Abstract
To cope with the dual nature of copper as being essential and toxic for cells, plants temporarily adapt the expression of copper homeostasis components to assure its delivery to cuproproteins while avoiding the interference of potential oxidative damage derived from both copper uptake and photosynthetic reactions during light hours. The circadian clock participates in the temporal organization of coordination of plant nutrition adapting metabolic responses to the daily oscillations. This timely control improves plant fitness and reproduction and holds biotechnological potential to drive increased crop yields. Hormonal pathways, including those of abscisic acid, gibberellins, ethylene, auxins, and jasmonates are also under direct clock and light control, both in mono and dicotyledons. In this review, we focus on copper transport in Arabidopsis thaliana and Oryza sativa and the presumable role of hormones in metal homeostasis matching nutrient availability to growth requirements and preventing metal toxicity. The presence of putative hormone-dependent regulatory elements in the promoters of copper transporters genes suggests hormonal regulation to match special copper requirements during plant development. Spatial and temporal processes that can be affected by hormones include the regulation of copper uptake into roots, intracellular trafficking and compartmentalization, and long-distance transport to developing vegetative and reproductive tissues. In turn, hormone biosynthesis and signaling are also influenced by copper availability, which suggests reciprocal regulation subjected to temporal control by the central oscillator of the circadian clock. This transcriptional regulatory network, coordinates environmental and hormonal signaling with developmental pathways to allow enhanced micronutrient acquisition efficiency.
Keywords: Arabidopsis thaliana, Oryza sativa, copper homeostasis, copper transporters, hormone biosynthesis, hormone signaling, circadian clock, oxidative stress
Introduction
Mineral nutrition is an important environmental constraint that influences diverse developmental processes in plants. Both deficient and excess nutrient availability are considered abiotic stresses that can cause deleterious effects on plant physiology and metabolism. Plants employ complex homeostatic networks to increase uptake and cope with non-optimal nutrient supply. These mechanisms are especially relevant for transition metal nutrients, such as copper (Cu), since this metal acts as a double-edged sword in living beings. Cu is essential as a redox-active cofactor in multiple biological processes, but is toxic in excess given its role in the production of highly reactive oxygen species (ROS; Puig and Peñarrubia, 2009; Ravet and Pilon, 2013). Both local root responses and systemic signaling have to be integrated in order to drive optimized metal nutrient acquisition under changing environmental conditions which, in many cases, alter the whole plant morphology and metabolism (López-Bucio et al., 2003). In order to orchestrate morphological, physiological, and molecular adaptive responses to soil mineral bioavailabilities, phytohormones act as major endogenous cues. Nutrients affect multiple levels and components in hormone biosynthesis, perception, and signaling pathways. In turn, hormones influence root growth, stomatal movements and stress tolerance, among other processes, and have a huge impact on plant mineral nutrition. Thus, there is a close interrelation between hormonal stimuli and nutritional homeostasis (Rubio et al., 2009) which underlies current strategies based on co-application of hormones and mineral fertilizers to increase crop yield (Zaman et al., 2014).
Widespread inappropriate agricultural practices, such as overusing fungicides with high Cu concentrations, release of industrial wastewater and mining activities, have caused Cu contamination in cultivated soils and irrigating waters at specific locations (Marschner and Marschner, 2012). On the opposite side, the boost of carbohydrate synthesis in plants, due to the growing levels of atmospheric CO2, results in a general loss of the mineral nutritional quality of vegetable food. Indeed recent meta-analyses have indicated that Cu and other metal deficiencies would be exacerbated in forthcoming years due to a rise in CO2, with increasing obesity and “hidden hunger” problems in the world (Loladze, 2014; Myers et al., 2014). Since plants constitute one of the main entrances of micronutrients into trophic chains, deciphering the regulatory mechanisms underlying dynamical hormonal interactions with Cu uptake and distribution to edible plant parts is relevant for optimal plant development and to avoid plant nutritional deficiencies or excesses from being transferred to consumers.
Environmental factors, mainly light and temperature, show daily and seasonal variations, which impose a specific temporal order to the plant’s biological functions orchestrated by the circadian clock. Thus, hormone biosynthesis, perception and signaling pathways are under the control of the circadian clock, which explains its pervasive effects on plant growth and development (Robertson et al., 2009). Many abiotic stresses, such as cold, salt, drought and heat, result from daily light/dark cycles and the circadian clock influences responses to such stresses (Sanchez et al., 2011). Abiotic stress signaling pathways are highly interconnected because of common concurrent processes, where stress-related hormones are major components (Fujita et al., 2006). Plant nutrient requirements vary along daily cycles with plant development stages and during stress responses (Marschner and Marschner, 2012). The circadian regulation of ion channels and nutrient transporters involved in the transport of carbohydrates, nitrogen, sulfate, phosphate, and micronutrients is a pervasive phenomenon. These processes have been proposed to regulate downstream targets to further spread circadian signaling while, in turn, these processes provide feedback to the central oscillator (Ko et al., 2009; Haydon et al., 2011). Hormones can also affect time-of-day-dependent changes in metal fluxes, a phenomenon known as metal muffling. This term refers to the non-steady state dynamics of metal ions that involves temporal expression changes in homeostatic components, affecting uptake, efflux, and intracellular compartmentalization (Colvin et al., 2010). Due to its role in the basic metabolic processes determining the energy status of the plant (photosynthesis and respiration) Cu deficiency results in decreased plant growth. But its effects on reproductive growth are even more important. In cereals it leads to reduced pollen viability and increase in spikelet sterility, thus developing many unfilled grains and yield losses (Azouaou and Souvré, 1993; Dobermann and Fairhurst, 2000; Marschner and Marschner, 2012). But as increasing Cu concentrations may easily result in toxic effects, understanding the mechanisms that may optimize Cu use by the plant is a need. The study of those involved in achieving a coherent temporal integration of nutrient homeostasis and hormone responses will become increasingly relevant for food production. This is particularly important under the predicted effects of climate change on agriculture. Indeed it is around temperate agricultural where environmental stress conditions areas could have a massive impact on food production.
We herein collect disperse data on the interaction between Cu homeostasis and plant hormones, mainly abscisic acid (ABA) and ethylene which are related to abiotic stress. Besides, an in silico search for the previously described hormone-responsive cis-regulatory elements has been performed among the promoters of the family members from the high-affinity Cu transporters, termed COPT, in Arabidopsis thaliana and Oryza sativa. Finally, a model of the effect of putative modulators on target expression has been developed as a first step for deciphering the spatiotemporal codes for metalloprotein regulation in plants.
High-Affinity Copper Influx in Plants
Under aerobic conditions, Cu2+ is the most abundant form of copper in soil solution and probably enters plant root cells through divalent cation low-affinity transporters, such as some members of the ZIP family (ZIP2 and ZIP4; Wintz et al., 2003). However, this still has to be proven in vivo. Under metal deficiencies, plants acidify the external medium by using H+-ATPases (AHA; Santi and Schmidt, 2009). When Cu is scarce, plants use a Cu+-specific transport system based on Cu2+ reduction by plasma membrane NADPH-dependent cupric reductases FRO4 and FRO5 (Bernal et al., 2012) and on cytosolic uptake by high-affinity CTR-like transporters, denoted COPTs in plants (Sancenón et al., 2003; Figure 1). COPT substrate availability depends on both free external Cu (not bound to inorganic and organic complexes) and the Cu+/Cu2+ ratio according to external redox status conditions and the enzymatic activity of cuprooxidoreductases. The energetically expensive reductive strategy used for Cu+ uptake has been shown to be the predominant and ubiquitous mechanism for Cu acquisition in dicotyledons (Jouvin et al., 2012; Ryan et al., 2013). This redox strategy in Cu+ uptake could be an adaptation possibly required for specific high-affinity monovalent cation selection or/and for meeting specific Cu+ intracellular needs.
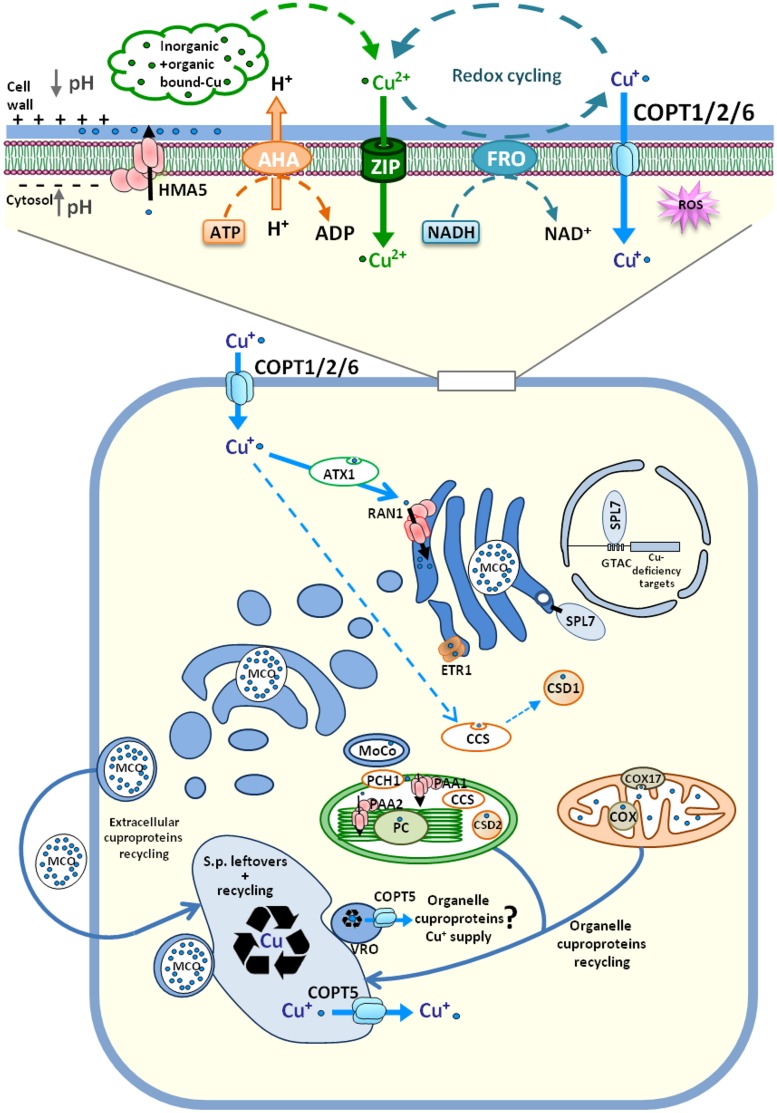
FIGURE 1.
Overview of Arabidopsis thaliana cellular Cu homeostasis. Cu+ uptake through plasma membrane transporters COPT1/COPT2/COPT6 depends on the activity of AHA H+-ATPase and FRO cuproreductases. COPT-mediated Cu+ transport is coupled to metallochaperones transfer and its delivery to targets. Cuprochaperone CCS provides Cu+ to cytosolic superoxide dismutase CSD1. ATX1 transfers Cu+ to P-type ATPase RAN1, located at the ER, where Cu+ is probably acquired by cuproproteins, such as multicopper oxidases (MCOs), the ethylene receptor (ETR1), and the molybdenum cofactor (MoCo). The Cu resulting from recycling and from the secretory pathway leftovers converges into the vacuole or into vacuolar-related organelles (VROs). The Cu+ supply to chloroplasts and mitochondria can take place from the lumen through the COPT5 efflux function. See the main text for details. The direction of Cu+ traffic is indicated by arrows and Cu content is indicated by different intensities of blue.
CTR-like Cu+ permeases function as trimers of small polypeptides. They have three transmembrane domains that contain the different conserved motifs involved in Cu+ binding from the extracytosolic compartment, translocation through the pore they form across the membrane and modulation of Cu+ delivery to metallochaperones for targeted distribution (Puig and Thiele, 2002; Pope et al., 2012). The aim of the present work is not to review currently available data on Arabidopsis COPT family members since they have been recently revised elsewhere (Peñarrubia et al., 2010; Puig, 2014). Briefly, Arabidopsis has a six-member family of COPTs (COPT1-COPT6). Each family member shows a tissue-specific expression pattern and performs specialized functions, as denoted by the phenotypes associated with copt mutant plants. Certain COPT proteins are regulated by Cu deficiency and are located in the plasma membrane (COPT1, COPT2, and COPT6), where they mediate Cu uptake from the external medium. COPT1 is expressed mostly in the root apex and pollen, where it participates in Cu+ uptake from soil and in Cu redistribution to reproductive organs (Sancenón et al., 2004). COPT2 is the most expressed member of the family, is present in the root elongation zone and responds to both Cu and iron (Fe) deficiencies. copt2 mutants are more resistant to double deficiency in Cu and Fe than wild type. The involvement of COPT2 in Fe metabolism could not result from a presumptive role in transporting Cu for a putative Cu-containing ferroxidase, which constitutes a common Fe–Cu connection in other organisms (Puig, 2014). Instead, a new and uncharacterized Cu–Fe crosstalk process has been suggested, where phosphate metabolism is also involved (Perea-García et al., 2013). COPT6 has been localized mainly at the vasculature of green tissues and reproductive organs where it can facilitate Cu redistribution under Cu scarcity (Jung et al., 2012; Garcia-Molina et al., 2013). Another COPT-type protein (COPT5) has been localized in the membrane of the prevacuolar/vacuolar compartment, where it is involved in the mobilization of Cu+ from the lumen to the cytosol in response to extreme Cu deficiency conditions (Garcia-Molina et al., 2011; Klaumann et al., 2011). COPT4 lacks the key methionine residues that are essential for Cu+ transport, which questions a possible role in Cu homeostasis. COPT3 is expressed at low levels and its function remains unsolved.
The characterization of the COPT family in rice (O. sativa), composed of seven members (OsCOPT1–OsCOPT7), uncovers the co-expression requirement of at least two family members to fully complement the yeast ctr1Δctr3Δ defect in high-affinity Cu uptake, except for OsCOPT7 (Yuan et al., 2011). OsCOPT2, OsCOPT3, or OsCOPT4 have to cooperate with OsCOPT6 to mediate an efficient Cu transport, which is consistent with their physical interactions analyzed by the split-ubiquitin system (Yuan et al., 2011). On the other hand, with proteins OsCOPT1 and OsCOPT5, the cooperation with a third component, the MtN3/saliva-type protein XA13, is required to mediate the low-affinity Cu transport both in yeast and rice (Yuan et al., 2010). OsCOPT6 and OsCOPT7 relate more to Arabidopsis tonoplast located COPT5. However, the regulation of the different rice members by Cu deficiency and their tissue expression patterns do not allow predicting their intracellular locations. Bimolecular fluorescence complementation (BiFC) demonstrated that the Vitis vinifera AtCOPT5 homolog VvCTr1 monomers self-interact (Martins et al., 2014). Since according to the Irving–Williams series Cu has the highest capacity for binding to organic compounds, the endogenous concentrations of other metals, such as Fe, manganese (Mn), or zinc (Zn), may also influence Cu homeostasis by affecting OsCOPTs expression (Yuan et al., 2011), probably aimed at avoiding Cu competence under other metals scarcity. In this sense, during Zn limitation Chlamydomonas reinhardtii sequesters Cu in lysosome-related compartments and this strategy has been suggested to prevent Zn protein mismetallation by Cu when Zn is scarce (Hong-Hermesdorf et al., 2014).
Despite the prevailing dogma of protein–protein interactions mediating Cu+ delivery from transporters to target cuproproteins, under specific situations, such as adaptation to variable metal environmental levels or during diurnal fluctuations in transporters expression (see the Temporal aspects in hormone and metal homeostasis section), the OH⋅ damage derived from Cu+ traffic could affect the molecules adjacent to Cu+ transporters. Thus, COPT-mediated Cu+ traffic has been shown to produce rapid increases in Ca2+ influx and K+-efflux (Rodrigo-Moreno et al., 2013). Membranes and/or directly Ca2+/K+ channels could be OH⋅ targets, and could rapidly respond to Cu+ entrance and initiate subsequent signaling pathways where Ca2+, ROS and ABA play crucial roles (Gilroy et al., 2014). As a result, plants incorporate Cu+ under Cu deficiency by paying both a high energy cost and the subsequent damage caused by high Cu+ reactivity (Ravet and Pilon, 2013). Cu deficiency also causes increased oxidative stress in plants through photosynthetic electron transport chain (PETC) blockage at the essential cuproprotein plastocyanin level (Ravet and Pilon, 2013; Yruela, 2013). Although the specific signaling pathways of the diverse ROS produced at different cellular locations are starting to be identified (Gilroy et al., 2014), a complex scenario is envisaged under Cu deficiency since plant cells would experience the ROS signaling crosstalk deriving from both the metal scarcity at chloroplasts and that caused by an increased COPT-mediated Cu+-entrance to the plasma membrane.
Intracellular Copper Traffic
Under non-stressed conditions, COPT-mediated Cu+ uptake is tightly coupled to its subsequent metabolic use. During this process, metallochaperones play an important role in transferring the metal between molecules, a process that kinetically competes with Cu+ dissociation in the solvent, which results in its immediate toxicity (Robinson and Winge, 2010; Figure 1). ATX1 is the cuprochaperone that delivers Cu+ to P-type ATPases (Cu+-ATPases), such as RAN1 located at the endoplasmic reticulum (ER). Subsequently, RAN1 pumps Cu+ into the lumen, where it is acquired by ER cuproproteins (Hirayama et al., 1999). Most extracellular and endomembrane cuproproteins follow the endocytic pathway to their final destinations and probably incorporate Cu upon transiting through the ER lumen or the trans-Golgi network. Thus, based on the relative abundance of cuproproteins, endocytic compartments are expected to display higher Cu levels than the nucleo-cytoplasmic space and, consequently, the main Cu+ flux should follow the COPT-ATX1-RAN1 pathway. In line with this, Cu delivery to cytosolic superoxide dismutase (Cu/ZnSOD) by the CCS cuprochaperone could represent a quantitatively minor Cu route (Robinson and Winge, 2010). Another Cu+-ATPase is HMA5. It is located at the plasma membrane, where it loads Cu+ into the xylem in roots and other organs which, under Cu excess conditions, functions in metal detoxification in both Arabidopsis and rice (Andrés-Colás et al., 2006; Kobayashi et al., 2008; Deng et al., 2013; Figure 1). Taken together, these facts predictably lead to Cu+ distribution between the nucleo-cytoplasmic and endocytic compartments by one or the other being favored, depending on the relative influx–efflux transport activity of COPT and Cu+-ATPases, respectively.
Chloroplasts are major consumers of Cu in plants, where it is incorporated into plastocyanin and Cu/ZnSOD, among other proteins (Ravet and Pilon, 2013). Once inside the chloroplast intermembrane space, the Cu+-loaded PCH1 cuprochaperone delivers Cu+ to the internal membrane-located P-type ATPase PAA1 (Blaby-Haas et al., 2014), which pumps Cu+ into the stroma (Shikanai and Fujii, 2013). PCH1 evolves by an alternative splicing event of the pre-mRNA encoding PAA1 (Blaby-Haas et al., 2014). Once inside the stroma, the CCS chaperone delivers Cu to chloroplastic Cu/ZnSOD and also to PAA2, which is the Cu+ P-type ATPase that delivers Cu to the thylakoids for plastocyanin supply (Abdel-Ghany et al., 2005; Blaby-Haas et al., 2014). The chloroplast caseinolytic protease (Clp) system is involved in specific PAA2 turnover under Cu excess in the stroma (Tapken et al., 2015). In mitochondria, Cu is required mainly for the assembly and activity of cytochrome c oxidase (COX) of the respiratory chain (Garcia et al., 2014). Cu delivery and insertion into COX is a complex process mediated by different metallochaperones present in the mitochondrial intermembrane space, such as COX17 (Balandin and Castresana, 2002; Attallah et al., 2007; Figure 1).
How Cu reaches organelles from an endosymbiotic origin, such as mitochondria and chloroplasts, is a poorly understood process. Since free Cu+ levels are extremely low in the cytosol (Rae et al., 1999), at least under Cu-limiting conditions, there must be other intracellular sources of Cu to ensure the arrival of Cu to organelles. Thus under nutrient deprivation, a putative Cu source could be the vacuole or vacuolar-related organelles (VROs). In these compartments, the valued metal arising from recycling metalloproteins and the scarce leftovers from secretory/endocytic pathways would converge (Figure 1). In addition, these compartments have been recently shown to participate in dynamic intracellular metal homeostasis (Blaby-Haas and Merchant, 2014; Hong-Hermesdorf et al., 2014). An increase in interorganellar communications with the membrane contact sites between mitochondria and the vacuole under nutrient deprivation stress has been recently described in yeast, which mainly serves for lipid and ion exchanges (Elbaz-Alon et al., 2014). If this were also the case in plants, organelles would be at “the end of the line” of the secretory pathway to acquire Cu which, under Cu deficiency, could lead to a competitive balance between previous Cu incorporation by in transit cuproproteins on the secretory pathway and the Cu leftovers available for the Cu supply of organelles.
Regulation of Gene Expression under Copper Deficiency
Cu deficiency in plants induces the reprogramming of a number of metabolic processes, which represent an adaptive mechanism that has developed to survive under adverse conditions. The transcriptional response to Cu deficiency in Arabidopsis is mediated by a Zn finger transcription factor family member named SQUAMOSA-PROMOTER BINDING-LIKE PROTEIN 7 (SPL7). SPL7 is essential for the response to Cu deficiency in vivo through its binding to GTAC motifs in the promoters of target genes (Yamasaki et al., 2009; Bernal et al., 2012). The repression mechanism in the presence of Cu could be mediated by the displacement of Zn2+ by Cu2+ from the Zn fingers of the SPL7 transcription factor (Sommer et al., 2010). SPL7 has been recently shown to interact with KIN17, a conserved curved DNA-binding domain protein that promotes Cu-deficiency responses and alleviates oxidative stress responses, perhaps by preserving cell integrity and plant growth under Cu scarcity (Garcia-Molina et al., 2014a). SPL7 displays an operative transmembrane domain that has been shown to insert the protein into endomembranes, most probably at the ER. ER stress, as a result of Cu deficiency, also activates the SPL7 function by changing its location to the nucleus through its functional bipartite nuclear localization sequence (NLS) overlapping zinc-finger2 (ZF2) within the SPL7 SBP-domain. SPL7 dimerization adds another regulatory feedback mechanism to the ER-stress and SPL7-mediated Cu homeostasis response since it could affect its nuclear localization (Garcia-Molina et al., 2014b). These processes render SPL7 a crucial Cu sensor molecule in two topological spaces where Cu is initially distributed (the nucleo-cytoplasmic and the lumen of secretory pathway compartments). Thus, SPL7 could be able to perceive both ER stress, mediated by Cu deficiency through its C-terminal part, and Cu status in the nucleo-cytoplasmic space, through its SBP domain, maybe driving a converging and regulated response under Cu scarcity. This response could be even more complex if other components of the 16 SPL family members participate in the heterodimerization regulatory mechanism with SPL7 (Garcia-Molina et al., 2014b).
It is worth mentioning that the SPL7-mediated miRNA expression serves Cu redistribution in order to establish a priority ranking for Cu delivery to essential cuproproteins by avoiding Cu incorporation in non-essential proteins. Hence, one of the SPL7-mediated strategies used when Cu is limiting consists in replacing non-essential cuproproteins by other metalloproteins, usually Fe proteins, which play a similar role, probably in order to save Cu for essential cuproproteins, such as plastocyanin. With superoxide dismutases (SODs), substituting the Cu form (Cu/ZnSOD) with the Fe counterpart (FeSOD) is done by SPL7 under Cu-limited conditions by expressing FeSOD mRNA (FSD1) and miR398, since miR398 targets Cu/ZnSOD mRNAs (CSD1 and CSD2) for degradation (Abdel-Ghany et al., 2005; Ravet and Pilon, 2013). Apart from metalloprotein substitution, another strategy for establishing priority ranking in Cu delivery under deficiency is the miRNA-mediated elimination of the first located cuproproteins on the pathway of Cu incorporation. In this case, other denoted Cu-miRNAs, such as miR408, target laccases (LAC3, LAC12, and LAC13; Yamasaki et al., 2007; Abdel-Ghany and Pilon, 2008). These cuproproteins predictably acquire Cu in the secretory pathway when transiting to their final destinations. Strikingly, miR408 accumulation and the subsequent down-regulation of the miR408 target genes in transgenic plants rescue developmental defects of the spl7 mutant. This indicates that diminished Cu delivery to the endomembrane system to en route laccases redounds in increased Cu acquisition by plastocyanin at chloroplasts (Zhang and Li, 2013; Zhang et al., 2014). These results further reinforce the suggested hypothesis for chloroplasts’ Cu supply dependency on secretory pathway leftovers under Cu deficiency conditions. Accordingly, the miR408 strategy could consist in shortening “the Cu metallation line” by eliminating cuproproteins, located at the beginning of the spatiotemporal Cu delivery pathway, in order to allow further Cu delivery to other cuproproteins. Thus in this way, the scarce Cu atoms would arrive at the essential cuproproteins in the organelles situated later in the intracellular Cu delivery pathway. Besides acting at the intracellular level, miRNA-mediated Cu redistribution also affects the extracellular Cu content through changes in laccase expression and it could even act at the systemic level as a phloem-mobile long-distance signals in response to nutrient deprivation (Buhtz et al., 2010).
Role of Copper Homeostasis in Hormone Biosynthesis and Perception
The role of Cu in hormone biosynthesis and perception has long since been known, mainly for its structural role in ethylene receptors (Rodríguez et al., 1999), and for molybdenum cofactor (MoCo) formation (Kuper et al., 2004) which is required for the biosynthesis of ABA and auxin indol-3-acetic (IAA) via indol-3-aldehyde, and because it is involved in the degradation of polyamines (PAs). More recent evidence has shown that Cu homeostasis also plays a prominent role on the salicylic acid (SA) signaling pathway (Wu et al., 2012; Yan and Dong, 2014) and is involved in ABA signal transduction as well as in the induction of nitric oxide (NO; Wimalasekera et al., 2011), an important and almost universal signaling molecule in plants.
As for other hormone-biosynthetic genes, data on the effect of Cu on ethylene production are accumulating. Thus, Cu excess has been reported to induce ethylene biosynthesis in broccoli seedlings (Jakubowicz et al., 2010) and in different organs of Arabidopsis plants (Arteca and Arteca, 2007), though this effect was not observed in the oldest leaves (Arteca and Arteca, 2007) or in seedlings (Lequeux et al., 2010). The Cu-inducible expression of ACC synthase (ACS) genes has been described in several species, such as potatoes, garden geraniums, and different tobacco cultivars (Schlagnhaufer et al., 1997). A range of Cu concentrations have also produced high ethylene levels, accompanied by toxicity symptoms in leaves and adventitious root formation in white poplars (Franchin et al., 2007). Therefore, in spite of some contrasting results, which may depend on the species or the organ studied, as well as on other factor such as the dosage and timing of the metal applied, it can be concluded that ethylene evolution is observed generally in response to Cu and other metals within a wide range of plant species (Maksymiec, 2007).
Five types of enzymes containing molybdenum (Mo) have been identified in plants to date. Among the plant enzymes containing MoCo, aldehyde oxidases merit special mention because several isoenzymes present a wide range of specificity for different aldehydes, and are also involved in the metabolism and signaling of different plant regulators. Thus Arabidopsis ALDEHYDE OXIDASE3 (AAO3) catalyzes the last step of ABA biosynthesis in conjunction with MoCo sulfurase (ABA3) and ALDEHYDE OXIDASE1 (AAO1) is involved in the biosynthetic pathway of auxins (Schwarz and Mendel, 2006). In these proteins, metal is bonded to pterins to form MoCo, whose biosynthesis has been widely studied and is closely related to the homeostasis of other metallic elements, such as Cu (Tejada-Jiménez et al., 2013). Although the role of Cu has not been fully elucidated, it is required for the activity of Cnx1G, an enzyme that catalyzes the insertion of Mo into molybdopterin. To date, this step of exchanging Cu and Mo appears to depend on unidentified cytoplasmic chaperones (Mendel and Kruse, 2012). In agreement with the Cu requirement for MoCo biosynthesis, increased Mo uptake has been observed under Cu deficiency in Brassica napus (Billard et al., 2014), although Cu treatment has been reported to inhibit in vitro MoCo biosynthesis (Kuper et al., 2004). This adds a second aspect to the interrelationship of Mo and Cu metabolisms, with Cu acting both positively and negatively during this process. In contrast, Cu-containing amine oxidases (CuAO) catalyze the oxidative de-amination of PA, such as putrescine and cadaverine, hence, degrading these plant regulators and affecting a number of the physiological processes they are involved in Cona et al. (2006).
In relation to the role of Cu in hormone perception and signaling, different components on both the ethylene biosynthesis and signal transduction pathways have been identified, some of which are differentially regulated depending on metal homeostasis, mainly Fe and Cu (Iqbal et al., 2013). The family of ethylene receptors (one of its members is ETR1) are cuproproteins where the Cu cofactor is necessary for hormone perception (Rodríguez et al., 1999). Further evidence for the importance of this metal on the ethylene-signaling pathway emerged with the discovery that EIN2, a central signal transducer on the ethylene-signaling pathway, has a significant homology to NRAMP divalent cation transporters (Alonso et al., 1999). It has been shown that both ETR1 and EIN2 are also involved in some ABA responses, and serve as integration nodes between both hormones signaling pathways (Wilkinson and Davies, 2010). In this context, it is also interesting to note that CuAO1 contributes to ABA- and PA-induced NO biosynthesis, and also influences ABA signal transduction (Wimalasekera et al., 2011).
In turn, SA is involved mainly in the systemic-acquired response (SAR), which is considered a plant immune response to pathogens. Transcriptional coregulator NONEXPRESSOR OF PATHOGENESIS-RELATED GENES1 (NPR1) activates the expression of SA-dependent defense genes, and Cu ions have been reported to be involved in its binding to SA in plants (Wu et al., 2012). NPR1 is a redox-regulated signaling protein and SA activates thioredoxin, which leads to NPR1 reduction, thus converting it into active monomers that are translocated from the cytosol to the nucleus by activating the defense gene expression (Tada et al., 2008). NPR1 and its homologs NPR3 and NPR4 are SA receptors (Pajerowska-Mukhtar et al., 2013). NPR1 functions in the crosstalk between SA and jasmonic acid (JA) signaling and is modulated by ethylene (Leon-Reyes et al., 2009), which indicates that NPR1 is a key player in integrating redox, metal and hormonal signaling (see the Putative modulators of the hormonal and copper homeostasis crosstalk section).
As previously indicated, although Cu is required for hormone biosynthesis and perception, an excess of this metal is not always associated with an increased accumulation or a higher sensitivity to the hormone but with toxicity symptoms. Indeed, under Cu excess, complex signal transduction networks produced by different hormonal interactions are involved in the morphological responses induced by Cu and other heavy metals. Auxins, ethylene, and ROS have been identified as major components of these morphological alterations (Potters et al., 2009) and in Arabidopsis, NO has been also shown to participate in the Cu excess-induced responses (Xiong et al., 2010; Peto et al., 2011). However, some results contradict one another probably due to differences in the concentration of the heavy metal applied, the treatment conditions, the age of the plant and the variety of tissues examined. In this sense, our current understanding of the molecular mechanisms involved in heavy metal toxicity and their interactions with phytohormones is quite limited and requires further studies.
Effects of Phytohormones on the Copper High Affinity COPT Transporters
Little is known about how hormones influence Cu homeostasis to adapt nutrient availability to the growth requirements while avoiding its toxic effects. Due to the scarce literature at this respect, as an approach to address hormone influence on Cu homeostasis we have undertaken an in silico search of the putative hormone-responsive elements in the promoters of the high-affinity COPT transporters in both A. thaliana and O. sativa (Table 1). The most abundant hormone-responsive elements present in the promoters from COPT family members were those involved in ABA and gibberellin (GA) signaling. While in Arabidopsis the highest number corresponded to GA-related elements, the opposite occurred in Oryza, in which the number of ABA-related elements was highest. Ethylene and auxin presented a lower number of cis-elements, similar in both species. JA-related elements were the less abundant in both Arabidopsis and Oryza, although this number was larger in rice (Table 1).
Table 1.
Analysis of the putative hormone-responsive cis-elements in the COPTs promoters.
| Arabidopsis thaliana | Oryza sativa | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hormones | Motifs | COPT1 | COPT2 | COPT3 | COPT5 | COPT6 | COPT1 | COPT2 | COPT3 | COPT4 | COPT5 | COPT6 | COPT7 | ||
| ABA | ABRE | 4 | 3 | – | 3 | 8 | 18 | 8 | 5 | 9 | 4 | 3 | 8 | 3 | 40 |
| DPBF | 1 | – | 1 | 2 | 2 | 6 | 5 | – | – | 2 | 2 | 1 | 1 | 11 | |
| DREB | – | – | – | 1 | – | 1 | – | – | – | – | – | – | 1 | 1 | |
| MYB-MYC BOX | 6 | 6 | 2 | 1 | 4 | 19 | 3 | 1 | 5 | 6 | 1 | 1 | 1 | 18 | |
| Others | – | – | – | – | 1 | 1 | – | – | 3 | – | 2 | 3 | 1 | 9 | |
| Total | 11 | 9 | 3 | 7 | 15 | 45 | 16 | 6 | 17 | 12 | 8 | 13 | 7 | 79 | |
| GA | CARE | – | 1 | – | 1 | 1 | 3 | 2 | 1 | – | – | – | – | – | 3 |
| GARE | 1 | 5 | 8 | 2 | 2 | 18 | – | 1 | – | – | 2 | – | – | 3 | |
| WRKY | 3 | 4 | 9 | 9 | 9 | 34 | 6 | 7 | 13 | 7 | 3 | 7 | 5 | 38 | |
| Others | 3 | 1 | 3 | 3 | 2 | 12 | 1 | 1 | 2 | – | 3 | – | – | 7 | |
| Total | 7 | 11 | 20 | 15 | 14 | 67 | 9 | 10 | 15 | 7 | 8 | 7 | 5 | 51 | |
| Ethylene | ERE | – | 1 | – | – | – | 1 | – | – | – | – | – | – | 1 | 1 |
| WBOX ERF3 | 2 | 4 | 4 | 4 | 7 | 21 | 2 | 4 | 5 | 1 | 1 | 3 | 2 | 18 | |
| Others | 1 | – | – | 1 | – | 2 | – | 1 | 1 | – | 1 | 2 | – | 5 | |
| Total | 3 | 5 | 4 | 5 | 7 | 24 | 2 | 5 | 6 | 1 | 2 | 5 | 3 | 24 | |
| AUXIN | ASF1 | – | 3 | 3 | 3 | 1 | 10 | 2 | 1 | 2 | 1 | 1 | 2 | – | 9 |
| SAUR | – | – | – | – | – | – | 1 | – | 1 | 1 | – | – | 1 | 4 | |
| SURE | – | – | 1 | 6 | 1 | 8 | 2 | – | 1 | 1 | 1 | 1 | 3 | 9 | |
| Others | – | – | – | 4 | – | 4 | – | 1 | – | – | – | – | – | 1 | |
| Total | – | 3 | 4 | 13 | 1 | 22 | 5 | 2 | 4 | 3 | 2 | 3 | 4 | 23 | |
| JA | GCC CORE | – | – | – | – | – | – | 1 | 1 | – | – | 7 | 3 | – | 12 |
| T/G BOX | 1 | – | – | – | – | 1 | 1 | – | – | – | – | – | – | 1 | |
| Total | 1 | – | – | – | – | 1 | 2 | 1 | – | – | 7 | 3 | – | 13 | |
The COPTs genomic sequences from Arabidopsis thaliana and Oryza sativa were obtained from the phytozome database. The promoter sequences were defined as the 1,000 bp upstream of the five prime untranslated region (5′-UTR) from the corresponding COPTs genes. The in silico analysis of the cis-elements present in the promoter regions was performed in the PLACE database. The commonest and most abundant cis-elements are shown for each hormone, including ABRE (ACGTGG/TC), DPBF (ACACNNG), DREB (A/GCCGACNT), MYB (CNGTTR), MYC (CANNTG), CARE (CAACTC), GARE (TAACAAA), WRKY (C/T)TGAC(T/C), ERE (AWTTCAAA), WBOX ERF3 (TGACY), ASF1 (TGACG), the SAUR motif (CATATG), SURE (GAGAC), the GCC core (GCCGCC) and T/G BOX (AACGTG). All the other cis-elements are included in the “Others” group.
The cis-acting elements and trans-acting factors involved in ABA-induced gene expression have been extensively analyzed and the main ABA responsive cis-elements are ABRE (ABA-responsive element), MYB/MYC and DPBF (Dc3-Promoter Binding Factor; Nakashima et al., 2014). The ABRE motifs have been identified in the promoter region of ABA-inducible genes and several basic leucine zipper (bZIP) proteins have been shown to bind these motifs (Fujita et al., 2011). The presence of ABRE motifs in the Arabidopsis COPTs promoters of the members located at the plasma membrane (Table 1) could be related to an early response to dehydration in vegetative parts with dark-induced senescence, as suggested by Simpson et al. (2003). The presence of drought- and ABA-related MYB and MYC motifs, mostly in the COPTs promoter members of the Arabidopsis plasma membrane-located transporters (Table 1), also suggests that these transporters could be regulated under developmental or stressful conditions by increasing ABA content. DPBF and DREB (dehydration responsive element binding) motifs, which have been described to play an important role in the ABA response in seeds and during early seedling establishment stages (Lopez-Molina and Chua, 2000), though scarce, are present in most Arabidopsis COPTs members (Table 1).
In the dehydration response context, it is worth noting that ABA plays an important role in the regulation of the stomatal behavior and gas exchange of dehydrated plants and, hence, in long-distance transport of nutrients. The effects of ABA on H+-ATPases in guard cells are well-known during stomata closure, and may involve Ca2+, light and ROS signaling (Brault et al., 2004; Zhang et al., 2004a). Ca2+ is located both up- and downstream of ABA signaling in guard cells, and Cu induces a peak in Ca2+ uptake and an increase in ROS (Rodrigo-Moreno et al., 2013). The convergence of both signaling pathways suggests that the ABA-mediated drought response and long-distance transport of nutrients might influence Cu homeostasis to some extent. Romero et al. (2012) reported that an ABA-deficient orange mutant, which is prone to fruit dehydration, was unable to induce early molecular responses to moderate water stress observed in wild-type fruit. Among the transcriptomic responses described, Citrus COPT1, COPT2, and COPT5 transporters were induced in parental, but not in the ABA-deficient fruit. Other di- and trivalent inorganic cation transporters were also induced only in parental fruit under those conditions, such as the Citrus Fe transporters IRT1, NRAMP1, NRAMP3, and FERRITIN. These results support the idea of an ABA/drought-mediated regulation of the genes involved in these metals homeostasis. Further research would be necessary to unravel whether the response to stresses increasing endogenous ABA levels in Arabidopsis plants also involves the regulation of these metal transporters. Within this context, studies including ABA-deficient plants, such as the aba2 mutant, would be even more explanatory. Nevertheless, the presence of ABA-related elements in the promoter sequences of COPTs in both Arabidopsis and Oryza (Table 1) points to a common regulation of these genes among different species.
Gibberellin is an essential phytohormone that controls many aspects of plant development and its antagonist role with ABA is well-known (Weiss and Ori, 2007). The WRKY family is one of the largest transcription factor families involved in GA and ABA responses, and in defense against pathogens and senescence in Arabidopsis (Eulgem et al., 2000). Accordingly, many putative cis-elements are present in COPTs promoters in both Arabidopsis and Oryza (Table 1). Most published WRKY proteins bind to the cognate cis-acting element containing the W-box in the promoter (Xie et al., 2005). For instance, OsWRKY71 is highly expressed in rice aleurone cells, where it represses the GA-induced Amy32bα-amylase promoter (Zhang et al., 2004b). The GA response GARE cis-element and the pyrimidine box (included in “Others”; Table 1) are partially involved in sugar repression in rice embryos (Morita et al., 1998). These GARE elements are required in Arabidopsis to modulate endogenous GA concentrations during seed germination (Ogawa et al., 2003).
ETHYLENE INSENSITIVE3 (EIN3) is a transcription factor that binds to the promoters of the target genes denoted ETHYLENE RESPONSIVE FACTORS (ERFs). ERFs might also play a role in ABA signaling, and they encode transcription factors that bind to the promoters containing a GCC motif by either activating or repressing target genes (Nakano et al., 2006). Ethylene WBOX ERF3 is the prevalent cis-element in COPTs promoters (Table 1). ERFs are often involved in the transcription of the defense genes that encode antifungal proteins, including chitinases and glucanases, in response to ethylene and fungal elicitors (Nishiuchi et al., 2004). Since differential Cu sensitivity between plants and fungal pathogens is often used for Cu-based fungicide treatments, the characterization of the interaction between ethylene signaling and Cu homeostasis could be relevant for optimizing fungal infected crops defense responses.
Among other environmental stimuli, root growth and development is highly dependent on nutrient availability. Changes in the root architecture have been reported at different nutrient levels, mainly phosphate, sulfate and nitrate, with specific effects on primary and lateral roots that are dependent on each nutrient (López-Bucio et al., 2003; Tian et al., 2014). However, responses to micronutrient levels, such as Cu, have also been observed (Kazan, 2013). Available data indicate that auxins seem to be the main hormonal mediators of root responses at soil nutrient levels. According to Lequeux et al. (2010), Cu excess remodelates root auxin distribution, and thus affects the mitotic activity of the meristem. Yuan et al. (2013) reported that this Cu-induced auxin redistribution involves the efflux carrier PIN1, which is responsible for root acropetal auxin transport. Spatio-temporal asymmetric auxin distribution has been indicated as a means of coordinating plant development (Tanaka et al., 2006). Auxin cis-elements have a similar distribution in both Arabidopsis and Oryza. SURE and ASF1 are the prevalent elements (Table 1) and are related to sulfur transport in the root epidermis and cortex cells (Maruyama-Nakashita et al., 2005). The SAUR motif is frequent in Oryza, but not in Arabidopsis (Table 1). This cis-element may play a significant role in secondary growth, especially secondary phloem and bark formation (Oh et al., 2003).
Cu is an essential nutrient for reproductive development, mainly due to diminished pollen viability under Cu scarcity, considered a typical symptom of Cu deficiency (Marschner and Marschner, 2012), although other effects, such as anther dehiscence and filament elongation, contribute to male fertility decrease. Accordingly, COPTs have been shown to be highly expressed in pollen and the anther filament (Sancenón et al., 2004; Garcia-Molina et al., 2011; Jung et al., 2012; Gayomba et al., 2013; Perea-García et al., 2013), and lack of COPT1 produces pollen defects (Sancenón et al., 2004). JA appears to be a critical signal for anther dehiscence, but other hormones, such as auxins and gibberellins, are also involved (Wilson et al., 2011). The JA receptor degrades a jasmonate-ZIM domain protein repressor (JAZ) of the JA responsive genes (Wasternack and Hause, 2013). This repressor binds to specific MYC and MYB transcription factors (Wager and Browse, 2012). Thus MYB21 and MYB24 function as direct targets of JAZs to regulate male fertility in A. thaliana (Song et al., 2011). Despite JA motifs being scarce in COPTs promoters, a GCC core element is present in four genes of the Oryza transporters, although not in Arabidopsis. This element, originally described in a gene encoding a plant defensin, is commonly used as a marker for the characterization of JA-dependent defense responses (Brown et al., 2003). JA, together with SA and ethylene, constitutes the main defense line against biotic stresses in plants. JA has been shown to act as a potent inducer of the expression of CuAO, induced by wounding and antagonizing with SA and ABA. Their action is mediated by oxidative stress since the wounding induction of CuAO leads to H2O2 production (Rea et al., 2002). Interestingly, the Cu-sensitive rice pathogen Xanthomonas oryzae pv oryzae (Xoo) strain exploits O. sativa Cu+- transport mechanisms through COPT1 and COPT5 to remove Cu from xylem vessels, where Xoo multiplies and spreads to cause disease (Yuan et al., 2010). So the different Cu sensitivity exhibited by distinct organisms suggests that modification of Cu homeostasis can be used by plants to fight pathogens.
Temporal Aspects in Hormone and Metal Homeostasis
Plant growth and development are regulated by the clock, hormonal changes and nutrient signals, suggesting a complex reciprocal relationship between the clock and metabolic signaling processes (Bolouri Moghaddam and Van den Ende, 2013). Understanding temporary regulation is essential to highlight the dynamic aspects in the homeostasis of transition metals and their integration with other processes. The central oscillator temporarily orders biological processes to adapt them to daily environmental cycles of light and temperature through a wide variety of regulatory mechanisms (Seo and Mas, 2014). Ninety percent of Arabidopsis genes exhibit diurnal or circadian regulation, and at least 30% of the transcriptome is regulated by the circadian clock (Michael et al., 2008). Most hormones fluctuate during light/dark cycles, which leads to a significant enrichment of circadian-regulated hormone-responsive genes (Nováková et al., 2005). Microarray analyses indicate that the transcripts involved in hormonal metabolism, perception and signaling are regulated by the circadian clock, which gates hormonal signals to better adapt daily physiological changes (Robertson et al., 2009). In turn, different phytohormones influence distinct plant circadian clock parameters, a fact that evidences a reciprocal interaction between hormone signaling and the clock. Whereas brassinosteroid shortens clock periodicity, ABA prolongs the circadian period in a light-dependent manner. Cytokinins delay the circadian phase and auxins regulate circadian amplitude and clock precision. Accordingly, hormone mutants exhibited predictable clock phenotypes (Hanano et al., 2006).
Ethylene biosynthesis is clock-regulated and peaks at the subjective day, which correlates with increased ACC SYNTHASE 8 (ACS8) transcript levels, subjected to circadian clock control. However, ethylene mutants do not alter circadian rhythms (Thain et al., 2004). Certain ACS and ACC OXIDASE (ACO) genes, which participate in ethylene biosynthesis, are circadianly regulated with a similar phase to ethylene emissions. Ethylene signaling components, such as EIN3, also display a similar oscillatory pattern of expression in response to light and sugar availability (Yanagisawa et al., 2003; Lee et al., 2006). XAP5 CIRCADIAN TIMEKEEPER (XCT) is also involved in blue light-dependent ethylene responses in Arabidopsis shoots (Ellison et al., 2011).
The expression of ABA- and JA-responsive genes also oscillates diurnally (Mizuno and Yamashino, 2008). The genes implicated in the synthesis of geranylgeranyl diphosphate (GGDP), an intermediate metabolite in isoprenoids synthesis, which leads to the production of chlorophylls, carotenoids, tocopherols, ABA and GA, are clock-regulated. Five of these genes peak in the subjective morning, as do ABA metabolic genes NINE-CIS-EPOXYCAROTENOID DIOXYGENASE3 (NCED3) and ABA DEFICIENT2 (ABA2). ABA levels fluctuate with diurnal rhythms in different species, and a significant overlap has been reported between the genes induced by either ABA or JA and the genes that oscillate with light/dark cycles. More than 40% of ABA-induced genes are circadianly regulated, and most peak in the subjective morning, in accordance with ABA levels (Covington et al., 2008). A number of ABA signaling genes also fluctuate during the day cycle. Thus several ABA receptors, some PP2CA-type constitutive repressors, the positive effectors of the ABA signaling pathway, such as SnRK2s and some members of the canonical ABF transcription factors, display diurnal cycles (Seung et al., 2012). It is noteworthy that unlike the above-described ABA biosynthetic genes, those involved in ABA signaling rarely peak at the same time, not even those that belong to the same protein family, and they display overlapping functions (Seung et al., 2012). The circadian clock regulates the diurnal expression of ABA-related gene ABAR/CHLH/GUN5 through TOC1 directly binding to its promoter (Legnaioli et al., 2009). TOC1 is a key repressor of gene expression (Huang et al., 2012). Conversely, ABA treatment induces TOC1 at midday, the subsequent down-regulation of clock gene expression and the lengthening of the free running clock period reveal a feedback loop that reciprocally links ABAR and TOC1 expressions (Legnaioli et al., 2009; Pokhilko et al., 2013). ABI3 also physically interacts with TOC1 (Kurup et al., 2000; Dekkers et al., 2008). Taken together, these results suggest that the ABA response is gated by the circadian clock for the fine tuning of environmental fluctuations in water availability, which occur mainly at midday (Seung et al., 2012).
The temporal aspects of Cu homeostasis are starting to be elucidated (Andrés-Colás et al., 2010; Peñarrubia et al., 2010; Perea-García et al., 2010). The requirements of metals vary during the diurnal cycles of light and darkness since photosynthesis is the process with the greatest metals requirements. To reconcile the dynamic changes between supply and demand, there are complex homeostasis networks whose aim is to maintain adequate metal levels in different tissues and developmental stages (Puig et al., 2007; Palmer and Guerinot, 2009; Puig and Peñarrubia, 2009). The fact that plasma membrane COPTs are regulated by Cu status through SPL7 has led to hypothesize that an intracellular oscillation of Cu is feasible since, under Cu-deficient conditions, induced plasma membrane COPT members are involved in metal uptake until sufficient Cu levels are reached and their expressions are inhibited. This self-regulatory feedback loop of Cu on its own transporters expression can cause oscillation in COPT expression driving to a cycling Cu concentration (Peñarrubia et al., 2010). Accordingly, Arabidopsis COPT1 and COPT2 transcripts are circadianly regulated (Haydon et al., 2011). If, as recently suggested, SPL7-mediated Cu deficiency stress responses can be perceived in the ER lumen (Garcia-Molina et al., 2014b), oscillations in Cu levels can take place in this compartment and perhaps at other subcellular locations, which depend on the endomembrane system for Cu supply. In this sense, cycling Cu uptake fluxes might drive the complex Cu muffling processes that take place in the entire cell.
Experimental data reinforce the interconnection between Cu homeostasis and the circadian rhythms in organisms other than plants (Perea-García et al., 2010). Recently, Yamada and Prosser (2014) reported the effects of Cu availability on the circadian clock of suprachiasmatic nuclei (SCN) in humans, where it appears to modulate the phase through glutamate signaling. In several animal systems, hormones, such as pineal-secreted melatonin, are connected with the circadian clock (Gamble et al., 2014). Melatonin has metal-chelating properties, which may contribute to reduce metal-induced toxicity (Romero et al., 2014). The wide distribution of melatonin in biological systems, including plants (Arnao and Hernández-Ruiz, 2014), delineates a putative hormonal, metal and clock interconnection that deserves further characterization. On the other hand, although the physiological and molecular mechanisms are obviously different in each group of organisms, the interconnections of the circadian clock with metals and hormones seem to be conserved. Plants are excellent models to study the effects of metal homeostasis on the circadian rhythms due to the widely presence of clocks in the different cells and tissues of vegetal organisms and the variety of well-established circadianly regulated processes.
Like hormones, different metals distinctly affect circadian clock parameters. The oscillation amplitude of the gene expression of two of the main components of the central oscillator, CIRCADIAN CLOCK ASSOCIATED1 (CCA1) and LATE ELONGATED HYPOCOTYL (LHY), increases under Cu deficiency, but their period remains mostly unaffected (Andrés-Colás et al., 2010). Magnesium (Mg) shortage also affects CCA1 and LHY1 expression, which increases at the end of the light period (Hermans et al., 2010). In contrast, Fe deficiency provokes period lengthening and Fe transport forms an interconnected loop with the central oscillator (Chen et al., 2013; Hong et al., 2013; Salomé et al., 2013; Tissot et al., 2014). It has been suggested that phytochromes and the functional state of chloroplasts would participate in a new retrograde Fe sensor-dependent route, which constitutes a central oscillator loop (Salomé et al., 2013).
Due to the pervasive oscillating nature in the expression of ion channels and nutrient transporters, the circadian signal spreads and, in turn, the nutritional status affects the circadian clock (Ko et al., 2009; Haydon et al., 2011). This mutual interaction between nutrient homeostasis and circadian clock components integrates temporary information and rhythmically coordinates, and also optimizes metabolism and physiology (Sanchez et al., 2011; Haydon et al., 2013). Therefore, understanding the mechanisms that continuously adjust the circadian clock is essential for improving plant fitness (Hotta et al., 2007). If the mutual influence between metal homeostasis and circadian rhythms is a widespread fact among higher eukaryotes, a wide range of new experimental approaches in plants is foreseen to address important biological questions, where plant research could facilitate the analysis of the temporal dimension contribution to metal-dependent cell processes.
Putative Modulators of the Hormonal and Copper Homeostasis Crosstalk
In this section we seek the putative spatiotemporal regulators that are the best candidates for the integration of signaling from circadian and/or light cycles, ROS, hormones and Cu homeostasis. These regulators, either activators or repressors, can respectively enhance or attenuate the gene expression of multiple target genes at different levels. Here we emphasize mainly the transcriptional and post-transcriptional regulation that contributes to temporarily adapt their functions (Figure 2).
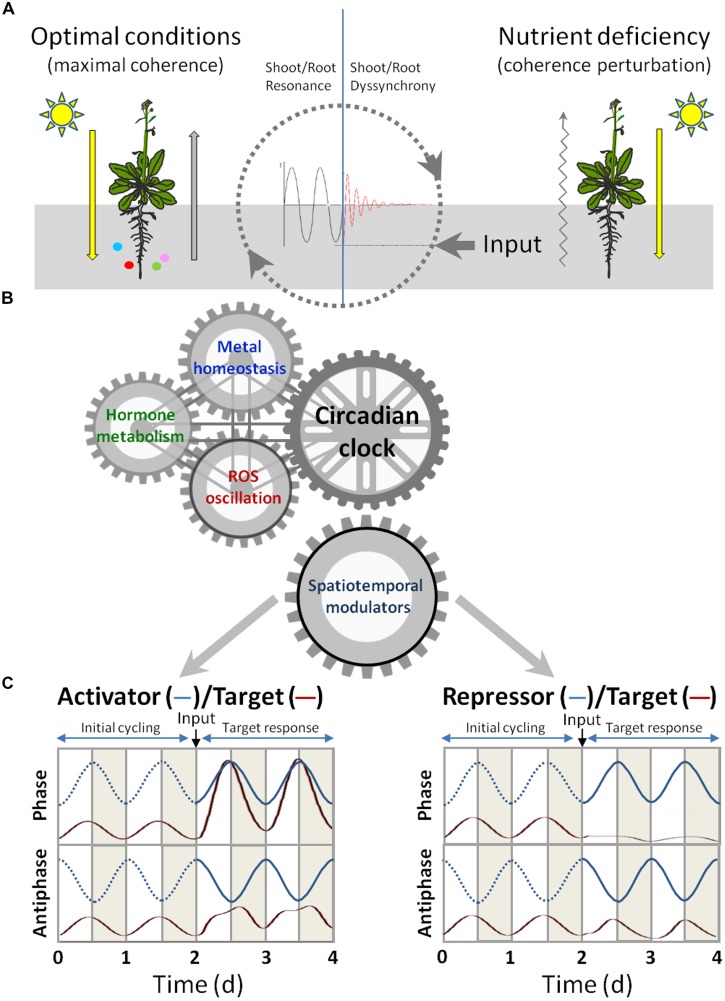
FIGURE 2.
Signaling crosstalk among circadian clock, hormones and metal homeostasis. (A) Under optimal conditions (left), light signaling influences the nutritional responses in roots and signals from adequate nutrient supply are coordinated with light signaling to drive maximal coherence between roots and shoots development. Under nutrient deficiency (right), a coherence perturbation is produced. (B) The circadian clock is a key integrator of environmental processes, such as light cycles and nutrient availability, and of endogenous cycles, such as ROS oscillation and hormone metabolism. The aim of the circadian clock-mediated regulation of spatio-temporal modulators is to optimize plant growth under both optimal cycles and altered conditions. (C) Model for the effect of activators and repressors on target gene expression. The time course of mRNA accumulation from modulators’ (blue lines) target genes (red lines) under circadian control is shown throughout four daily cycles (12 h light in white and 12 h dark in gray). The circadian oscillating levels of the activator (left) and repressor (right), running in phase (upper panel) or in antiphase (lower panel) with their targets, are shown for two cycles. The dotted lines indicate lack of modulator activity. The effects on the target expression of an input (indicated by an arrow) that activates the modulator is shown during the next two cycles. The changes underwent by the mRNA levels of the target genes are modeled by the differential equation shown in the main text. The numerical integration of Eq. 1 (with auxiliary Eq. 2) was performed by the COPASI program (Hoops et al., 2006) with σ = 0 (q = 0) for time 0–2 (i.e., the first two cycles) and with σ = 0.125 (q = 1.5) from time 2 onward. The values for other constants were A = 12, α = 5.
In previous sections, some putative modulators, such as NPR1 or melatonin, and their integrative functions in light, metal, hormonal and redox processes have been described. Other relevant candidates for these integrative functions are considered herein. Among them we find zinc finger (ZF) proteins, both classical transcriptional regulators, including Cu deficiency master regulator SPL7 and non-classical ZF, that act at the post-transcriptional level (Lee and Michel, 2014). In these metallo-regulatory proteins, metals ions can serve as “switches” during their nucleic acid recognition process. In fact, ZF proteins are potential targets for toxic metals, which may affect their structure and function (Hartwig, 2001). By way of example, it has been suggested that excess Cu2+ disrupts the glycine-rich proteins containing the RNA recognition motifs that are essential for post-transcriptional regulation and may impair the development of plants or animals (Qin et al., 2014). Although ZF proteins are usually identified also by the presence of similar sequence elements, most provide no in vivo evidence for the native metals ions that they bind and about the physiological consequences of metal substitution. In certain ZF proteins, transition metal coordination has been reported to be associated with rapid oxidation through radical formation by Fenton chemistry (Lee and Michel, 2014). This fact, in addition to the inability of metal binding by oxidized Cys residues, makes these proteins putative candidates for integrating metal and oxidative stress responses. ROS produced by metal deficiencies or during metal trafficking could act as potent intermediates in the crosstalk between metal homeostasis and the circadian clock. Indeed photosynthetic organisms cyclically suffer from the oxidative stress derived from light utilization in chloroplasts, and ROS cycling is integrated into the circadian clock. In fact peroxiredoxin oxidation–reduction cycles constitute a universal marker for circadian rhythms in all domains of life, including Arabidopsis (Edgar et al., 2012), and the genes involved in the synthesis of those compounds that prevent ROS production are clock-regulated and peak near subjective dawn (Lai et al., 2012). Consequently, it is predictable that, whenever possible, other oxidative processes could be temporarily separated from the intense light period in order to avoid both the saturation of antioxidative plant system capacity and interference with other ROS signaling pathways.
Recent research is uncovering the key integrators between light and hormone pathways, which may be also influenced by metal status. Among the factors that link the light signaling to GA and ABA antagonistic signaling we find PHYTOCHROME INTERACTING FACTOR3-LIKE5 (PIL5), a basic helix-loop-helix protein. The interaction of PIL5 with phytochromes induces its degradation through the 26S proteasome and promotes seed germination (Oh et al., 2006). PIL5 up-regulates GA and down-regulates ABA levels by modulating the expression of their metabolic genes (Seo et al., 2009). This regulation is mediated by non-classical ZF protein TZP (Tandem Zinc Protein) SOMNUS (Kim et al., 2008) which could be affected by Cu levels providing a putative link between metal homeostasis and hormone signaling. Another TZP protein, termed Cth2, is considered a master regulator of the stability of many target mRNAs to mediate global metabolic remodeling in Saccharomyces cerevisiae when Fe is scarce (Sanvisens et al., 2011). Cth2 is conserved in yeast, human and plants, which points to TZP proteins as putative mediators between hormones and metal homeostasis.
bHLH transcription factor ELONGATED HYPOCOTYL 5 (HY5) has been shown to integrate the light, clock and hormones, mainly auxin and cytokinin, signaling pathways by promoting the expression of negative regulators and by controlling the protein stability of HY5, respectively (Cluis et al., 2004; Vandenbussche et al., 2007). HY5 physically interacts with core clock component CCA1 (Andronis et al., 2008). In fact HY5 binds to ∼40% of the coding loci in the Arabidopsis genome, including miR408, a Cu-deficiency target involved in the post-transcriptional degradation of its target multicopper oxidases Laccase-type (LAC12 and LAC13; Lee et al., 2007; Zhang et al., 2011). It is noteworthy that SPL7 and HY5 have been shown to physically interact and co-regulate the expression of a large cohort of genes, including miR408, which integrate both transcriptional and post-transcriptional regulations in response to light and Cu status (Zhang et al., 2014). In addition, the sulfate assimilation regulatory circuit and glutathione accumulation are transcriptionally regulated by light and cross-interact with ABA (Cao et al., 2014). A possible interplay of HY5 with another transcription factor has been shown to participate in the regulation of sulfate assimilation by light (Koprivova et al., 2014). Interactions between light and hormone signaling pathways have long been observed, being HY5 one of the main integrators (Lau and Deng, 2010). HY5 binds the G-box (CACGTG) and G-box-related cis-elements as well as other factors, some of them related with ABA signaling (Wind et al., 2013). A competence in binding to G-box-related elements could drive a crosstalk between light and hormonal signaling. In this sense, HY5 interaction with the Cu deficiency master regulator SPL7 would participate in this competence, where the arrangement of Cu-deficiency and G-box-like cis-elements in the promoters of target genes could play an important role. Taken together, these results indicate that HY5 is a central, temporal integrator that adapts plant development to cycling nutrient homeostasis, hormone signaling and light/dark cycles, and that it is also a spatio-temporal modulator of multiple targets for oscillatory adaptation to changing environmental cues.
MicroRNAs are also key candidates for dynamic and long-distance abiotic stress signaling and ABA induce the accumulation of a number of small RNAs, including miR168. Its exclusive target is ARGONAUTE1 (AGO1), a key component of the RNA-inducing silencing complex. AGO1 alterations have been involved in ABA sensitivity, although the exact mechanism remains unclear (Earley et al., 2010). Nonetheless, much is known about how AGO1 is regulated by the miR168-mediated feedback loop during ABA responses. ABRE-binding transcription factors ABF (1–4) activate miR168a expression through the ABRE cis-element present in its promoter (Li et al., 2012). As mentioned before, several Cu-miRNAs are responsive to Cu-deficiency and govern the priority ranking for Cu-acquisition that ABA-dependent AGO1 regulation can alter. Since miR398 controls antioxidant activities, in addition to Cu-deficiency, other processes involving enhanced ROS regulate its expression (Zhu et al., 2011). Dynamic miR398 expression regulation under drought stress remains a controversial matter as it hinders assigning a clear role for ABA during the process (Ding et al., 2013).
Another key issue is how metal status signals, wherever perceived, are intracellularly communicated with the nucleus. A putative candidate for this communication is AP2-type transcription factor ABI4, which plays a central role in mediating mitochondrial and chloroplast retrograde signaling (Koussevitzky et al., 2007; Giraud et al., 2009; Wind et al., 2013). ABI4 was originally characterized for its role in ABA signaling (Finkelstein et al., 1998). ABA biosynthesis and signaling extensively interact with sugars. ABI4 directly regulates plastidial Cu+-ATPase PAA1 through binding to the PAA1 promoter. In fact, PAA1 and ABI4 expressions are mutually affected and lack of the PAA1 function provokes insensitivity to high glucose. It has been suggested that PAA1 functions in the bidirectional communication between the plastid and nucleus due to its essential plastid activity, and perhaps affects ROS, sugar levels, or even both (Lee et al., 2012).
During the evolution of eukaryotic organisms, including higher plants, the ability to modulate cycling changes in intracellular compartmentalized metal concentrations and metal traffic and efflux, denoted as metal muffling, had to be coordinated with other cyclic responses to environmental and endogenous cues for a certain developmental coherence to be achieved. Light is the main environmental signal for plant development, which is perceived in shoots, but also influences root growth. Nutrient uptake takes place in roots, but is regulated by light conditions. During these long-distance signaling processes, the crosstalk among ROS, hormones and the circadian clock are known to play a crucial function whose aim is to coordinate a temporary coherent developmental program between roots and shoots. Under optimal environmental conditions, global plant growth presents maximal coherence, whereas non-optimal conditions drive to different degrees of stress, depending on the severity and length of stress. Root abiotic stress, such as metal deficiency, can be considered to distort maximal coherence (Figure 2A).
The interplay among ROS, hormones and the circadian clock, and metal influence, during all these processes could play a key role in temporal responses to stress. In order to recover coherence, the effect of spatio-temporal modulators, acting at different levels of gene expression on their respective targets, should be considered to understand the dynamics of attaining plant fitness under stress conditions (Figure 2B). Modulators can be divided into activators and repressors by increasing or decreasing the expression of their target genes, respectively. Frequently, the expression of both modulators and their target genes are subjected to circadian regulation. Hence, their expression will be oscillatory with the same period (i.e., 1 day) but with a certain phase difference. For simplicity we will consider only two situations (phase and antiphase) to illustrate these interactions (Figure 2C). The following differential equation was used to model the change in the amount of target mRNA (m) in time (t, in days) when a specific signal or input, such as deficient metal supply, triggered the expression of the modulator:
| (1) |
where A is a positive constant that represents the circadianly regulated transcriptional strength, and α is the first-order kinetic constant for mRNA degradation. The effect of the modulator was introduced by the factor M. If the modulator is an activator driven by the circadian clock, then M equals B defined as:
| (2) |
where f is the phase-shift with the target (e.g., f = 0 for phase, f = π for antiphase) and q represents the strength of the modulator effect on the transcriptional rate of the target. Thereby, the oscillatory expression of the activator will act as a multiplicative factor on the target mRNA synthesis term in Eq. 1. On the other hand, if the modulator is a repressor, then M equals 1/B and the oscillatory expression of the repressor will attenuate the synthesis of the target mRNA. The relative strength of either modulator may be expressed by σ = q/A, which is the ratio of the modulator to circadian transcriptional strengths (see the legend of Figure 2C for further details of the parameter values). If the modulator is an activator, the phase synchrony between the activator and its target genes will redound on “constructive interference,” in analogy to the physical concept of waves adding up their amplitudes when in phase. As a result, the transcription of a target gene that oscillates with the same phase as the activator will be enhanced (Figure 2C). On the other hand, the target genes that oscillate in anti-phase with activators will display only a moderately increased and somewhat distorted transcriptional response. In contrast, the temporal phase synchrony between a repressor and its target gene will result in “destructive interference” (i.e., waves annihilating each other when interacting with opposite phases), which will lead to gross attenuation, or even the suppression, of the target’s oscillatory behavior, while the genes that shift to anti-phase will be much less affected by the repressor (Figure 2C). This oversimplified model emphasizes the importance of the initial relative phase of modulators and their target genes in the response to an input that triggers the modulator function. This mathematical approach can also help understand the differential target responses based on interactions under the cyclic gene expression of both modulators and their targets. Moreover, this model predicts that changes in the modulators phase would profoundly impact on their target responses and consequently these wave interferences can serve to modulate and integrate endogenous responses in order to improve plant fitness under stress conditions.
In summary, these observations highlight the importance of understanding the synchronization of the endogenous circadian system with environmental, nutritional and hormonal factors. Disrupted orchestration of circadian, nutritional, and hormonal rhythms occurs readily through environmental and/or genetic perturbations, and results in a state of circadian dyssynchrony. Chronic dyssynchrony ensues if endogenous hormonal or/and nutritional cycles are frequently altered, and modulators do not have the time required to complete resynchronization with environmental cycles. Impairment of the normal circadian rhythmicity produced by aberrant metabolic homeostasis could negatively affect a plethora of biological processes and drive to a misalignment of metabolic cycles with the central oscillator. If modulators of the circadian clock mechanism and their role in the integration with hormonal and nutrient cycles are identified, the prospect of targeting these mechanisms in plants offers much potential.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. S. Puig for critical reading of the manuscript. The authors gratefully acknowledge support from funds from Grant BIO2011-24848 (LP and AS), the predoctoral FPI fellowship to AC-S from the Spanish Ministry of Economy and Competitiveness, and the FEDER funds from the European Union.
References
- Abdel-Ghany S. E., Burkhead J. L., Gogolin K. A., Andrés-Colás N., Bodecker J. R., Puig S., et al. (2005). AtCCS is a functional homolog of the yeast copper chaperone Ccs1/Lys7. FEBS Lett. 579 2307–2312 10.1016/j.febslet.2005.03.025 [DOI] [PubMed] [Google Scholar]
- Abdel-Ghany S. E., Pilon M. (2008). MicroRNA-mediated systemic down-regulation of copper protein expression in response to low copper availability in Arabidopsis. J. Biol. Chem. 283 15932–15945 10.1074/jbc.M801406200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso J. M., Hirayama T., Roman G., Nourizadeh S., Ecker J. R. (1999). EIN2 a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284 2148–2152 10.1126/science.284.5423.2148 [DOI] [PubMed] [Google Scholar]
- Andrés-Colás N., Perea-García A., Puig S., Peñarrubia L. (2010). Deregulated copper transport affects Arabidopsis development especially in the absence of environmental cycles. Plant Physiol. 153 170–184 10.1104/pp.110.153676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrés-Colás N., Sancenón V., Rodríguez-Navarro S., Mayo S., Thiele D. J., Ecker J. R., et al. (2006). The Arabidopsis heavy metal P-type ATPase HMA5 interacts with metallochaperones and functions in copper detoxification of roots. Plant J. 45 225–236 10.1111/j.1365-313X.2005.02601.x [DOI] [PubMed] [Google Scholar]
- Andronis C., Barak S., Knowles S. M., Sugano S., Tobin E. M. (2008). The clock protein CCA1 and the bZIP transcription factor HY5 physically interact to regulate gene expression in Arabidopsis. Mol. Plant 1 58–67 10.1093/mp/ssm005 [DOI] [PubMed] [Google Scholar]
- Arnao M. B., Hernández-Ruiz J. (2014). Melatonin: plant growth regulator and/or biostimulator during stress? Trends Plant Sci. 19 789–797 10.1016/j.tplants.2014.07.006 [DOI] [PubMed] [Google Scholar]
- Arteca R. N., Arteca J. M. (2007). Heavy-metal-induced ethylene production in Arabidopsis thaliana. J. Plant Physiol. 164 1480–1488 10.1016/j.jplph.2006.09.006 [DOI] [PubMed] [Google Scholar]
- Attallah C. V., Welchen E., Pujol C., Bonnard G., Gonzalez D. H. (2007). Characterization of Arabidopsis thaliana genes encoding functional homologues of the yeast metal chaperone Cox19p, involved in cytochrome c oxidase biogenesis. Plant Mol. Biol. 65 343–355 10.1007/s11103-007-9224-9221 [DOI] [PubMed] [Google Scholar]
- Azouaou Z., Souvré A. (1993). Effects of copper deficiency on pollen fertility and nucleic acids in the durum wheat anther. Sex. Plant Reprod. 6 199–204 10.1007/BF00228649 [DOI] [Google Scholar]
- Balandin T., Castresana C. (2002). AtCOX17 an Arabidopsis homolog of the yeast copper chaperone COX17. Plant Physiol. 129 1852–1857 10.1104/pp.010963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal M., Casero D., Singh V., Wilson G. T., Grande A., Yang H., et al. (2012). Transcriptome sequencing identifies SPL7-regulated copper acquisition genes FRO4/FRO5 and the copper dependence of iron homeostasis in Arabidopsis. Plant Cell 24 738–761 10.1105/tpc.111.090431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billard V., Ourry A., Maillard A., Garnica M., Coquet L., Jouenne T., et al. (2014). Copper-deficiency in Brassica napus induces copper remobilization, molybdenum accumulation and modification of the expression of chloroplastic proteins. PLoS ONE 9:e109889 10.1371/journal.pone.0109889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaby-Haas C. E., Merchant S. S. (2014). Lysosome-related organelles as mediators of metal homeostasis. J. Biol. Chem. 289 28129–28136 10.1074/jbc.R114.592618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaby-Haas C. E., Padilla-Benavides T., Stübe R., Argüello J. M., Merchant S. S. (2014). Evolution of a plant-specific copper chaperone family for chloroplast copper homeostasis. Proc. Natl. Acad. Sci. U.S.A. 111:E5480-7 10.1073/pnas.1421545111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolouri Moghaddam M. R., Van den Ende W. (2013). Sugars, the clock and transition to flowering. Front. Plant Sci. 4:22 10.3389/fpls.2013.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault M., Amiar Z., Pennarun A. M., Monestiez M., Zhang Z., Cornel D., et al. (2004). Plasma membrane depolarization induced by abscisic acid in Arabidopsis suspension cells involves reduction of proton pumping in addition to anion channel activation, which are both Ca2+ dependent. Plant Physiol. 135 231–243 10.1104/pp.104.039255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. L., Kazan K., Mcgrath K. C., Maclean D. J., Manners J. M. (2003). A role for the GCC-box in jasmonate-mediated activation of the PDF1.2 gene of Arabidopsis. Plant Physiol. 132 1020–1032 10.1104/pp.102.017814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhtz A., Pieritz J., Springer F., Kehr J. (2010). Phloem small RNAs, nutrient stress responses, and systemic mobility. BMC Plant Biol. 10:64 10.1186/1471-2229-10-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M. J., Wang Z., Zhao Q., Mao J. L., Speiser A., Wirtz M., et al. (2014). Sulfate availability affects ABA levels and germination response to ABA and salt stress in Arabidopsis thaliana. Plant J. 77 604–615 10.1111/tpj.12407 [DOI] [PubMed] [Google Scholar]
- Cluis C. P., Mouchel C. F., Hardtke C. S. (2004). The Arabidopsis transcription factor HY5 integrates light and hormone signaling pathways. Plant J. 38 332–347 10.1111/j.1365-313X.2004.02052.x [DOI] [PubMed] [Google Scholar]
- Colvin R. A., Holmes W. R., Fontaine C. P., Maret W. (2010). Cytosolic zinc buffering and muffling: their role in intracellular zinc homeostasis. Metallomics 2 306–317 10.1039/B926662C [DOI] [PubMed] [Google Scholar]
- Cona A., Rea G., Angelini R., Federico R., Tavladoraki P. (2006). Functions of amine oxidases in plant development and defence. Trends Plant Sci. 11 80–88 10.1016/j.tplants.2005.12.009 [DOI] [PubMed] [Google Scholar]
- Covington M. F., Maloof J. N., Straume M., Kay S. A., Harmer S. L. (2008). Global transcriptome analysis reveals circadian regulation of key pathways in plant growth and development. Genome Biol. 9:R130 10.1186/gb-2008-9-8-r130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. Y., Wang Y., Shin L. J., Wu J. F., Shanmugam V., Tsednee M., et al. (2013). Iron is involved in the maintenance of circadian period length in Arabidopsis. Plant Physiol. 161 1409–1420 10.1104/pp.112.212068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekkers B. J. W., Schuurmans J. A. M. J., Smeekens S. C. M. (2008). Interaction between sugar and abscisic acid signalling during early seedling development in Arabidopsis. Plant Mol. Biol. 67 151–167 10.1007/s11103-008-9308-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng F., Yamaji N., Xia J., Ma J. F. (2013). A Member of the heavy metal P-type ATPase OsHMA5 is involved in xylem loading of copper in rice. Plant Physiol. 163 1353–1362 10.1104/pp.113.226225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Tao Y., Zhu C. (2013). Emerging roles of microRNAs in the mediation of drought stress response in plants. J. Exp. Bot. 64 3077–3086 10.1093/jxb/ert164 [DOI] [PubMed] [Google Scholar]
- Dobermann A., Fairhurst J. (2000). Rice: Nutrient Disorders and Nutrient Management. Philippines: Potash & Phosphate Institute of Canada (PPIC) and International Rice Research Institute. [Google Scholar]
- Earley K., Smith M. R., Weber R., Gregory B. D., Poethig R. S. (2010). An endogenous F-box protein regulates ARGONAUTE1 in Arabidopsis thaliana. Silence 1:15 10.1186/1758-907X-1-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R. S., Green E. W., Zhao Y., Van Ooijen G., Olmedo M., Qin X., et al. (2012). Peroxiredoxins are conserved markers of circadian rhythms. Nature 485 459–464 10.1038/nature11088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaz-Alon Y., Rosenfeld-Gur E., Shinder V., Futerman A. H., Geiger T., Schuldiner M. (2014). A dynamic interface between vacuoles and mitochondria in yeast. Dev. Cell 30 95–102 10.1016/j.devcel.2014.06.007 [DOI] [PubMed] [Google Scholar]
- Ellison C. T., Vandenbussche F., Van Der Straeten D., Harmer S. L. (2011). XAP5 CIRCADIAN TIMEKEEPER regulates ethylene responses in aerial tissues of Arabidopsis. Plant Physiol. 155 988–999 10.1104/pp.110.164277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulgem T., Rushton P. J., Robatzek S., Somssich I. E. (2000). The WRKY superfamily of plant transcription factors. Trends Plant Sci. 5 199–206 10.1016/S1360-1385(00)01600-9 [DOI] [PubMed] [Google Scholar]
- Finkelstein R. R., Li Wang M., Lynch T. J., Rao S., Goodman H. M. (1998). The Arabidopsis abscisic acid response locus ABI4 encodes an APETALA2 domain protein. Plant Cell 10 1043–1054 10.2307/3870689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchin C., Fossati T., Pasquini E., Lingua G., Castiglione S., Torrigiani P., et al. (2007). High concentrations of zinc and copper induce differential polyamine responses in micropropagated white poplar (Populus alba). Physiol. Plant. 130 77–90 10.1111/j.1399-3054.2007.00886.x [DOI] [Google Scholar]
- Fujita M., Fujita Y., Noutoshi Y., Takahashi F., Narusaka Y., Yamaguchi-Shinozaki K., et al. (2006). Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr. Opin. Plant Biol. 9 436–442 10.1016/j.pbi.2006.05.014 [DOI] [PubMed] [Google Scholar]
- Fujita Y., Fujita M., Shinozaki K., Yamaguchi-Shinozaki K. (2011). ABA-mediated transcriptional regulation in response to osmotic stress in plants. J. Plant Res. 124 509–525 10.1007/s10265-011-0412-3 [DOI] [PubMed] [Google Scholar]
- Gamble K. L., Berry R., Frank S. J., Young M. E. (2014). Circadian clock control of endocrine factors. Nat. Rev. Endocrinol. 10 466–475 10.1038/nrendo.2014.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Molina A., Andrés-Colás N., Perea-García A., Del Valle-Tascón S., Peñarrubia L., Puig S. (2011). The intracellular Arabidopsis COPT5 transport protein is required for photosynthetic electron transport under severe copper deficiency. Plant J. 65 848–860 10.1111/j.1365-313X.2010.04472.x [DOI] [PubMed] [Google Scholar]
- Garcia-Molina A., Andrés-Colás N., Perea-García A., Neumann U., Dodani S. C., Huijser P., et al. (2013). The Arabidopsis COPT6 transport protein functions in copper distribution under copper-deficient conditions. Plant Cell Physiol. 54 1378–1390 10.1093/pcp/pct088 [DOI] [PubMed] [Google Scholar]
- Garcia-Molina A., Xing S., Huijser P. (2014a). A conserved KIN17 curved DNA-binding domain protein assembles with SQUAMOSA PROMOTER-BINDING PROTEIN-LIKE7 to adapt Arabidopsis growth and development to limiting copper availability. Plant Physiol. 164 828–840 10.1104/pp.113.228239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Molina A., Xing S., Huijser P. (2014b). Functional characterisation of Arabidopsis SPL7 conserved protein domains suggests novel regulatory mechanisms in the Cu deficiency response. BMC Plant Biol. 14:231 10.1186/s12870-014-0231-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia L., Welchen E., Gonzalez D. H. (2014). Mitochondria and copper homeostasis in plants. Mitochondrion 19 269–274 10.1016/j.mito.2014.02.011 [DOI] [PubMed] [Google Scholar]
- Gayomba S. R., Jung H. I., Yan J., Danku J., Rutzke M. A., Bernal M., et al. (2013). The CTR/COPT-dependent copper uptake and SPL7-dependent copper deficiency responses are required for basal cadmium tolerance in A. thaliana. Metallomics 5 1262–1275 10.1039/c3mt00111c [DOI] [PubMed] [Google Scholar]
- Gilroy S., Suzuki N., Miller G., Choi W. G., Toyota M., Devireddy A. R., et al. (2014). A tidal wave of signals: calcium and ROS at the forefront of rapid systemic signaling. Trends Plant Sci. 19 623–630 10.1016/j.tplants.2014.06.013 [DOI] [PubMed] [Google Scholar]
- Giraud E., Van Aken O., Ho L. H., Whelan J. (2009). The transcription factor ABI4 is a regulator of mitochondrial retrograde expression of ALTERNATIVE OXIDASE1a. Plant Physiol. 150 1286–1296 10.1104/pp.109.139782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanano S., Domagalska M. A., Nagy F., Davis S. J. (2006). Multiple phytohormones influence distinct parameters of the plant circadian clock. Genes Cells 11 1381–1392 10.1111/j.1365-2443.2006.01026.x [DOI] [PubMed] [Google Scholar]
- Hartwig A. (2001). Zinc finger proteins as potential targets for toxic metal ions: differential effects on structure and function. Antioxid. Redox Signal. 3 625–634 10.1089/15230860152542970 [DOI] [PubMed] [Google Scholar]
- Haydon M. J., Bell L. J., Webb A. A. R. (2011). Interactions between plant circadian clocks and solute transport. J. Exp. Bot. 62 2333–2348 10.1093/jxb/err040 [DOI] [PubMed] [Google Scholar]
- Haydon M. J., Hearn T. J., Bell L. J., Hannah M. A., Webb A. A. R. (2013). Metabolic regulation of circadian clocks. Semin. Cell Dev. Biol. 24 414–421 10.1016/j.semcdb.2013.03.007 [DOI] [PubMed] [Google Scholar]
- Hermans C., Vuylsteke M., Coppens F., Craciun A., Inzé D., Verbruggen N. (2010). Early transcriptomic changes induced by magnesium deficiency in Arabidopsis thaliana reveal the alteration of circadian clock gene expression in roots and the triggering of abscisic acid-responsive genes. New Phytol. 187 119–131 10.1111/j.1469-8137.2010.03258.x [DOI] [PubMed] [Google Scholar]
- Hirayama T., Kieber J. J., Hirayama N., Kogan M., Guzman P., Nourizadeh S., et al. (1999). RESPONSIVE-TO-ANTAGONIST1 a Menkes/Wilson disease-related copper transporter, is required for ethylene signaling in Arabidopsis. Cell 97 383–393 10.1016/S0092-8674(00)80747-3 [DOI] [PubMed] [Google Scholar]
- Hong S., Kim S. A., Guerinot M. L., McClung C. R. (2013). Reciprocal interaction of the circadian clock with the iron homeostasis network in Arabidopsis. Plant Physiol. 161 893–903 10.1104/pp.112.208603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong-Hermesdorf A., Miethke M., Gallaher S. D., Kropat J., Dodani S. C., Chan J., et al. (2014). Subcellular metal imaging identifies dynamic sites of Cu accumulation in Chlamydomonas. Nat. Chem. Biol. 10 1034–1042 10.1038/nchembio.1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoops S., Sahle S., Gauges R., Lee C., Pahle J., Simus N., et al. (2006). COPASI — a COmplex PAthway SImulator. Bioinformatics 22 3067–3074 10.1093/bioinformatics/btl485 [DOI] [PubMed] [Google Scholar]
- Hotta C. T., Gardner M. J., Hubbard K. E., Baek S. J., Dalchau N., Suhita D., et al. (2007). Modulation of environmental responses of plants by circadian clocks. Plant Cell Environ. 30 333–349 10.1111/j.1365-3040.2006.01627.x [DOI] [PubMed] [Google Scholar]
- Huang W., Pérez-García P., Pokhilko A., Millar A. J., Antoshechkin I., Riechmann J. L., et al. (2012). Mapping the core of the Arabidopsis circadian clock defines the network structure of the oscillator. Science 335 75–79 10.1126/science.1219075 [DOI] [PubMed] [Google Scholar]
- Iqbal N., Trivellini A., Masood A., Ferrante A., Khan N. A. (2013). Current understanding on ethylene signaling in plants: the influence of nutrient availability. Plant Physiol. Biochem. 73 128–138 10.1016/j.plaphy.2013.09.011 [DOI] [PubMed] [Google Scholar]
- Jakubowicz M., Gałgańska H., Nowak W., Sadowski J. (2010). Exogenously induced expression of ethylene biosynthesis, ethylene perception, phospholipase D, and Rboh-oxidase genes in broccoli seedlings. J. Exp. Bot. 61 3475–3491 10.1093/jxb/erq177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouvin D., Weiss D. J., Mason T. F., Bravin M. N., Louvat P., Zhao F., et al. (2012). Stable isotopes of Cu and Zn in higher plants: evidence for Cu reduction at the root surface and two conceptual models for isotopic fractionation processes. Environ. Sci. Technol. 46 2652–2660 10.1021/es202587m [DOI] [PubMed] [Google Scholar]
- Jung H. I., Gayomba S. R., Rutzke M. A., Craft E., Kochian L. V., Vatamaniuk O. K. (2012). COPT6 is a plasma membrane transporter that functions in copper homeostasis in Arabidopsis and is a novel target of SQUAMOSA promoter-binding protein-like 7. J. Biol. Chem. 287 33252–33267 10.1074/jbc.M112.397810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazan K. (2013). Auxin and the integration of environmental signals into plant root development. Ann. Bot. 112 1655–1665 10.1093/aob/mct229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. H., Yamaguchi S., Lim S., Oh E., Park J., Hanada A., et al. (2008). SOMNUS, a CCCH-type Zinc finger protein in Arabidopsis, negatively regulates light-dependent seed germination downstream of PIL5. Plant Cell 20 1260–1277 10.1105/tpc.108.058859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaumann S., Nickolaus S. D., Fürst S. H., Starck S., Schneider S., Ekkehard Neuhaus H., et al. (2011). The tonoplast copper transporter COPT5 acts as an exporter and is required for interorgan allocation of copper in Arabidopsis thaliana. New Phytol. 192 393–404 10.1111/j.1469-8137.2011.03798.x [DOI] [PubMed] [Google Scholar]
- Ko G. Y., Shi L., Ko M. L. (2009). Circadian regulation of ion channels and their functions. J. Neurochem. 110 1150–1169 10.1111/j.1471-4159.2009.06223.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y., Kuroda K., Kimura K., Southron-Francis J. L., Furuzawa A., Iuchi S., et al. (2008). Amino acid polymorphisms in strictly conserved domains of a P-type ATPase HMA5 are involved in the mechanism of copper tolerance variation in Arabidopsis. Plant Physiol. 148 969–980 10.1104/pp.108.119933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprivova A., Calderwood A., Lee B. R., Kopriva S. (2014). Do PFT1 and HY5 interact in regulation of sulfate assimilation by light in Arabidopsis? FEBS Lett. 588 1116–1121 10.1016/j.febslet.2014.02.031 [DOI] [PubMed] [Google Scholar]
- Koussevitzky S., Nott A., Mockler T. C., Hong F., Sachetto-Martins G., Surpin M., et al. (2007). Signals from chloroplasts converge to regulate nuclear gene expression. Science 316 715–719 10.1126/science.1140516 [DOI] [PubMed] [Google Scholar]
- Kuper J., Llamas A., Hecht H. J., Mendel R. R., Schwarz G. (2004). Structure of the molybdopterin-bound Cnx1G domain links molybdenum and copper metabolism. Nature 430 803–806 10.1038/nature02681 [DOI] [PubMed] [Google Scholar]
- Kurup S., Jones H. D., Holdsworth M. J. (2000). Interactions of the developmental regulator ABI3 with proteins identified from developing Arabidopsis seeds. Plant J. 21 143–155 10.1046/j.1365-313x.2000.00663.x [DOI] [PubMed] [Google Scholar]
- Lai A. G., Doherty C. J., Mueller-Roeber B., Kay S. A., Schippers J. H., Dijkwel P. P. (2012). Circadian Clock-Associated 1 regulates ROS homeostasis and oxidative stress responses. Proc. Natl. Acad. Sci. U.S.A. 109 17129–17134 10.1073/pnas.1209148109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau O. S., Deng X. W. (2010). Plant hormone signaling lightens up: integrators of light and hormones. Curr. Opin. Plant Biol. 13 571–577 10.1016/j.pbi.2010.07.001 [DOI] [PubMed] [Google Scholar]
- Lee J. H., Deng X. W., Kim W. T. (2006). Possible role of light in the maintenance of EIN3/EIL1 stability in Arabidopsis seedlings. Biochem. Biophys. Res. Commun. 350 484–491 10.1016/j.bbrc.2006.09.074 [DOI] [PubMed] [Google Scholar]
- Lee J., He K., Stolc V., Lee H., Figueroa P., Gao Y., et al. (2007). Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell 19 731–749 10.1105/tpc.106.047688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. J., Michel S. L. (2014). Structural metal sites in nonclassical zinc finger proteins involved in transcriptional and translational regulation. Acc. Chem. Res. 47 2643–2650 10.1021/ar500182d [DOI] [PubMed] [Google Scholar]
- Lee S. A., Yoon E. K., Heo J. O., Lee M. H., Hwang I., Cheong H., et al. (2012). Analysis of Arabidopsis glucose insensitive growth mutants reveals the involvement of the plastidial copper transporter PAA1 in glucose-induced intracellular signaling. Plant Physiol. 159 1001–1012 10.1104/pp.111.191726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legnaioli T., Cuevas J., Mas P. (2009). TOC1 functions as a molecular switch connecting the circadian clock with plant responses to drought. EMBO J. 28 3745–3757 10.1038/emboj.2009.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon-Reyes A., Spoel S. H., De Lange E. S., Abe H., Kobayashi M., Tsuda S., et al. (2009). Ethylene modulates the role of NONEXPRESSOR OF PATHOGENESIS-RELATED GENES1 in cross talk between salicylate and jasmonate signaling. Plant Physiol. 149 1797–1809 10.1104/pp.108.133926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lequeux H., Hermans C., Lutts S., Verbruggen N. (2010). Response to copper excess in Arabidopsis thaliana: impact on the root system architecture, hormone distribution, lignin accumulation and mineral profile. Plant Physiol. Biochem. 48 673–682 10.1016/j.plaphy.2010.05.005 [DOI] [PubMed] [Google Scholar]
- Li W., Cui X., Meng Z., Huang X., Xie Q., Wu H., et al. (2012). Transcriptional regulation of Arabidopsis MIR168a and ARGONAUTE1 homeostasis in abscisic acid and abiotic stress responses. Plant Physiol. 158 1279–1292 10.1104/pp.111.188789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loladze I. (2014). Hidden shift of the ionome of plants exposed to elevated CO2 depletes minerals at the base of human nutrition. eLife 3:e02245 10.7554/eLife.02245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bucio J., Cruz-Ramírez A., Herrera-Estrella L. (2003). The role of nutrient availability in regulating root architecture. Curr. Opin. Plant Biol. 6 280–287 10.1016/S1369-5266(03)00035-9 [DOI] [PubMed] [Google Scholar]
- Lopez-Molina L., Chua N. H. (2000). A null mutation in a bZIP factor confers ABA-insensitivity in Arabidopsis thaliana. Plant Cell Physiol. 41 541–547 10.1093/pcp/41.5.541 [DOI] [PubMed] [Google Scholar]
- Maksymiec W. (2007). Signaling responses in plants to heavy metal stress. Acta Physiol. Plant. 29 177–187 10.1007/s11738-007-0036-3 [DOI] [Google Scholar]
- Marschner H., Marschner P. (2012). Marschner’s mineral nutrition of higher plants. Waltham: Academic press. [Google Scholar]
- Martins V., Bassil E., Hanana M., Blumwald E., Gerós H. (2014). Copper homeostasis in grapevine: functional characterization of the Vitis vinifera copper transporter 1. Planta 240 91–101 10.1007/s00425-014-2067-5 [DOI] [PubMed] [Google Scholar]
- Maruyama-Nakashita A., Nakamura Y., Watanabe-Takahashi A., Inoue E., Yamaya T., Takahashi H. (2005). Identification of a novel cis-acting element conferring sulfur deficiency response in Arabidopsis roots. Plant J. 42 305–314 10.1111/j.1365-313X.2005.02363.x [DOI] [PubMed] [Google Scholar]
- Mendel R. R., Kruse T. (2012). Cell biology of molybdenum in plants and humans. Biochim. Biophys. Acta 1823 1568–1579 10.1016/j.bbamcr.2012.02.007 [DOI] [PubMed] [Google Scholar]
- Michael T. P., Mockler T. C., Breton G., Mcentee C., Byer A., Trout J. D., et al. (2008). Network discovery pipeline elucidates conserved time-of-day-specific cis-regulatory modules. PLoS Genetics 4:e14 10.1371/journal.pgen.0040014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T., Yamashino T. (2008). Comparative transcriptome of diurnally oscillating genes and hormone-responsive genes in Arabidopsis thaliana: insight into circadian clock-controlled daily responses to common ambient stresses in plants. Plant Cell Physiol. 49 481–487 10.1093/pcp/pcn008 [DOI] [PubMed] [Google Scholar]
- Morita A., Umemura T. A., Kuroyanagi M., Futsuhara Y., Perata P., Yamaguchi J. (1998). Functional dissection of a sugar-repressed α-amylase gene (RAmy1A) promoter in rice embryos. FEBS Lett. 423 81–85 10.1016/S0014-5793(98)00067-2 [DOI] [PubMed] [Google Scholar]
- Myers S. S., Zanobetti A., Kloog I., Huybers P., Leakey A. D., Bloom A. J., et al. (2014). Increasing CO2 threatens human nutrition. Nature 510 139–142 10.1038/nature13179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T., Suzuki K., Ohtsuki N., Tsujimoto Y., Fujimura T., Shinshi H. (2006). Identification of genes of the plant-specific transcription-factor families cooperatively regulated by ethylene and jasmonate in Arabidopsis thaliana. J. Plant Res. 119 407–413 10.1007/s10265-006-0287-x [DOI] [PubMed] [Google Scholar]
- Nakashima K., Yamaguchi-Shinozaki K., Shinozaki K. (2014). The transcriptional regulatory network in the drought response and its crosstalk in abiotic stress responses including drought, cold, and heat. Front. Plant Sci. 5:170 10.3389/fpls.2014.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiuchi T., Shinshi H., Suzuki K. (2004). Rapid and transient activation of transcription of the ERF3 gene by wounding in tobacco leaves: possible involvement of NtWRKYs and autorepression. J. Biol. Chem. 279 55355–55361 10.1074/jbc.M409674200 [DOI] [PubMed] [Google Scholar]
- Nováková M., Motyka V., Dobrev P. I., Malbeck J., Gaudinová A., Vanková R. (2005). Diurnal variation of cytokinin, auxin and abscisic acid levels in tobacco leaves. J. Exp. Bot. 56 2877–2883 10.1093/jxb/eri282 [DOI] [PubMed] [Google Scholar]
- Ogawa M., Hanada A., Yamauchi Y., Kuwahara A., Kamiya Y., Yamaguchi S. (2003). Gibberellin biosynthesis and response during Arabidopsis seed germination. Plant Cell 15 1591–1604 10.1105/tpc.011650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E., Yamaguchi S., Kamiya Y., Bae G., Chung W. I., Choi G. (2006). Light activates the degradation of PIL5 protein to promote seed germination through gibberellin in Arabidopsis. Plant J. 47 124–139 10.1111/j.1365-313X.2006.02773.x [DOI] [PubMed] [Google Scholar]
- Oh S., Park S., Han K.-H. (2003). Transcriptional regulation of secondary growth in Arabidopsis thaliana. J. Exp. Bot. 54 2709–2722 10.1093/jxb/erg304 [DOI] [PubMed] [Google Scholar]
- Pajerowska-Mukhtar K. M., Emerine D. K., Mukhtar M. S. (2013). Tell me more: roles of NPRs in plant immunity. Trends Plant Sci. 18 402–411 10.1016/j.tplants.2013.04.004 [DOI] [PubMed] [Google Scholar]
- Palmer C. M., Guerinot M. L. (2009). Facing the challenges of Cu, Fe and Zn homeostasis in plants. Nat. Chem. Biol. 5 333–340 10.1038/nchembio.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñarrubia L., Andrés-Colás N., Moreno J., Puig S. (2010). Regulation of copper transport in Arabidopsis thaliana: a biochemical oscillator? J. Biol. Inorg. Chem. 15 29–36 10.1007/s00775-009-0591-8 [DOI] [PubMed] [Google Scholar]
- Perea-García A., Andrés-Colás N., Peñarrubia L. (2010). Copper homeostasis influences the circadian clock in Arabidopsis. Plant Signal. Behav. 5 1237–1240 10.4161/psb.5.10.12920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea-García A., Garcia-Molina A., Andrés-Colás N., Vera-Sirera F., Pérez-Amador M. A., Puig S., et al. (2013). Arabidopsis copper transport protein COPT2 participates in the cross talk between iron deficiency responses and low-phosphate signaling. Plant Physiol. 162 180–194 10.1104/pp.112.212407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peto A., Lehotai N., Lozano-Juste J., León J., Tari I., Erdei L., et al. (2011). Involvement of nitric oxide and auxin in signal transduction of copper-induced morphological responses in Arabidopsis seedlings. Ann. Bot. 108 449–457 10.1093/aob/mcr176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokhilko A., Mas P., Millar A. J. (2013). Modelling the widespread effects of TOC1 signalling on the plant circadian clock and its outputs. BMC Syst. Biol. 7:23 10.1186/1752-0509-7-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope C. R., Flores A. G., Kaplan J. H., Unger V. M. (2012). Structure and function of copper uptake transporters. Curr. Top. Membr. 69 97–112 10.1016/B978-0-12-394390-3.00004-5 [DOI] [PubMed] [Google Scholar]
- Potters G., Pasternak T. P., Guisez Y., Jansen M. A. K. (2009). Different stresses, similar morphogenetic responses: integrating a plethora of pathways. Plant Cell Environ. 32 158–169 10.1111/j.1365-3040.2008.01908.x [DOI] [PubMed] [Google Scholar]
- Puig S. (2014). Function and regulation of the plant COPT family of high-affinity copper transport proteins. Adv. Bot. 2014:9 10.1155/2014/476917 [DOI] [Google Scholar]
- Puig S., Andrés-Colás N., García-Molina A., Peñarrubia L. (2007). Copper and iron homeostasis in Arabidopsis: responses to metal deficiencies, interactions and biotechnological applications. Plant Cell Environ. 30 271–290 10.1111/j.1365-3040.2007.01642.x [DOI] [PubMed] [Google Scholar]
- Puig S., Peñarrubia L. (2009). Placing metal micronutrients in context: transport and distribution in plants. Curr. Opin. Plant Biol. 12 299–306 10.1016/j.pbi.2009.04.008 [DOI] [PubMed] [Google Scholar]
- Puig S., Thiele D. J. (2002). Molecular mechanisms of copper uptake and distribution. Curr. Opin. Chem. Biol. 6 171–180 10.1016/S1367-5931(02)00298-3 [DOI] [PubMed] [Google Scholar]
- Qin X., Huang Q., Zhu L., Xiao H., Yao G., Huang W., et al. (2014). Interaction with Cu2+ disrupts the RNA binding affinities of RNA recognition motif containing protein. Biochem. Biophys. Res. Commun. 444 116–120 10.1016/j.bbrc.2014.01.006 [DOI] [PubMed] [Google Scholar]
- Rae T. D., Schmidt P. J., Pufahl R. A., Culotta V. C., O’Halloran T. V. (1999). Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science 284 805–808 10.1126/science.284.5415.805 [DOI] [PubMed] [Google Scholar]
- Ravet K., Pilon M. (2013). Copper and iron homeostasis in plants: the challenges of oxidative stress. Antioxid. Redox Signal. 19 919–932 10.1089/ars.2012.5084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea G., Metoui O., Infantino A., Federico R., Angelini R. (2002). Copper amine oxidase expression in defense responses to wounding and Ascochyta rabiei invasion. Plant Physiol. 128 865–875 10.1104/pp.010646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson F. C., Skeffington A. W., Gardner M. J., Webb A. A. (2009). Interactions between circadian and hormonal signalling in plants. Plant Mol. Biol. 69 419–427 10.1007/s11103-008-9407-4 [DOI] [PubMed] [Google Scholar]
- Robinson N. J., Winge D. R. (2010). Copper metallochaperones. Annu. Rev. Biochem. 79 537–562 10.1146/annurev-biochem-030409-143539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo-Moreno A., Andrés-Colás N., Poschenrieder C., Gunsé B., Peñarrubia L., Shabala S. (2013). Calcium- and potassium-permeable plasma membrane transporters are activated by copper in Arabidopsis root tips: linking copper transport with cytosolic hydroxyl radical production. Plant Cell Environ. 36 844–855 10.1111/pce.12020 [DOI] [PubMed] [Google Scholar]
- Rodríguez F. I., Esch J. J., Hall A. E., Binder B. M., Eric Schaller G., Bleecker A. B. (1999). A copper cofactor for the ethylene receptor ETR1 from Arabidopsis. Science 283 996–998 10.1126/science.283.5404.996 [DOI] [PubMed] [Google Scholar]
- Romero A., Ramos E., De Los Ríos C., Egea J., Del Pino J., Reiter R. J. (2014). A review of metal-catalyzed molecular damage: protection by melatonin. J. Pineal Res. 56 343–370 10.1111/jpi.12132 [DOI] [PubMed] [Google Scholar]
- Romero P., Rodrigo M. J., Alférez F., Ballester A. R., González-Candelas L., Zacarías L., et al. (2012). Unravelling molecular responses to moderate dehydration in harvested fruit of sweet orange (Citrus sinensis L. Osbeck) using a fruit-specific ABA-deficient mutant. J. Exp. Bot. 63 2753–2767 10.1093/jxb/err461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio V., Bustos R., Irigoyen M. L., Cardona-López X., Rojas-Triana M., Paz-Ares J. (2009). Plant hormones and nutrient signaling. Plant Mol. Biol. 69 361–373 10.1007/s11103-008-9380-y [DOI] [PubMed] [Google Scholar]
- Ryan B. M., Kirby J. K., Degryse F., Harris H., Mclaughlin M. J., Scheiderich K. (2013). Copper speciation and isotopic fractionation in plants: uptake and translocation mechanisms. New Phytol. 199 367–378 10.1111/nph.12276 [DOI] [PubMed] [Google Scholar]
- Salomé P. A., Oliva M., Weigel D., Krämer U. (2013). Circadian clock adjustment to plant iron status depends on chloroplast and phytochrome function. EMBO J. 32 511–523 10.1038/emboj.2012.330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancenón V., Puig S., Mateu-Andrés I., Dorcey E., Thiele D. J., Peñarrubia L. (2004). The Arabidopsis copper transporter COPT1 functions in root elongation and pollen development. J. Biol. Chem. 279 15348–15355 10.1074/jbc.M313321200 [DOI] [PubMed] [Google Scholar]
- Sancenón V., Puig S., Mira H., Thiele D., Peñarrubia L. (2003). Identification of a copper transporter family in Arabidopsis thaliana. Plant Mol. Biol. 51 577–587 10.1023/a:1022345507112 [DOI] [PubMed] [Google Scholar]
- Sanchez A., Shin J., Davis S. J. (2011). Abiotic stress and the plant circadian clock. Plant Signal. Behav. 6 223–231 10.4161/psb.6.2.14893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi S., Schmidt W. (2009). Dissecting iron deficiency-induced proton extrusion in Arabidopsis roots. New Phytol. 183 1072–1084 10.1111/j.1469-8137.2009.02908.x [DOI] [PubMed] [Google Scholar]
- Sanvisens N., Bañó M. C., Huang M., Puig S. (2011). Regulation of ribonucleotide reductase in response to iron deficiency. Mol. Cell 44 759–769 10.1016/j.molcel.2011.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlagnhaufer C. D., Arteca R. N., Pell E. J. (1997). Sequential expression of two 1-aminocyclopropane-1-carboxylate synthase genes in response to biotic and abiotic stresses in potato (Solanum tuberosum L.) leaves. Plant Mol. Biol. 35 683–688 10.1023/A:1005857717196 [DOI] [PubMed] [Google Scholar]
- Schwarz G., Mendel R. R. (2006). Molybdenum cofactor biosynthesis and molybdenum enzymes. Annu. Rev. Plant Biol. 57 623–647 10.1146/annurev.arplant.57.032905.105437 [DOI] [PubMed] [Google Scholar]
- Seo M., Nambara E., Choi G., Yamaguchi S. (2009). Interaction of light and hormone signals in germinating seeds. Plant Mol. Biol. 69 463–472 10.1007/s11103-008-9429-y [DOI] [PubMed] [Google Scholar]
- Seo P. J., Mas P. (2014). Multiple layers of posttranslational regulation refine circadian clock activity in Arabidopsis. Plant Cell 26 79–87 10.1105/tpc.113.119842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seung D., Risopatron J. P., Jones B. J., Marc J. (2012). Circadian clock-dependent gating in ABA signalling networks. Protoplasma 249 445–457 10.1007/s00709-011-0304-3 [DOI] [PubMed] [Google Scholar]
- Shikanai T., Fujii S. (2013). Function of PPR proteins in plastid gene expression. RNA Biol. 10 1263–1273 10.4161/rna.25207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson S. D., Nakashima K., Narusaka Y., Seki M., Shinozaki K., Yamaguchi-Shinozaki K. (2003). Two different novel cis-acting elements of erd1 a clpA homologous Arabidopsis gene function in induction by dehydration stress and dark-induced senescence. Plant J. 33 259–270 10.1046/j.1365-313X.2003.01624.x [DOI] [PubMed] [Google Scholar]
- Sommer F., Kropat J., Malasarn D., Grossoehme N. E., Chen X., Giedroc D. P., et al. (2010). The CRR1 nutritional copper sensor in Chlamydomonas contains two distinct metal-responsive domains. Plant Cell 22 4098–4113 10.1105/tpc.110.080069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S., Qi T., Huang H., Ren Q., Wu D., Chang C., et al. (2011). The jasmonate-ZIM domain proteins interact with the R2R3-MYB transcription factors MYB21 and MYB24 to affect jasmonate-regulated stamen development in Arabidopsis. Plant Cell 23 1000–1013 10.1105/tpc.111.083089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada Y., Spoel S. H., Pajerowska-Mukhtar K., Mou Z., Song J., Wang C., et al. (2008). Plant immunity requires conformational charges of NPR1 via S-nitrosylation and thioredoxins. Science 321 952–956 10.1126/science.1156970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H., Dhonukshe P., Brewer P. B., Friml J. (2006). Spatiotemporal asymmetric auxin distribution: a means to coordinate plant development. Cell. Mol. Life Sci. 63 2738–2754 10.1007/s00018-006-6116-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapken W., Kim J., Nishimura K., Van Wijk K. J., Pilon M. (2015). The Clp protease system is required for copper ion-dependent turnover of the PAA2/HMA8 copper transporter in chloroplasts. New Phytol. 205 511–517 10.1111/nph.13093 [DOI] [PubMed] [Google Scholar]
- Tejada-Jiménez M., Chamizo-Ampudia A., Galván A., Fernández E. and Llamas, Á. (2013). Molybdenum metabolism in plants. Metallomics 5 1191–1203 10.1039/c3mt00078h [DOI] [PubMed] [Google Scholar]
- Thain S. C., Vandenbussche F., Laarhoven L. J., Dowson-Day M. J., Wang Z. Y., Tobin E. M., et al. (2004). Circadian rhythms of ethylene emission in Arabidopsis. Plant Physiol. 136 3751–3761 10.1104/pp.104.042523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H., De Smet I., Ding Z. (2014). Shaping a root system: regulating lateral versus primary root growth. Trends Plant Sci. 19 426–431 10.1016/j.tplants.2014.01.007 [DOI] [PubMed] [Google Scholar]
- Tissot N., Przybyla-Toscano J., Reyt G., Castel B., Duc C., Boucherez J., et al. (2014). Iron around the clock. Plant Sci. 224 112–119 10.1016/j.plantsci.2014.03.015 [DOI] [PubMed] [Google Scholar]
- Vandenbussche F., Habricot Y., Condiff A. S., Maldiney R., Straeten D. V., Ahmad M. (2007). HY5 is a point of convergence between cryptochrome and cytokinin signalling pathways in Arabidopsis thaliana. Plant J. 49 428–441 10.1111/j.1365-313X.2006.02973.x [DOI] [PubMed] [Google Scholar]
- Wager A., Browse J. (2012). Social network: JAZ protein interactions expand our knowledge of jasmonate signaling. Front. Plant Sci. 3:41 10.3389/fpls.2012.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C., Hause B. (2013). Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 111 1021–1058 10.1093/aob/mct067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss D., Ori N. (2007). Mechanisms of cross talk between gibberellin and other hormones. Plant Physiol. 144 1240–1246 10.1104/pp.107.100370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S., Davies W. J. (2010). Drought, ozone, ABA and ethylene: new insights from cell to plant to community. Plant Cell Environ. 33 510–525 10.1111/j.1365-3040.2009.02052.x [DOI] [PubMed] [Google Scholar]
- Wilson Z. A., Song J., Taylor B., Yang C. (2011). The final split: the regulation of anther dehiscence. J. Exp. Bot. 62 1633–1649 10.1093/jxb/err014 [DOI] [PubMed] [Google Scholar]
- Wimalasekera R., Villar C., Begum T., Scherer G. F. (2011). COPPER AMINE OXIDASE1 (CuAO1) of Arabidopsis thaliana contributes to abscisic acid-and polyamine-induced nitric oxide biosynthesis and abscisic acid signal transduction. Mol. Plant 4 663–678 10.1093/mp/ssr023 [DOI] [PubMed] [Google Scholar]
- Wind J. J., Peviani A., Snel B., Hanson J., Smeekens S. C. (2013). ABI4: versatile activator and repressor. Trends Plant Sci. 18 125–132 10.1016/j.tplants.2012.10.004 [DOI] [PubMed] [Google Scholar]
- Wintz H., Fox T., Wu Y. Y., Feng V., Chen W., Chang H. S., et al. (2003). Expression profiles of Arabidopsis thaliana in mineral deficiencies reveal novel transporters involved in metal homeostasis. J. Biol. Chem. 278 47644–47653 10.1074/jbc.M309338200 [DOI] [PubMed] [Google Scholar]
- Wu Y., Zhang D., Chu J., Boyle P., Wang Y., Brindle I., et al. (2012). The Arabidopsis NPR1 protein is a receptor for the plant defense hormone salicylic acid. Cell Rep. 1 639–647 10.1016/j.celrep.2012.05.008 [DOI] [PubMed] [Google Scholar]
- Xie Z., Zhang Z. L., Zou X., Huang J., Ruas P., Thompson D., et al. (2005). Annotations and functional analyses of the rice WRKY gene superfamily reveal positive and negative regulators of abscisic acid signaling in aleurone cells. Plant Physiol. 137 176–189 10.1104/pp.104.054312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J., Fu G., Tao L., Zhu C. (2010). Roles of nitric oxide in alleviating heavy metal toxicity in plants. Arch. Biochem. Biophys. 497 13–20 10.1016/j.abb.2010.02.014 [DOI] [PubMed] [Google Scholar]
- Yamada Y., Prosser R. A. (2014). Copper chelation and exogenous copper affect circadian clock phase resetting in the suprachiasmatic nucleus in vitro. Neuroscience 256 252–261 10.1016/j.neuroscience.2013.10.033 [DOI] [PubMed] [Google Scholar]
- Yamasaki H., Abdel-Ghany S. E., Cohu C. M., Kobayashi Y., Shikanai T., Pilon M. (2007). Regulation of copper homeostasis by micro-RNA in Arabidopsis. J. Biol. Chem. 282 16369–16378 10.1074/jbc.M700138200 [DOI] [PubMed] [Google Scholar]
- Yamasaki H., Hayashi M., Fukazawa M., Kobayashi Y., Shikanai T. (2009). SQUAMOSA PROMOTER BINDING PROTEIN-LIKE7 is a central regulator for copper homeostasis in Arabidopsis. Plant Cell 21 347–361 10.1105/tpc.108.060137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S., Dong X. (2014). Perception of the plant immune signal salicylic acid. Curr. Opin. Plant Biol. 20 64–68 10.1016/j.pbi.2014.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa S., Yoo S. D., Sheen J. (2003). Differential regulation of EIN3 stability by glucose and ethylene signalling in plants. Nature 425 521–525 10.1038/nature01984 [DOI] [PubMed] [Google Scholar]
- Yruela I. (2013). Transition metals in plant photosynthesis. Metallomics 5 1090–1109 10.1039/c3mt00086a [DOI] [PubMed] [Google Scholar]
- Yuan H. M., Xu H. H., Liu W. C., Lu Y. T. (2013). Copper regulates primary root elongation through PIN1-mediated auxin redistribution. Plant Cell Physiol. 54 766–778 10.1093/pcp/pct030 [DOI] [PubMed] [Google Scholar]
- Yuan M., Li X., Xiao J., Wang S. (2011). Molecular and functional analyses of COPT/Ctr-type copper transporter-like gene family in rice. BMC Plant Biol. 11:69 10.1186/1471-2229-11-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M., Wang S., Chu Z., Li X., Xu C. (2010). The bacterial pathogen Xanthomonas oryzae overcomes rice defenses by regulating host copper redistribution. Plant Cell 22 3164–3176 10.1105/tpc.110.078022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman M., Kurepin L. V., Catto W., Pharis R. P. (2014). Enhancing crop yield with the use of N-based fertilizers co-applied with plant hormones or growth regulators. J. Sci. Food Agric. 10.1002/jsfa.6938 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Zhang H., He H., Wang X., Yang X., Li L., Deng X. W. (2011). Genome-wide mapping of the HY5-mediated gene networks in Arabidopsis that involve both transcriptional and post-transcriptional regulation. Plant J. 65 346–358 10.1111/j.1365-313X.2010.04426.x [DOI] [PubMed] [Google Scholar]
- Zhang H., Li L. (2013). SQUAMOSA promoter binding protein-like7 regulated microRNA408 is required for vegetative development in Arabidopsis. Plant J. 74 98–109 10.1111/tpj.12107 [DOI] [PubMed] [Google Scholar]
- Zhang H., Zhao X., Li J., Cai H., Deng X. W., Li L. (2014). MicroRNA408 is critical for the HY5-SPL7 gene network that mediates coordinated response to light and copper. Plant Cell 26 4933–4953 10.1105/tpc.114.127340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Wang H., Takemiya A., Song C.-P., Kinoshita T., Shimazaki K. I. (2004a). Inhibition of blue light-dependent H+ pumping by abscisic acid through hydrogen peroxide-induced dephosphorylation of the plasma membrane H+-ATPase in guard cell protoplasts. Plant Physiol. 136 4150–4158 10.1104/pp.104.046573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z. L., Xie Z., Zou X., Casaretto J., Ho T.-H. D., Shen Q. J. (2004b). A rice WRKY gene encodes a transcriptional repressor of the gibberellin signaling pathway in aleurone cells. Plant Physiol. 134 1500–1513 10.1104/pp.103.034967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C., Ding Y., Liu H. (2011). MiR398 and plant stress responses. Physiol. Plant. 143, 1–9 10.1111/j.1399-3054.2011.01477.x [DOI] [PubMed] [Google Scholar]