C., Widhalm J. R., Dale M. A., Wertz J., Basset G. J. (2010). A bimodular oxidoreductase mediates the specific reduction of phylloquinone (vitamin K) in chloroplasts. Plant J. 64 38–46 10.1111/j.1365-313X.2010.04305.x [DOI] [PubMed] [Google Scholar]
- Gopalan G., He Z., Balmer Y., Romano P., Gupta R., Heroux A., et al. (2004). Structural analysis uncovers a role for redox in regulating FKBP13 an immunophilin of the chloroplast thylakoid lumen. Proc. Natl. Acad. Sci. U.S.A. 101 13945–13950 10.1073/pnas.0405240101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M., Mata-Cabana A., Akerlund H. E., Florencio F. J., Schroder W. P., Lindahl M., et al. (2010). Thioredoxin targets of the plant chloroplast lumen and their implications for plastid function. Proteomics 10 987–1001 10.1002/pmic.200900654 [DOI] [PubMed] [Google Scholar]
- Han H., Gao S., Li B., Dong X. C., Feng H. L., Meng Q. W. (2010). Overexpression of violaxanthin de-epoxidase gene alleviates photoinhibition of PSII and PSI in tomato during high light and chilling stress. J. Plant Physiol. 167 176–183 10.1016/j.jplph.2009.08.009 [DOI] [PubMed] [Google Scholar]
- Huesgen P. F., Schuhmann H., Adamska I. (2006). Photodamaged D1 protein is degraded in Arabidopsis mutants lacking the Deg2 protease. FEBS Lett. 580 6929–6932 10.1016/j.febslet.2006.11.058 [DOI] [PubMed] [Google Scholar]
- Jiang M., Zhang J. (2002). Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J. Exp. Bot. 53 2401–2410 10.1093/jxb/erf090 [DOI] [PubMed] [Google Scholar]
- Jocelyn P. C. (1967). The standard redox potential of cysteine-cystine from the thiol-disulphide exchange reaction with glutathione and lipoic acid. Eur. J. Biochem. 2 327–331 10.1111/j.1432-1033.1967.tb00142.x [DOI] [PubMed] [Google Scholar]
- Kang C. B., Hong Y., Dhe-Paganon S., Yoon H. S. (2008). FKBP family proteins: immunophilins with versatile biological functions. Neurosignals 16 318–325 10.1159/000123041 [DOI] [PubMed] [Google Scholar]
- Karamoko M., Cline S., Redding K., Ruiz N., Hamel P. P. (2011). Lumen thiol oxidoreductase1 a disulfide bond-forming catalyst, is required for the assembly of photosystem II in Arabidopsis. Plant Cell 23 4462–4475 10.1105/tpc.111.089680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamoko M., Gabilly S. T., Hamel P. P. (2013). Operation of trans-thylakoid thiol-metabolizing pathways in photosynthesis. Front. Plant Sci. 4:476 10.3389/fpls.2013.00476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieselbach T. (2013). Oxidative folding in chloroplasts. Antioxid. Redox. Signal. 19 72–82 10.1089/ars.2012.4582 [DOI] [PubMed] [Google Scholar]
- Kirilovsky D., Etienne A. L. (1991). Protection of reaction center II from photodamage by low temperature and anaerobiosis in spinach chloroplasts. FEBS Lett. 279 201–204 10.1016/0014-5793(91)80149-W [DOI] [PubMed] [Google Scholar]
- Krause G. H., Weis E. (1991). Chlorophyll fluorescence and photosynthesis: the basics. Annu. Rev. Plant Phys. 42 313–349 10.1146/annurev.pp.42.060191.001525 [DOI] [Google Scholar]
- Latowski D., Akerlund H. E., Strzalka K. (2004). Violaxanthin de-epoxidase, the xanthophyll cycle enzyme, requires lipid inverted hexagonal structures for its activity. Biochemistry 43 4417–4420 10.1021/bi049652g [DOI] [PubMed] [Google Scholar]
- Li W., Schulman S., Dutton R. J., Boyd D., Beckwith J., Rapoport T. A. (2010). Structure of a bacterial homologue of vitamin K epoxide reductase. Nature 463 507–512 10.1038/nature08720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Hall D. A., Last R. L. (2011). A small zinc finger thylakoid protein plays a role in maintenance of photosystem II in Arabidopsis thaliana. Plant Cell 23 1861–1875 10.1105/tpc.111.085456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Peng J. J., Yu Z. B., Du J. J., Xu J. N., Wang X. Y. (2014). Thylakoid membrane oxidoreductase LTO1/AtVKOR is involved in ABA-mediated response to osmotic stress in Arabidopsis. Physiol. Plant. 10.1111/ppl.12268 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Lu Y., Wang H. R., Li H., Cui H. R., Feng Y. G., Wang X. Y. (2013). A chloroplast membrane protein LTO1/AtVKOR involving in redox regulation and ROS homeostasis. Plant Cell Rep. 32 1427–1440 10.1007/s00299-013-1455-9 [DOI] [PubMed] [Google Scholar]
- Lunch C. K., Lafountain A. M., Thomas S., Frank H. A., Lewis L. A., Cardon Z. G. (2013). The xanthophyll cycle and NPQ in diverse desert and aquatic green algae. Photosynth. Res. 115 139–151 10.1007/s11120-013-9846-x [DOI] [PubMed] [Google Scholar]
- Murata N., Takahashi S., Nishiyama Y., Allakhverdiev S. I. (2007). Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta 1767 414–421 10.1016/j.bbabio.2006.11.019 [DOI] [PubMed] [Google Scholar]
- Murchie E. H., Niyogi K. K. (2011). Manipulation of photoprotection to improve plant photosynthesis. Plant Physiol. 155 86–92 10.1104/pp.110.168831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahara N. (2011). Catalytic site cysteines of thiol enzyme: sulfurtransferases. J. Amino. Acids 2011 709404 10.4061/2011/709404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath K., Jajoo A., Poudyal R. S., Timilsina R., Park Y. S., Aro E. M., et al. (2013). Towards a critical understanding of the photosystem II repair mechanism and its regulation during stress conditions. FEBS Lett. 587 3372–3381 10.1016/j.febslet.2013.09.015 [DOI] [PubMed] [Google Scholar]
- Nishiyama Y., Allakhverdiev S. I., Murata N. (2006). A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim. Biophys. Acta 1757 742–749 10.1016/j.bbabio.2006.05.013 [DOI] [PubMed] [Google Scholar]
- Nishiyama Y., Allakhverdiev S. I., Murata N. (2011). Protein synthesis is the primary target of reactive oxygen species in the photoinhibition of photosystem II. Physiol. Plant. 142 35–46 10.1111/j.1399-3054.2011.01457.x [DOI] [PubMed] [Google Scholar]
- Nishiyama Y., Allakhverdiev S. I., Yamamoto H., Hayashi H., Murata N. (2004). Singlet oxygen inhibits the repair of photosystem II by suppressing the translation elongation of the D1 protein in Synechocystis sp. PCC 6803. Biochemistry 43 11321–11330 10.1021/bi036178q [DOI] [PubMed] [Google Scholar]
- Nishiyama Y., Yamamoto H., Allakhverdiev S. I., Inaba M., Yokota A., Murata N. (2001). Oxidative stress inhibits the repair of photodamage to the photosynthetic machinery. EMBO J. 20 5587–5594 10.1093/emboj/20.20.5587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi K. K., Grossman A. R., Bjorkman O. (1998). Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell 10 1121–1134 10.1105/tpc.10.7.1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenburg J., Bevans C. G., Muller C. R., Watzka M. (2006). Vitamin K epoxide reductase complex subunit 1 (VKORC1): the key protein of the vitamin K cycle. Antioxid. Redox. Signal. 8 347–353 10.1089/ars.2006.8.347 [DOI] [PubMed] [Google Scholar]
- Onda Y. (2013). Oxidative protein-folding systems in plant cells. Int. J. Cell Biol. 2013 585431 10.1155/2013/585431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts I. N., Lam X. T., Miranda H., Kieselbach T., Funk C. (2012). Degradation of PsbO by the Deg protease HhoA Is thioredoxin dependent. PLoS ONE 7:e45713 10.1371/journal.pone.0045713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero L. C., Aroca M. A., Laureano-Marin A. M., Moreno I., Garcia I., Gotor C. (2014). Cysteine and cysteine-related signaling pathways in Arabidopsis thaliana. Mol. Plant 7 264–276 10.1093/mp/sst168 [DOI] [PubMed] [Google Scholar]
- Roose J. L., Yocum C. F., Popelkova H. (2010). Function of PsbO, the photosystem II manganese-stabilizing protein: probing the role of aspartic acid 157. Biochemistry 49 6042–6051 10.1021/bi100303f [DOI] [PubMed] [Google Scholar]
- Takahashi S., Whitney S. M., Badger M. R. (2009). Different thermal sensitivity of the repair of photodamaged photosynthetic machinery in cultured Symbiodinium species. Proc. Natl. Acad. Sci. U.S.A. 106 3237–3242 10.1073/pnas.0808363106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan C. M., Yang X. J., Du J. J., Lu Y., Yu Z. B., Feng Y. G., et al. (2014). Identification and characterization of SlVKOR, a disulfide bond formation protein from Solanum lycopersicum, and bioinformatic analysis of plant VKORs. Biochemistry (Mosc) 79 440–449 10.1134/S0006297914050083 [DOI] [PubMed] [Google Scholar]
- Wang X., Dutton R. J., Beckwith J., Boyd D. (2011). Membrane topology and mutational analysis of Mycobacterium tuberculosis VKOR, a protein involved in disulfide bond formation and a homologue of human vitamin K epoxide reductase. Antioxid. Redox. Signal. 14 1413–1420 10.1089/ars.2010.3558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouters M. A., Fan S. W., Haworth N. L. (2010). Disulfides as redox switches: from molecular mechanisms to functional significance. Antioxid. Redox. Signal. 12 53–91 10.1089/ARS.2009.2510 [DOI] [PubMed] [Google Scholar]
- Yang X. J., Cui H. R., Yu Z. B., Du J. J., Xu J. N., Wang X. Y. (2015). Key Amino Acids of Arabidopsis VKOR in the activity of phylloquinone reduction and disulfide bond formation. Protein Pept. Lett. 22 81–86 10.2174/0929866521666140926115347 [DOI] [PubMed] [Google Scholar]
- Yu Z. B., Lu Y., Du J. J., Peng J. J., Wang X. Y. (2014). The chloroplast protein LTO1/AtVKOR is involved in the xanthophyll cycle and the acceleration of D1 protein degradation. J. Photochem. Photobiol. B 130 68–75 10.1016/j.jphotobiol.2013.11.003 [DOI] [PubMed] [Google Scholar]