Summary
Inflammatory caspases play a central role in innate immunity by responding to cytosolic signals and initiating a twofold response. First, caspase-1 induces the activation and secretion of the two prominent pro-inflammatory cytokines, interleukin-1β (IL-1β) and IL-18. Second, either caspase-1 or caspase-11 can trigger a form of lytic, programmed cell death called pyroptosis. Pyroptosis operates to remove the replication niche of intracellular pathogens, making them susceptible to phagocytosis and killing by a secondary phagocyte. However, aberrant, systemic activation of pyroptosis in vivo may contribute to sepsis. Emphasizing the efficiency of inflammasome detection of microbial infections, many pathogens have evolved to avoid or subvert pyroptosis. This review focuses on molecular and morphological characteristics of pyroptosis and the individual inflammasomes and their contribution to defense against infection in mice and humans.
Keywords: caspase-1, caspase-11, inflammasome, pyroptosis, inflammatory caspases
Introduction
Different forms of cell death can be classified based on whether they are lytic or non-lytic. Also, cell death can be programmed by specific signaling events or result from accidental injury. Caspases are ancient cysteine proteases that trigger two distinct types of programmed cell death: apoptosis or pyroptosis. Apoptotic caspases include initiator caspases (caspase-2, -8, -9, -10) that respond to an extrinsic or intrinsic apoptotic signal. These cleave and activate the effector caspases (caspase-3, -6, -7), which in turn cleave target proteins to orchestrate apoptotic cell death. In contrast, both the initiator and effector functions are contained within one inflammatory pyroptotic caspases (caspase-1, -4, -5, -11).
This review focuses on pyroptosis in vivo. We describe the morphological and molecular determinants and the upstream signaling required to initiate pyroptosis. Pyroptosis plays an important role in the defense against microbial infections, but systemic caspases activation can be harmful. We review the balancing act between protective and detrimental roles of pyroptosis and conclude by discussing the evasion strategies employed by microbes to avoid pyroptosis altogether.
Morphological and molecular determinants of pyroptosis
Pyroptosis was first described in 1992 by Zychlinsky and colleagues (1), who observed a lytic form of cell death in Shigella flexneri-infected macrophages. This cell death was at first termed apoptosis because it shared some characteristics with apoptosis, including DNA fragmentation, nuclear condensation, and caspase dependence. Later, as caspase-1-dependent cell death was further characterized, it was found to be distinct from apoptosis. Thus, caspase-1-dependent cell death was named pyroptosis in 2001. ‘Pyro’, meaning fire, reflects the inflammatory nature of this form of cell death, and ‘ptosis’, meaning falling, matches the terms used for other forms of programmed cell death (2).
Pyroptotic cell death is defined by several criteria. First, it is programmed by an inflammatory caspase. The proteolytic activity of the caspase is required, but autoproteolytic processing of the caspase is not. This is important to note, since processing of the caspase has often been used as a proxy for proteolytic activity. Yet, this is not necessarily the case in pyroptosis, at least when the caspase is activated directly via a CARD-containing inflammasome.
Second, inflammatory caspase activation results in pore formation in the plasma membrane, and the cell becomes permeable to small molecular weight, membrane-impermeable dyes such as 7-aminoactinomycin (7-AAD), ethidium bromide (EtBr), and propidium iodide (PI). These pores are likely between 1.1 and 2.4nm in diameter (3). In contrast, apoptotic cells remain intact and fragment into apoptotic bodies that do not stain with 7-AAD or PI.
Following permabilization of the plasma membrane, ions and water rush into the cell causing it to swell and lyse, resulting in the release of cytosolic contents (3). After membrane rupture, the inner leaflet of the plasma membrane is exposed to the extracellular fluid and thus can be stained with Annexin V, which binds to the inner leaflet-sequestered lipid phosphatidyl serine (PS) (4). In contract, during apoptosis, PS is translocated by a flippase to the extracellular surface of the plasma membrane, permitting Annexin V staining. Thus, Annexin V does not distinguish between apoptotic and pyroptotic cell death. The majority of the work addressing the morphological events that accompany pyroptosis has been performed with caspase-1 specific agonists, and it is assumed that these observations apply to caspase-11-induced pyroptosis as well. A detailed comparison is therefore needed to confirm this assumption.
Third, pyroptotic cells display DNA damage and are positive in a TUNEL assay, but at a lower intensity than apoptotic cells. Further, there is little DNA laddering present during pyroptosis. Chromatin condensation also occurs, but in comparison to apoptosis, the nucleus remains intact. Whereas DNA damage during apoptosis is dependent on the caspase-activated DNase (CAD), CAD remains bound to its inhibitor ICAD during pyroptosis even though caspase-1 can cleave ICAD in vitro. Yet, DNA damage is not required for pyroptosis, as inhibition of DNA fragmentation with a nuclease inhibitor does not prevent cell lysis (3).
Finally, during lytic, non-programmed, necrotic cell death, DNA damage activates ADP-ribose polymerase (PARP), which consumes NAD+, thereby depleting ATP. Apoptotic effector caspases cleave and inactivate PARP, maintaining the cellular levels of ATP needed to drive apoptosis. Unlike in apoptotic macrophages, PARP is not inactivated during S. typhimurium-induced pyroptosis, though caspase-1 is capable of cleaving PARP in vitro (5). It is unknown if caspase-11 can cleave either ICAD or PARP in vitro similar to caspase-1. Yet, Parp−/− macrophages readily undergoing pyroptosis (6), indicating that PARP activity is not required for pyroptosis. PARP-1 has been reported as a cofactor of nuclear factor-κB (NF-κΒ) to regulate lipopolysaccharide (LPS)-induced transcription of caspase-11 in vitro, but as shRNA knockdown of PARP-1 did not affect interferon-γ (IFN-γ)-induced caspase-11 transcription (7), PARP-1 is unlikely required for caspase-11-mediated pyroptosis. For a summary of the molecular and morphological characteristics of pyroptosis compared to apoptosis, see Table 1.
Table 1.
Morphological and molecular determinants of pyroptosis versus apoptosis
| Characteristic | Pyroptosis | Apoptosis |
|---|---|---|
| Inflammatory vs non-inflammatory | Inflammatory | Non-inflammatory |
| Lytic vs non-lytic | Lytic | Non-lytic |
| Initiator caspase | Caspase-1/4/5/11 | Caspase-2, 8, 9, 10 |
| Effector caspase | None | Caspase-3, 6, 7 |
| DNA damage | ||
| Laddering | No | Yes |
| TUNEL stain | Yes | Yes |
| ICAD cleavage | No | Yes |
| Chromatin condensation | Yes | Yes |
| Nucleus intact | Yes | No |
| Plasma membrane pore formation | Yes | No |
| PARP cleavage | No | Yes |
| Annexin V staining | Yes | Yes |
The caspase-1 canonical inflammasomes
Caspase-1 plays a central role in innate immunity by responding to cytosolic signals and initiating a twofold response. First, caspase-1 induces the activation and secretion of the two pro-inflammatory cytokines, interleukin-1β (IL-1β) and IL-18. Second, caspase-1 triggers pyroptosis. To date, both mouse and human macrophages and dendritic cells are the primary cells types known to undergo caspase-1-dependent pyroptosis (8, 9). Additionally, the majority of the work in understanding the composition and signaling of different inflammasomes has been done in macrophages. Nevertheless, caspase-1 expression and/or activity have been reported in many cell types such as keratinocytes (10) and intestinal epithelial cells (11), but it remains unclear if these cell types undergo pyroptosis in response to microbial ligands.
Caspase-1 is activated by various inflammasomes, which are multi-protein signaling complexes that assemble in response the detection of cellular perturbations or intracellular microbial ligands. The currently known inflammasomes are encompassed within three gene families: Nod-like receptors (NLRs) (4, 12, 13), AIM2-like receptors (ALRs) (14, 15), or tripartite motif family (TRIM)(of which only one member is an inflammasome: Pyrin) (16, 17). Each inflammasome contains either a caspase activation and recruitment domain (CARD) or a pyrin domain (PYD) that mediates the signaling event. Those with only a PYD exclusively signal through the ASC adapter, which is composed of a PYD and a CARD. The CARD domain of ASC then recruits caspase-1 via CARD-CARD interactions. Inflammasomes with a CARD domain (NLRC4, NLRP1, NLRP1a, NLRP1b) can signal either through ASC or directly to caspase-1. For CARD-containing inflammasomes, pyroptosis occurs independently of ASC and caspase-1 processing. For NLRC4, cytokine secretion requires ASC and caspase-1 processing, while for the CARD-containing NLRP1b, cytokine secretion occurs independently of ASC and caspase-1 processing (18, 19). The reasons why NLRC4 and NLRP1b would have differential requirements for ASC in cytokine processing are unknown.
Pyroptosis of the infected cell removes the pathogen’s protective, intracellular niche and makes them susceptible to killing by a secondary phagocyte. Indeed, we propose that pyroptosis is so effective in defending against intracellular infection that any intracellular pathogen must evade pyroptosis in vivo, lest the parasitized cell lyse. Although this concept is intuitively sound, there is limited experimental evidence that actually demonstrates it. Mice deficient in caspase-1 or caspase-11 are susceptible to a large number of bacterial pathogens that induce inflammasome formation. However, to understand the role of pyroptosis in the clearance of microbial infections, its effects must be experimentally isolated from the effects of IL-1β and IL-18.
NLRC4
The NLRC4 inflammasome was initially named ICE-protease-activating factor (IPAF) for its role in activating caspase-1 [originally named IL-1-converting enzyme (ICE)]. The NLRC4 inflammasome responds to three bacterial proteins when they enter the cytosol of host cells: flagellin, type III secretion system (T3SS) rod, and T3SS needle.
Flagellin is the monomeric subunit of the bacterial flagellum. In bacteria, flagella are assembled via a T3SS that is distinct from the virulence-associated T3SS that mediate translocation of effector proteins into host cells, though they share significant structural and sequence similarities. Given this conservation, flagellin is recognized and accidentally secreted by a T3SS that normally delivers bacterial effectors to the host cytosol where it is efficiently detected by NLRC4 (20, 21) (Fig. 1). Flagellin is also detected during Legionella pneumophila infection. Here, cytosolic flagellin is a marker of the type IV secretion system (T4SS) activity (22–24). It is less apparent whether flagellin is being injected across the phagosomal membrane by the T4SS or whether flagellin enters the cytosol by transient vacuolar permeabilization events occurring consequently to the T4SS activity. Cytosolic flagellin is also a marker for bacteria that directly invade the cytosol, such as Listeria monocytogenes (11, 25). Interestingly, S. typhimurium and L. monocytogenes both repress flagellin expression in vivo.
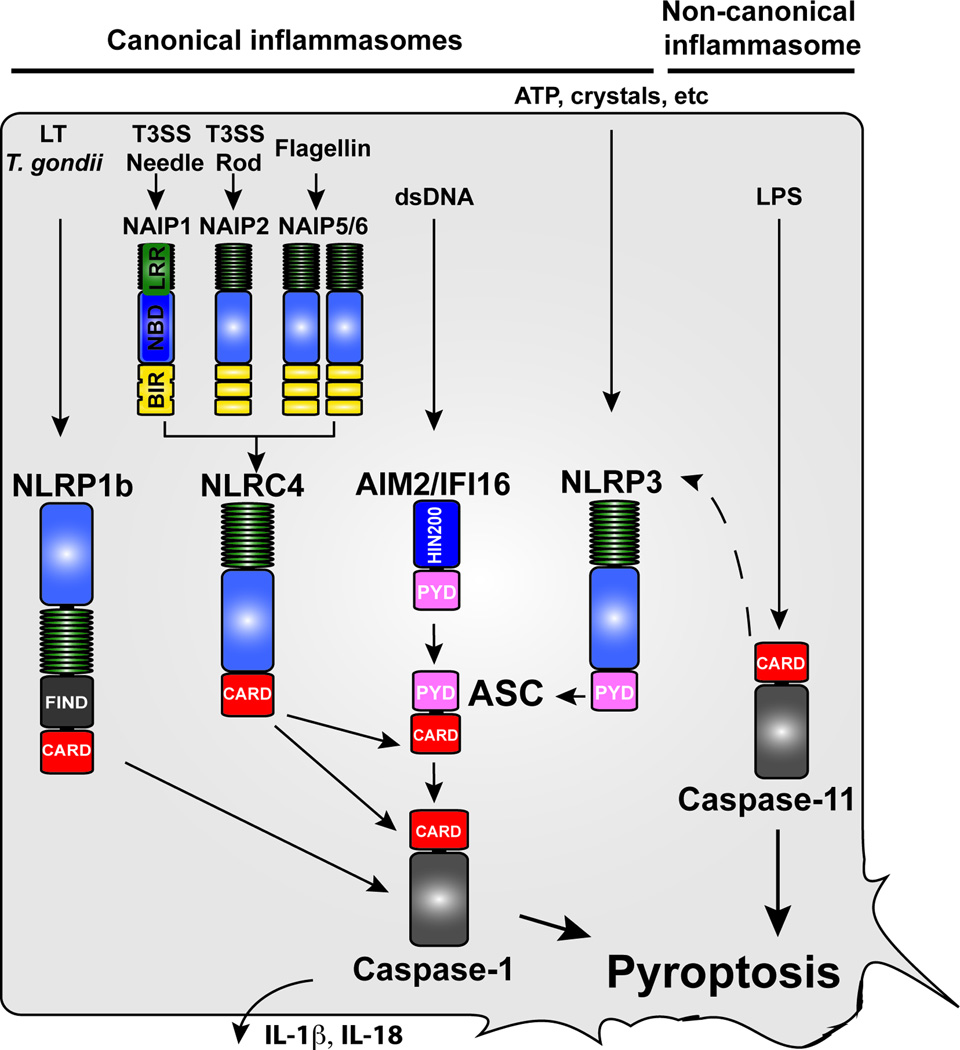
Fig. 1. Activation of inflammatory caspases by canonical and non-canonical inflammasomes.
Caspase-1 is activated by several inflammasomes, which are multi-protein signaling complexes that assemble in response the detection of cellular perturbations or intracellular microbial ligands. The currently known inflammasomes are encompassed within three gene families: Nod-like receptors (NLRs), AIM2-like receptors (ALRs), or tripartite motif family (TRIM) (of which only one member is an inflammasome: Pyrin). The noncanonical inflammasomes consists of caspase-11, which directly senses LPS. Activation of both the canonical and noncanonical inflammasome induces pyroptosis and the secretion of the pro-inflammatory cytokines IL-1β and IL-18. For CARD-containing inflammasomes, pyroptosis and cytokine secretion occurs independently and dependently of ASC, respectively, with the exception of NLRP1, which does not require ASC for cytokine secretion. Caspase-11 induces cytokine secretion via NLRP3/ASC by an unknown mechanism.
S. typhimurium strains deficient in flagellin expression still trigger NLRC4, indicating that NLRC4 is capable of responding to additional agonists (20). This observation was explained when it was demonstrated that NLRC4 is also capable of recognizing two additional bacteria ligands: the T3SS rod protein (26) and the T3SS needle protein (27) (Fig. 1). In the Salmonella SPI-1 T3SS these proteins are PrgJ and PrgI, respectively.
A fundamental question in the field was how the NLRC4 inflammasome responds to multiple bacterial ligands with little sequence similarity. Two groups independently demonstrated that the NLR family NAIP proteins govern the specificity of the NLRC4 inflammasome response to different ligands (27, 28). C57BL/6 mice have four NAIP genes: NAIP5 and NAIP6 bind directly to flagellin, NAIP2 binds the T3SS rod, and NAIP1 binds the T3SS needle (27–30)(Fig. 1). In contrast, humans have a single NAIP, which responds only to the T3SS needle protein (27). These NAIPs control ligand-dependent oligomerization of NLRC4; only a NAIP5/6-flagellin and NAIP2-PrgJ interact with NLRC4. The NAIP and NLRC4 monomers oligomerize together to form an inflammasome hub that includes NAIP and NLRC4 in roughly a 2:5 ratio (31). The stoichiometry of this complex likely increases the sensitivity of NAIP/NLRC4 inflammasomes in comparison to other NLR ligands. It is peculiar that humans are limited to detecting a single bacterial ligand, the T3SS needle protein. Both pathogenic and non-pathogenic bacteria express flagella. Thus, sensors for flagellin are perhaps more susceptible to false activation by a commensal microbe than are sensors for T3SS apparatus proteins, which are more specific for pathogens.
NLRC4 is phosphorylated at serine 533 by protein kinase Cδ, which has been implicated in its activation (32); however, this has been disputed (33). This phosphorylation event was also observed in the crystal structure of NLRC4, which was crystallized in the inactive state (34). Thus, the importance of the phosphorylation event remains to be further investigated.
S. typhimurium efficiently evades NLRC4 during systemic infection. It does so by repressing flagellin and expressing a variant T3SS whose rod and (presumably) needle proteins are not detected by NLRC4 (20, 26, 35). S. typhimurium engineered to constitutively activate the NLRC4 inflammasome have suggested a critical role for pyroptosis in innate immunity. Constitutive flagellin expression leads to rapid clearance of S. typhimurium in wildtype (WT) mice (36). Importantly, clearance is entirely dependent on NLRC4 but independent of IL-1β and IL-18. This indicates that pyroptosis is responsible for the clearance of the infection completely independent of pro-inflammatory cytokines. By monitoring the cell membrane permeability of infected macrophages, it was shown that cells infected with flagellin-expressing S. typhimurium became permeable low-molecular weight dyes (36). Pore-formation was NLRC4/caspase-1 dependent, indicating that these infected macrophages were in fact undergoing pyroptosis. This led to the eradication of the infecting bacteria. After release from the pyroptotic macrophage, these bacteria were phagocytosed and killed by neutrophils. Similar results were seen when S. typhimurium was engineered to express the SPI-1 T3SS rod protein, PrgJ during systemic infection (26). L. monocytogenes provides a second example that proves this point. Like S. typhimurium, L. monocytogenes efficiently evades inflammasomes in vivo by repressing flagellin expression (37, 38). Similar to the S. typhimurium results, L. monocytogenes strains engineered to express flagellin or T3SS rod protein in vivo were efficiently cleared. These engineered bacteria remained attenuated in Il1b−/−Il18−/− mice, suggesting a role for pyroptosis (39, 40).
Upon pyroptosis, neutrophils phagocytose the flagellin overexpressing S. typhimurium and kill the bacteria via ROS production by the NADPH phagocyte oxidase system (36). Mice deficient in the NADPH phagocyte oxidase component p47phox (Ncf1−/−) fail to clear a flagellin-expressing strain of S. typhimurium. Instead, S. typhimurium accumulates in neutrophils of Ncf1−/− mice, since these animals induce pyroptosis but fail to clear the pathogens via ROS. The secondary phagocyte could potentially induce pyroptosis as well if the phagocytosed bacteria continued to express flagellin, but the neutrophil is likely not susceptible to pyroptosis for several reasons. First, ROS production dramatically exceeds that of macrophages and is sufficient to kill S. typhimurium. Second, neutrophils express caspase-1 but not NLRC4 and are therefore unable to respond to bacterial flagellin or T3SS rod and needle protein (36, 41). Whether neutrophils express NLRC4 was recently contested, however (42), although NLRC4 expression would not preclude neutrophils from killing bacteria prior to activation of the inflammasome.
AIM2 and IFI16
AIM2 contains a HIN200 domain, which directly binds double stranded DNA (dsDNA) (43–46). It detects viral dsDNA, such as vaccinia virus and cytomegalovirus (CMV). It also detects bacterial dsDNA from cytosol-invasive bacteria, such as Francisella novicida or Listeria monocytogenes that lyse in the cytosolic compartment (47–53) (Fig. 1).
AIM2 activation induces pyroptosis in BMM in vitro in response to liposome-delivered dsDNA, but the contribution of pyroptosis versus cytokine secretion to host defense in vivo remains unclear. For example, AIM2-deficient mice have higher titers of CMV compared to WT mice, and IL-18 was implicated as the inflammasome-dependent effector conferring this phenotype (48), although a role for pyroptosis was not excluded.
Aim2−/− mice are more susceptible to F. novicida infection (47, 48, 52). Protection may be mediated by pyroptosis, as treating Casp1−/− Casp11−/− mice with recombinant IL-1β and IL-18 only partially rescues these mice (54). However, these results have not been verified using Il1b−/−Il18−/− mice, and further evidence of pyroptosis occurring in vivo has not been obtained. Similarly, Mycobacterium tuberculosis activates AIM2 in vitro, although one report suggests that a virulent M. tuberculosis strain but not the non-virulent M. smegmatis can suppress AIM2 activation in vitro (55–57). Saiga et al. (56) showed that Aim2−/− mice are highly susceptible to infection with M. tuberculosis. However, as the authors did not investigate susceptibility to infection in Il1b−/−Il18−/− mice, it is unclear is protection against M. tuberculosis is pyroptosis dependent.
AIM2 is the founding member of the ALR family, characterized by the presence of a HIN200 domain. Humans encode four ALRs, while the family is significantly expanded in mice (14). IFI16 is a human ALR that detects viral dsDNA in the nucleus and activates caspase-1 (58). It is unclear whether any of the murine ALRs are functional IFI16 homologs (14). Recently, IFI16 was shown to play a critical role in pyroptosis of CD4+ T cells during human immunodeficiency virus (HIV) infection. Depletion of CD4+ T cells is a hallmark of acquired immunodeficiency syndrome (AIDS) and occurs as a result of cell death in response to the accumulation of deficient cytosolic HIV virions (59). Doitsh et al. (60) recently demonstrated that CD4+ T-cell death is a result of caspase-1-dependent pyroptosis. Concurrently, two groups identified IFI16 as the sensor of reverse transcribed ssHIV DNA, leading to pyroptosis and cytokine secretion (61, 62). These observations therefore significantly extend the role of AIM2-related proteins in sensing and protection against retroviral infection.
NLRP3
Cumulative evidence suggests that NLRP3 does not directly sense the many signals that are capable of inducing NLRP3 inflammasome formation. Instead, fungal, bacterial, and viral pathogens, pore-forming toxins, crystals and DAMPs are thought to induce a cytosolic perturbation that is sensed by NLRP3 (Fig. 1). The work of several groups have identified potential converging signals, such as potassium efflux (63), mitochondrial ROS production (64), and changes in cell volume (65). To date, however, there is little evidence to indicate that pyroptosis acts in vivo downstream of NLRP3. We are not aware of reports that have shown an NLRP3-dependent protective effect against infection that was not dependent upon IL-1β and/or IL-18.
NLRP1
Humans express a single NLRP1 protein that has an unusual structure: it consists of an N-terminal PYD domain, an LRR domain, a function-to-find domain (FIIND), and a C-terminal CARD domain. Mice, however, express three homologues: NLRP1a, NLRP1b, and NLRP1c. NLRP1a and NLRP1b contain the CARD signaling domain but lack the PYD domain (66), while NLRP1c is truncated and has neither a CARD nor a PYD signaling domain (67). Another critical difference between the human and mouse NLRP1 is that certain alleles of mouse NLRP1b activate in response to the anthrax lethal toxin (LT), whereas human NLRP1 does not. LT cleaves the N-terminus of NLRP1b, activating the inflammasome and inducing pyroptosis and cytokine secretion. NLRP1b recruits caspase-1 via CARD-CARD interactions, and ASC is dispensable for induction of pyroptosis and IL-1β secretion (68). Similar to NLRC4-dependent pyroptosis, NLRP1b induces pyroptosis independent of caspase-1 autoprocessing (69). The three mouse homologues are highly polymorphic among mouse strains. For example, 129S1 mice do not express NLRP1a and NLRP1c and express a LT responsive NLRP1b allele. In contrast, C57BL/6 mice express all three genes, but the C57BL/6 NLRP1b allele does not respond to LT (66, 67). Interestingly, the NLRP1 family FIIND domain was recently shown to have autoproteolytic activity, which is required for inflammasome activation (70, 71).
Besides LT, NLRP1b responds to the parasite Toxoplasma gondii in vitro (72–74). In vitro knockdown of NLRP1b in a human monocytic cell line leads to decrease in parasite-mediated pyroptosis and cytokine secretion (74). Earlier reports indicated that human NLRP1 can detect muramyl-dipeptide (MDP) in vitro (75, 76), but it is unclear if this observation is physiologically relevant, as murine NLRP1 proteins do not detect MDP (68), and the relevance of MDP responses in vivo in humans has not been examined. Whether NLRP1 senses T. gondii by N-terminal cleavage events as in LT or via signals interacting with the LRR domain remain unknown.
The NLRP1 inflammasome protects against Bacillus anthracis-derived LT and T. gondii in vivo (77, 78). Infection with B. anthracis leads to rapid respiratory failure following lung tissue necrosis, circulatory collapse and pulmonary edema, largely mediated by LT (79). Kovarova et al. (68) demonstrated that NLRP1b mediates lung injury in response to LT injection in mice. The NLRP1b-driven pathology was independent of IL-1β; thus, pyroptosis is likely the primary mediator of LT-induced pathology.
Human NLRP1, however, detects T. gondii infection in vitro, and certain SNPs in the NLRP1 gene are associated with susceptibility to congenital toxoplasmosis (74). Toxoplasma is a macrophage tropic intracellular pathogen, thus pyroptosis would be expected to eliminate this intracellular niche and contribute to host defense. However, this protection is entirely dependent on IL-18, as Il18−/− and Asc−/− mice both succumb to T. gondii infection, suggesting that NLRP1b-mediated defense occurs via cytokine production (80, 81).
NLRP1-mediated pyroptosis has also been implicated in the development of immune suppression by depletion of hematopoietic cells (82). A murine germline gain-of-function mutation in NLRP1a induced pyroptosis of hematopoietic progenitor cells, IL-1β secretion and resulted in a lethal inflammatory disease. Pathology was independent of ASC and cytokine secretion, implicating NLRP1a-driven pyroptotic cell death as the primary mediator of inflammation. Notably, the human NLRP1 is genetically linked to several autoinflammatory diseases (83). Given the role of NLRP1-mediated pyroptosis in the depletion of hematopoietic cells, future work should be directed at determining if NLRP1-dependent cell death plays a role in the development of other autoinflammatory diseases.
The caspase-11 non-canonical inflammasomes
The inflammatory caspase locus in mice contains caspase-1, caspase-11, and caspase-12, all encoded in close proximity on chromosome 9. In humans, caspase-11 is duplicated as caspase-4 and caspase-5, and most humans encode a defective caspase-12 gene. Interestingly, Casp1−/−, Casp11−/−, and Casp12−/− mice are all resistant to endotoxic shock (84–86). The two caspase-1 knockout mouse lines (84, 87) were known to be deficient in caspase-11 expression (88), yet this garnered little attention in the published literature until 2011. Then, the caspase-11 deficiency was shown to arise from a natural mutation in the 129 mouse line that was back crossed into C57BL/6 along with the engineered Casp1−/− mutation; these two Casp1−/− mouse lines are now referred to as Casp1−/−Casp11−/− mice. The resistance of Casp1−/−Casp11−/− mice to endotoxic shock was attributed primarily to the caspase-11 deficiency, rather than caspase-1. Because caspase-11 independently directs pyroptosis, this pathway was termed the noncanonical inflammasome to contrast it with the canonical inflammasome pathway that activates caspase-1. Because of their 129 origin Casp12−/− mice backcrossed to the C57BL6 background are expected to have the same caveat and to be Casp11−/−Casp12−/− mice, and the resistance of these mice to endotoxic shock may arise from a caspase-11 mutation.
LPS within the cytosol was subsequently identified as the bona fide caspase-11 agonist (89, 90). Caspase-11 displays LPS structural specificity as the caspase is only activated in response to cytosolic lipid A with six acyl groups, but not lipid A species with four acyl groups. Thus, caspase-11 detects LPS in the cytosol, while Toll-like receptor 4 (TLR4) detects LPS in the extracellular or vacuolar space.
Prior to activation, caspase-11 must be primed via either type I interferon or IFN-γ (91–97). Besides pyroptosis, caspase-11 also induces IL-1β secretion in cooperation with NLRP3, ASC and caspase-1. The molecular mechanism linking caspase-11 and NLRP3-caspase-1 has yet to be established. The physiologic relevance of the link between caspase-11 and IL-1β secretion has not been demonstrated.
Until recently, all known initiator caspases besides caspase-11 were activated by an upstream caspase-activating platform: the inflammasome, apoptosome, PIDDosome, or DISC for caspase-1, caspase-9, caspase-2, and caspase-8, respectively. It was thus widely assumed that an upstream noncanonical inflammasome detected cytosolic LPS and in response recruited and activated caspase-11. A recent study, however, demonstrated that caspase-11 is itself the LPS sensor, upending the dogma of caspase activation (98). The caspase-11 CARD domain binds LPS and in response mediates caspase-11 oligomerization. Several basic residues within the caspase-11 CARD domain are required for LPS binding, presumably interacting with the acidic phosphate moieties of lipid A. The authors further showed that human caspase-4 and caspase-11 are bona fide homologues, inducing pyroptosis in response to LPS and supporting the importance of this cytosolic LPS detection pathway in both mice and humans (98). Why this pathway has been duplicated in humans remains to be elucidated.
Similar to caspase-1, the study of caspase-11 function has focused on macrophages. Humans encode two homologues of caspase-11, caspase-4 and caspase-5. Whether these two homologues both function similarly to caspase-11 has been unclear. Shi et al. (98) recently demonstrated that caspase-4 detects LPS in human monocytes, where it appears to be constitutively expressed. This is in contrast to caspase-11, which is inducible in myeloid cells, indicating that a priming step is not required for LPS-mediated endotoxic shock in humans. This model of caspase-11 expression restricted to macrophages was recently expanded by a publication demonstrating a role for caspase-11 pyroptosis in intestinal epithelial cells (99). Caspase-4 is moreover expressed and induces pyroptosis in keratinocytes and epithelial cells, but not in T-cells or pre-myeloid cells (98, 100, 101). Caspase-5 also binds LPS and can reconstitute pyroptosis Casp-11−/− deficient BMMs, yet caspase-5 was not expressed in macrophages. This may indicate that the two human inflammatory caspases are expressed in different cell types or under very different conditions. Whereas caspase-4 is constitutively expressed, caspase-5 may require priming by an unknown mechanism, such as IFN-γ as suggested by Lin et al. (100).
Caspase-11 induced pyroptosis has been implicated in the clearance of cytosolic bacterial pathogens in vivo (95). Mutant strains of both S. typhimurium and L. pneumophila that escape the vacuole (SifA and SdhA, respectively) are detected by caspase-11, and this has been shown to promote clearance of the ΔsifA S. typhimurium mutant in vivo (95). When macrophages are primed with LPS or IFN-γ, they rapidly undergo caspase-11 dependent pyroptosis during infection with a flagellin deficient mutant of L. pneumophila (94). However, WT L. pneumophila induces caspase-1-dependent pyroptosis in WT macrophages primed with LPS in vitro, presumably since the bacteria are confined to the structurally intact, stable vacuole and do not enter the cytosol. Yet, Akhter et al. (102) observed a mild contribution of caspase-4 in restricting WT L. pneumophila growth in vitro, perhaps reflecting a small number of bacteria that aberrantly enter the cytosol.
Since caspase-11 detects cytosolic LPS, it has remained unclear how these vacuolar bacteria are detected by caspase-11. One possibility is that the interferon inducible GTPases alter the integrity of the bacteria-containing vacuole, allowing some LPS to enter the cytosol (103, 104).
Caspase-11 also defends against infection by the cytosolic pathogen B. thailandensis and B. pseudomallei (95). However, it remains unclear to what extend pyroptosis plays in protection. Defense against B. pseudomallei in WT mice requires both pyroptosis and IL-18, whereas IL-1β actually has a detrimental effect (41). In contrast to B. pseudomallei, B. thailandensis infection is still lethal in, Il-1β−/− ll-18−/− mice, suggesting that pyroptosis is the dominant effector mechanism (95). As with F. novicida in vivo infection data, the occurrence of pyroptosis in vivo for these infections has not been fully characterized.
Two groups independently recently suggested a protective role for caspase-11-dependent pyroptosis in the intestinal epithelium, using two different models of intestinal inflammation. Demon et al. (105) showed that Casp11−/− mice are hypersusceptible to DSS-induced colitis, but it is unclear if protection is mediated by pyroptosis or cytokine secretion. The second report (106) established how caspase-11 protects against S. typhimurium infection of intestinal epithelial cells by promoting shedding of infected cells via pyroptosis. Since caspase-11 specifically detects LPS in the cytosol, it is likely that S. typhimurium is only detected by caspase-11 after vacuolar disruption.
Aberrant activation of pyroptosis in vivo
With the additional knowledge that LPS is the caspase-11 agonist, there are now two LPS detection pathways: cytosolic LPS is detected by caspase-11, and extracellular and vacuolar LPS is detected by TLR4, and Tlr4−/− are also resistant to LPS-mediated endotoxic shock (89, 90, 107). Endotoxic shock has therefore been proposed to occur in a two-step manner: (i) TLR4 detects LPS in the extracellular or vacuolar space, resulting in IFN-β secretion that primes caspase-11, and (ii) detection of aberrantly cytosolic LPS by caspase-11. Caspase-11-mediated pyroptosis then facilitates endotoxic shock. Treating mice with a low dose LPS results in TLR4 detection and TLR4-depepent priming of the caspase-11 pathway. These mice then become exceedingly susceptible to a subsequent challenge with a low dose LPS (89). In fact, the requirement of TLR4 can be bypassed, by priming the mice with the TLR3 agonist poly(I:C). Priming with poly(I:C) and subsequently challenging mice with a low dose LPS is also lethal. Hence, TLR4 can be bypassed in a mouse model of LPS-induce endotoxic shock, whereas caspase-11 is responsible for the mortality in this model. Since Nlrp3−/− mice do not show the same resistance as Casp11−/− mice (108), it is likely that IL-1β and IL-18 are not primary effectors of caspase-11-driven endotoxic shock. This would suggest pyroptosis as a primary driver in endotoxic shock, although this deserves further study.
As evidenced by the role of caspase-11 in sepsis, excessive, uncontrolled pyroptosis is detrimental to the host. This detriment is also evident in systemic activation of caspase-1. Flagellin can be delivered to the cytosol of host cells in vivo by replacing the catalytic subunit of anthrax lethal toxin with flagellin (FlaTox) (109). FlaTox is rapidly detected by NLRC4, activating caspase-1 and inducing pyroptosis. Mice treated with FlaTox systemically activate the NLRC4 inflammasome, leading to an eicosanoid storm, vascular permeability, and death. Systemic NLRC4 activation is independent of cytokines, as FlaTox-treated Il-1β−/− Il18−/− mice survive, suggesting pyroptosis is the driver of pathology. The COX inhibitor indomethacin can block production and secretion of these lipid mediators, and mice treated with indomethacin survive FlaTox treatment. In fact, inhibiting eicosanoid production also rescues mice primed with poly(I:C) and challenged with low dose LPS (89). Combined, these data indicate that eicosanoids are critical mediators of mortality in endotoxic shock downstream of caspase-1 and caspase-11. It remains unclear whether pyroptosis is directly related to eicosanoid production in these models, or whether instead caspase proteolytic activity activates a different pathway to drive eicosanoid production.
The detrimental effects of aberrant inflammasome activation have recently been well illustrated in humans. Aberrant NLRP3 activation and cytokine secretion has been implicated in several human autoimmune diseases (110). For example, gain of function mutations in NLRP3 causes a syndrome characterized by spontaneous inflammasome activation and increased secretion of IL-1β called cryopyrin-associated periodic syndromes (CAPS). Patients with CAPS are successfully treated with IL-1β blocking antibodies. Thus, like in CAPS, inflammasome-mediated cytokine secretion rather than pyroptosis seems to be the underlying mechanism of most NLRP3-mediated autoimmune disorders.
Recently three groups identified several de novo gain-of-function mutations in close proximity to each other in the nucleotide-binding domain (NBD) of NLRC4 that is associated with inflammatory syndromes in humans (111–113). These are the first reports of disease associations with genetic variants of NLRC4 in humans. Given the strong links between NLRC4 and pyroptosis, it was tempting to speculate that autoactivation of NLRC4 would result in a pyroptosis driven disease in vivo. However, this syndrome was successfully treated with IL-1 receptor antagonist, suggesting that the cytokines, rather than pyroptosis drive the clinical symptoms. However, we must keep in mind that two of these mutations are very mild (threonine to serine, and valine to alanine) (111, 112), while the third appears to be autoactivated only upon exposure to cold temperatures (113). We speculate that a stronger activating mutation may result in a pyroptosis-driven clinical syndrome.
In summary, we therefore propose that an early pyroptotic response during infection ablates the intracellular replicative niche that pathogens attempt to exploit. This is beneficial and results in clearance of the infection. In contrast, during sepsis where infection has failed to be controlled, aberrant cytosolic PAMP entry could trigger inappropriate pyroptosis on a broad scale, resulting in wholesale release of cytosolic DAMPs that drive shock.
Microbial pathogens evade pyroptosis
The importance of inflammasome activation and pyroptosis in expelling pathogens from their intracellular niche is highlighted by the fact that many pathogens have evolved to avoid activating this innate immune response. Pathogens employ multiple mechanisms to subvert detection. (i) Pathogens can restrict expression of the ligand to compartments devoid of inflammasome activity or render the ligand unavailable for binding to an NLR sensor. (ii) Pathogens modify the structure of the ligand detected by inflammasomes to evade detection. (iii) They directly inhibit inflammasome function. Whether it is most important for each pathogen to prevent IL-1β secretion, IL-18 secretion, pyroptosis, or a combination thereof will likely depend upon the pathogenic strategy of the particular pathogen.
Salmonella
S. typhimurium utilizes two distinct pathogenicity islands, SPI-1 and SPI-2, at specific times during infection. This permits the bacteria to secrete specific sets of effector proteins depending on the requirements imposed by the host environment. The T3SS apparatus encoded by SPI-1 functions primarily during epithelial cell invasion, is co-expressed with flagellin, and is readily detected by NLRC4. In contrast, the SPI-2 T3SS is expressed in a vacuolar compartment of both epithelial cells and macrophages. S. typhimurium suppresses transcription of flagellin during SPI-2inducing conditions (26), thereby avoiding detection by NLRC4.
A second strategy to avoid inflammasome detection involves modifying the ligand to prevent recognition by the sensor. The SPI-I T3SS rod protein PrgJ of S. typhimurium, but not the SPI-II T3SS SsaI, is detected by the NLRC4 inflammasome (26). NLRC4 activation in response to flagellin and the T3SS rod protein, two proteins that do not share a high degree of amino acid similarity, is mediated by the interaction of flagellin and T3SS rod protein with different NAIP proteins: NAIP5 and NAIP6 detect flagellin and NAIP2 detects T3SS rod protein (27, 28). Ligand-bound NAIP proteins then activate the NLRC4 inflammasome. NAIP2 detection of SsaI is abolished due to several amino acid substitutions in the region critical for recognition by NAIP2 (26). In fact, NAIP2 recognition of SsaI can be restored by substituting the eight carboxyl-terminal aa of SsaI with the homologues PrgJ sequence. In combination with flagellin suppression during SPI-II expression, modification of the SsaI rod protein ensures that S. typhimurium effectively subverts NLRC4 detection in the cytosol of infected cells.
Listeria
L. monocytogenes also avoids inflammasome detection by the NLRC4 inflammasomes. At 37°C (host temperature), L. monocytogenes suppresses flagellin expression via its transcriptional regulator MogR (38, 114), thus avoiding NLRC4 detection of flagellin shed from cytosolic bacteria that have escaped the vacuole. In vivo, Casp1−/−Casp11−/− mice were found to have an increased susceptibility to L. monocytogenes infection (115); however, this phenotype was found by other groups to be quite mild (39). That L. monocytogenes is primarily evading inflammasome detection in vivo is driven home by the severe attenuation of the bacteria when they are forced to express flagellin or T3SS rod protein in vivo (39, 40).
Vacuolar bacteria
Both S. typhimurium and L. pneumophila avoid caspase-11 detection by virtue of their replicative niche within a vacuole. The T3SS/T4SS effectors SifA and SdhA both maintain the integrity of the Salmonella and Legionella-containing vacuole respectively. SifA and-SdhA-deficient strains are detected by caspase-11 as these bacteria escape the vacuole, likely lysing and releasing LPS into the cytosol (95). Besides preventing caspase-11 detection of cytosolic L. pneumophila, ShdA regulation of vacuole integrity precludes AIM2 detection (116). A small number of L. pneumophila likely lyse within the pathogen-containing vacuole and the DNA may access the cytosol. Ge et al. (116) demonstrated that DNA release into the cytosol is enhanced in cells infected with an SdhA mutant and results in detection by AIM2.
Francisella
The importance of maintaining the structural integrity of the pathogen during infection to avoid inflammasome detection is evidenced by the study of F. novicida evasion of AIM2 and ASC. Sampson et al. (117) recently demonstrated that the clustered, regularly interspaced, short palindromic repeats-CRISPR associated (CRISPR-Cas) systems functions in maintaining bacterial envelope activity of F. novicida, and this CRISPR-Cas system is critical in F. novicida evasion of AIM2, ASC, and TLR2 in vitro and in vivo.
LPS is currently the only known ligand sensed by caspase-11. This implies that a variety of Gram-negative intracellular pathogens that either reside in or aberrantly enters the cytosol of infected cell can be detected via this pathway. It is therefore not surprising that the cytosolic bacteria Francisella novicida has evolved mechanisms to avoid caspase-11 detection of its LPS by only expressing tetra-acylated LPS (89).
Yersinia
A final strategy involves directly interfering with inflammasome assembly and activation of the inflammasome or caspase-1. The T3SS protein YopM of Yersinia pseudotuberculosis directly binds and inhibits capase-1 activation via a four aa region of YopM that is similar to YVAD, a substrate of caspase-1 (118). YopM is therefore serves as a pseudosubstrate of caspase-1. Interestingly, YopM can bind both inactive and activated capase-1, and inhibits pyroptosis induced by multiple inflammasomes. Transduced YopM directly inhibits caspase-1 downstream of any inflammasome. The anti-inflammasome function of YopM is paramount in vivo, as a YopM mutant only colonizes Casp1−/− Casp11−/− deficient mice but not WT mice. However, whether this phenotype is attributed to blockade of IL-1β, IL-18, and/or pyroptosis has not been investigated. The mechanism by which YopM inhibits caspase-1 appears twofold. First, YopM preferentially binds activated caspase-1 and prevents cleavage of its substrates. Even if a small portion of the total capase-1 available in a cell is activated, this enables Yersinia to halt the downstream signaling events that initiate pore-formation and lysis. Second, YopM prevents caspase-1 association with the inflammasome itself. The Yersinia secreted protein YopK has also been implicated in inflammasome evasion as yopK mutants are more readily detected by inflammasomes (119).
Shigella
The enteric pathogen Shigella flexneri uses its T3SS to translocate OspC3, which like YopM, directly inhibits caspase-4 (120). Infection of guinea pigs with an OspC3 mutant strain of S. flexneri showed that this effector protein inhibits caspase-4 activation in the intestinal epithelium. Inhibition of caspase-4 is likely to be a pre-requisite for the pathogenic strategy of this cytosol invasive Gram-negative bacterium, since its LPS is detected by the cytosolic LPS detection pathway. Thus, in contrast to Francisella, which modifies the structure of its LPS to evade detection, Shigella actively inhibits the caspase-4 sensor.
Viruses
Inflammasome inhibitors were first described in viruses that encode PYD-domain containing proteins that mimic ASC. Poxviruses express a number of PYD-containing proteins, of which the Myxoma M13L-PYD protein is the best described. Strains deficient in M13L-PYD are attenuated in vivo and ectopic expression in vitro efficiently inhibits caspase-1 activation and cytokine production (121). However, there is no evidence at present that PYD homologues inhibit pyroptosis in vitro or in vivo. Rather, prevailing evidence suggests that the benefit derived by the virus originates from curbing IL-1β and IL-18 secretion. Poxviruses, such as vaccinia virus and Cowpox, encode caspase-1 inhibitors called serpin-like protease inhibitors (SPI) (122). Like PYD-containing proteins, it remains unclear if CrmA and other SPI proteins inhibit pyroptosis besides preventing cytokine production.
In addition to inhibiting caspase-1 activity, viral proteins have also been shown to specifically inhibit NLRP1 inflammasome assembly. Orf16 encoded by Kaposi’s sarcoma herpes simplex virus (KHSV) is a viral homologue of NLRP1, but only contains the LRR and NBD domains, lacking PYD and CARD (76). Over-expression of Orf16 inhibits NLRP1-depepdent pyroptosis and cytokine secretion in human cells. Like Orf16, the vaccinia virus F1L directly binds NLRP1, preventing its activation of caspase-1 (123).
Given that the HIN200 domain of AIM2 binds and detects dsDNA, is it not surprising that AIM2 detects dsDNA viruses such as vaccinia virus and MCMV (48). It is therefore interesting that some viruses that produce cytosolic DNA as some stage during their life cycle, such as the dsDNA viruses HSV1 are not detected by AIM2. This may indicate that HSV1, and potentially other dsDNA viruses, suppresses AIM2 activation by a yet unknown mechanism. For a summary of microbial evasion strategies and effectors, see Table 2.
Table 2.
Evasion strategies by microbial pathogens
| Bacteria | Strategy | Effector |
Inflammasome/ caspase |
| S. typhimurium | Flagellin suppression | Flagellin | NLRC4 |
| Agonist modification | T3SS | NLRC4 | |
| Maintain vacuolar stability | SifA | NLRC4/NLRP3 | |
| F. novicida | Alternative LPS structure not recognized by Caspase-11 | Tetra-acylated LPS | Caspase-11 |
| Maintain cell wall stability | CRISPR-Cas | AIM2 | |
| L. monocytogenes | Flagellin suppression | Flagellin/MogR | NLRC4 |
| Restrict LLO expression to vacuole | LLO | NLRP3 | |
| Y. pseudotuberculosis | Inhibiting caspase-1 activation | YopM | Caspase-1 |
| L. pneumophila | Maintain vacuolar stability | ShdA | NLRC4, AIM2, Caspase-11 |
| S. flexneri | Inhibiting caspase-1 activation | OspC3 | Caspase-4 |
| Virus | Strategy | Effector | Inflammasome |
| Myxoma virus | Inhibit caspase-1 activation | M13L-PYD | Caspase-1 |
| Cowpox | Inhibit caspase-1 activation | CrmA | Caspase-1 |
| KHSV | Inhibit NLRP1 inflammasome | Orf16 | NLRP1 |
| Vaccinia virus | Inhibit NLRP1 inflammasome | F1L | NLRP1 |
Conclusions
Detecting and responding to intracellular pathogens that have invaded host cells is paramount in defending against infections. Programmed cell death pathways play a key role in this response. Pyroptosis of infected cells removes the protective, intracellular replicative niche of the bacteria, enabling the innate immune effector cells to kill the pathogen. A number of pathogens induce inflammasome activation, and pyroptosis and cytokine production can both play a role in clearance, depending on the infection. Significant work is required to further our understanding of the role of pyroptosis in vivo and the detrimental downstream mediators during systemic, uncontrolled pyroptosis, for example during sepsis. Only then can we begin to identify potential therapeutic strategies for use in treatment of sepsis.
Acknowledgements
This work was funded by NIH grants DK007737 (IJ) and AI097518 (EAM).
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- 2.Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends in microbiology. 2001;9:113–114. doi: 10.1016/s0966-842x(00)01936-3. [DOI] [PubMed] [Google Scholar]
- 3.Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cellular microbiology. 2006;8:1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 4.Siegel RM. Caspases at the crossroads of immune-cell life and death. Nature reviews Immunology. 2006;6:308–317. doi: 10.1038/nri1809. [DOI] [PubMed] [Google Scholar]
- 5.Shao W, Yeretssian G, Doiron K, Hussain SN, Saleh M. The caspase-1 digestome identifies the glycolysis pathway as a target during infection and septic shock. The Journal of biological chemistry. 2007;282:36321–36329. doi: 10.1074/jbc.M708182200. [DOI] [PubMed] [Google Scholar]
- 6.Malireddi RK, Ippagunta S, Lamkanfi M, Kanneganti TD. Cutting edge: proteolytic inactivation of poly(ADP-ribose) polymerase 1 by the Nlrp3 and Nlrc4 inflammasomes. Journal of immunology. 2010;185:3127–3130. doi: 10.4049/jimmunol.1001512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoo L, Hong S, Shin KS, Kang SJ. PARP-1 regulates the expression of caspase-11. Biochemical and biophysical research communications. 2011;408:489–493. doi: 10.1016/j.bbrc.2011.04.070. [DOI] [PubMed] [Google Scholar]
- 8.Fink SL, Bergsbaken T, Cookson BT. Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:4312–4317. doi: 10.1073/pnas.0707370105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edgeworth JD, Spencer J, Phalipon A, Griffin GE, Sansonetti PJ. Cytotoxicity and interleukin-1beta processing following Shigella flexneri infection of human monocyte-derived dendritic cells. European journal of immunology. 2002;32:1464–1471. doi: 10.1002/1521-4141(200205)32:5<1464::AID-IMMU1464>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 10.Feldmeyer L, Keller M, Niklaus G, Hohl D, Werner S, Beer HD. The inflammasome mediates UVB-induced activation and secretion of interleukin-1beta by keratinocytes. Current biology : CB. 2007;17:1140–1145. doi: 10.1016/j.cub.2007.05.074. [DOI] [PubMed] [Google Scholar]
- 11.Elinav E, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Molecular cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 13.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 14.Brunette RL, Young JM, Whitley DG, Brodsky IE, Malik HS, Stetson DB. Extensive evolutionary and functional diversity among mammalian AIM2-like receptors. The Journal of experimental medicine. 2012;209:1969–1983. doi: 10.1084/jem.20121960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cridland JA, et al. The mammalian PYHIN gene family: phylogeny, evolution and expression. BMC evolutionary biology. 2012;12:140. doi: 10.1186/1471-2148-12-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chae JJ, et al. Gain-of-function Pyrin mutations induce NLRP3 protein-independent interleukin-1beta activation and severe autoinflammation in mice. Immunity. 2011;34:755–768. doi: 10.1016/j.immuni.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu H, et al. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature. 2014;513:237–241. doi: 10.1038/nature13449. [DOI] [PubMed] [Google Scholar]
- 18.Broz P, von Moltke J, Jones JW, Vance RE, Monack DM. Differential requirement for Caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell host & microbe. 2010;8:471–483. doi: 10.1016/j.chom.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Opdenbosch N, Gurung P, Vande Walle L, Fossoul A, Kanneganti TD, Lamkanfi M. Activation of the NLRP1b inflammasome independently of ASC-mediated caspase-1 autoproteolysis and speck formation. Nature communications. 2014;5:3209. doi: 10.1038/ncomms4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miao EA, et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nature immunology. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 21.Franchi L, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nature immunology. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 22.Ren T, Zamboni DS, Roy CR, Dietrich WF, Vance RE. Flagellin-deficient Legionella mutants evade caspase-1- and Naip5-mediated macrophage immunity. PLoS pathogens. 2006;2:e18. doi: 10.1371/journal.ppat.0020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molofsky AB, et al. Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. The Journal of experimental medicine. 2006;203:1093–1104. doi: 10.1084/jem.20051659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amer A, et al. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. The Journal of biological chemistry. 2006;281:35217–35223. doi: 10.1074/jbc.M604933200. [DOI] [PubMed] [Google Scholar]
- 25.Warren SE, Mao DP, Rodriguez AE, Miao EA, Aderem A. Multiple Nod-like receptors activate caspase 1 during Listeria monocytogenes infection. Journal of immunology. 2008;180:7558–7564. doi: 10.4049/jimmunol.180.11.7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miao EA, et al. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:3076–3080. doi: 10.1073/pnas.0913087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Y, et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011;477:596–600. doi: 10.1038/nature10510. [DOI] [PubMed] [Google Scholar]
- 28.Kofoed EM, Vance RE. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature. 2011;477:592–595. doi: 10.1038/nature10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rayamajhi M, Zak DE, Chavarria-Smith J, Vance RE, Miao EA. Cutting edge: Mouse NAIP1 detects the type III secretion system needle protein. Journal of immunology. 2013;191:3986–3989. doi: 10.4049/jimmunol.1301549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang J, Zhao Y, Shi J, Shao F. Human NAIP and mouse NAIP1 recognize bacterial type III secretion needle protein for inflammasome activation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:14408–14413. doi: 10.1073/pnas.1306376110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tenthorey JL, Kofoed EM, Daugherty MD, Malik HS, Vance RE. Molecular basis for specific recognition of bacterial ligands by NAIP/NLRC4 inflammasomes. Molecular cell. 2014;54:17–29. doi: 10.1016/j.molcel.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qu Y, et al. Phosphorylation of NLRC4 is critical for inflammasome activation. Nature. 2012;490:539–542. doi: 10.1038/nature11429. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki S, et al. Shigella type III secretion protein MxiI is recognized by Naip2 to induce Nlrc4 inflammasome activation independently of Pkcdelta. PLoS pathogens. 2014;10:e1003926. doi: 10.1371/journal.ppat.1003926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu Z, et al. Crystal structure of NLRC4 reveals its autoinhibition mechanism. Science. 2013;341:172–175. doi: 10.1126/science.1236381. [DOI] [PubMed] [Google Scholar]
- 35.Cummings LA, Wilkerson WD, Bergsbaken T, Cookson BT. In vivo, fliC expression by Salmonella enterica serovar Typhimurium is heterogeneous, regulated by ClpX, and anatomically restricted. Molecular microbiology. 2006;61:795–809. doi: 10.1111/j.1365-2958.2006.05271.x. [DOI] [PubMed] [Google Scholar]
- 36.Miao EA, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nature immunology. 2010;11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Way SS, et al. Characterization of flagellin expression and its role in Listeria monocytogenes infection and immunity. Cellular microbiology. 2004;6:235–242. doi: 10.1046/j.1462-5822.2004.00360.x. [DOI] [PubMed] [Google Scholar]
- 38.Grundling A, Burrack LS, Bouwer HG, Higgins DE. Listeria monocytogenes regulates flagellar motility gene expression through MogR, a transcriptional repressor required for virulence. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:12318–12323. doi: 10.1073/pnas.0404924101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sauer JD, et al. Listeria monocytogenes engineered to activate the Nlrc4 inflammasome are severely attenuated and are poor inducers of protective immunity. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:12419–12424. doi: 10.1073/pnas.1019041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Warren SE, et al. Generation of a Listeria vaccine strain by enhanced caspase-1 activation. European journal of immunology. 2011;41:1934–1940. doi: 10.1002/eji.201041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ceballos-Olvera I, Sahoo M, Miller MA, Del Barrio L, Re F. Inflammasome-dependent pyroptosis and IL-18 protect against Burkholderia pseudomallei lung infection while IL-1beta is deleterious. PLoS pathogens. 2011;7:e1002452. doi: 10.1371/journal.ppat.1002452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen KW, et al. The neutrophil NLRC4 inflammasome selectively promotes IL-1beta maturation without pyroptosis during acute Salmonella challenge. Cell reports. 2014;8:570–582. doi: 10.1016/j.celrep.2014.06.028. [DOI] [PubMed] [Google Scholar]
- 43.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hornung V, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burckstummer T, et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nature immunology. 2009;10:266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 46.Roberts TL, et al. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science. 2009;323:1057–1060. doi: 10.1126/science.1169841. [DOI] [PubMed] [Google Scholar]
- 47.Fernandes-Alnemri T, et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nature immunology. 2010;11:385–393. doi: 10.1038/ni.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rathinam VA, et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nature immunology. 2010;11:395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Warren SE, et al. Cutting edge: Cytosolic bacterial DNA activates the inflammasome via Aim2. Journal of immunology. 2010;185:818–821. doi: 10.4049/jimmunol.1000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu J, Fernandes-Alnemri T, Alnemri ES. Involvement of the AIM2, NLRC4, and NLRP3 inflammasomes in caspase-1 activation by Listeria monocytogenes. Journal of clinical immunology. 2010;30:693–702. doi: 10.1007/s10875-010-9425-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsuchiya K, et al. Involvement of absent in melanoma 2 in inflammasome activation in macrophages infected with Listeria monocytogenes. Journal of immunology. 2010;185:1186–1195. doi: 10.4049/jimmunol.1001058. [DOI] [PubMed] [Google Scholar]
- 52.Jones JW, et al. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9771–9776. doi: 10.1073/pnas.1003738107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sauer JD, Witte CE, Zemansky J, Hanson B, Lauer P, Portnoy DA. Listeria monocytogenes triggers AIM2-mediated pyroptosis upon infrequent bacteriolysis in the macrophage cytosol. Cell host & microbe. 2010;7:412–419. doi: 10.1016/j.chom.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mariathasan S, Weiss DS, Dixit VM, Monack DM. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. The Journal of experimental medicine. 2005;202:1043–1049. doi: 10.1084/jem.20050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang Y, et al. the AIM2 inflammasome is involved in macrophage activation during infection with virulent Mycobacterium bovis strain. The Journal of infectious diseases. 2013;208:1849–1858. doi: 10.1093/infdis/jit347. [DOI] [PubMed] [Google Scholar]
- 56.Saiga H, et al. Critical role of AIM2 in Mycobacterium tuberculosis infection. International immunology. 2012;24:637–644. doi: 10.1093/intimm/dxs062. [DOI] [PubMed] [Google Scholar]
- 57.Shah S, et al. Cutting edge: Mycobacterium tuberculosis but not nonvirulent mycobacteria inhibits IFN-beta and AIM2 inflammasome-dependent IL-1beta production via its ESX-1 secretion system. Journal of immunology. 2013;191:3514–3518. doi: 10.4049/jimmunol.1301331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kerur N, et al. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi Sarcoma-associated herpesvirus infection. Cell host & microbe. 2011;9:363–375. doi: 10.1016/j.chom.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomas C. Roadblocks in HIV research: five questions. Nature medicine. 2009;15:855–859. doi: 10.1038/nm0809-855. [DOI] [PubMed] [Google Scholar]
- 60.Doitsh G, et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2014;505:509–514. doi: 10.1038/nature12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jakobsen MR, et al. IFI16 senses DNA forms of the lentiviral replication cycle and controls HIV-1 replication. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E4571–E4580. doi: 10.1073/pnas.1311669110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Monroe KM, et al. IFI16 DNA sensor is required for death of lymphoid CD4 T cells abortively infected with HIV. Science. 2014;343:428–432. doi: 10.1126/science.1243640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Munoz-Planillo R, Kuffa P, Martinez-Colon G, Smith BL, Rajendiran TM, Nunez G. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 65.Compan V, et al. Cell volume regulation modulates NLRP3 inflammasome activation. Immunity. 2012;37:487–500. doi: 10.1016/j.immuni.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 66.Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nature genetics. 2006;38:240–244. doi: 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- 67.Sastalla I, Crown D, Masters SL, McKenzie A, Leppla SH, Moayeri M. Transcriptional analysis of the three Nlrp1 paralogs in mice. BMC genomics. 2013;14:188. doi: 10.1186/1471-2164-14-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kovarova M, et al. NLRP1-dependent pyroptosis leads to acute lung injury and morbidity in mice. Journal of immunology. 2012;189:2006–2016. doi: 10.4049/jimmunol.1201065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guey B, Bodnar M, Manie SN, Tardivel A, Petrilli V. Caspase-1 autoproteolysis is differentially required for NLRP1b and NLRP3 inflammasome function. Proceedings of the National Academy of Sciences of the United States of America. 2014 doi: 10.1073/pnas.1415756111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Finger JN, et al. Autolytic proteolysis within the function to find domain (FIIND) is required for NLRP1 inflammasome activity. The Journal of biological chemistry. 2012;287:25030–25037. doi: 10.1074/jbc.M112.378323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.D'Osualdo A, Weichenberger CX, Wagner RN, Godzik A, Wooley J, Reed JC. CARD8 and NLRP1 undergo autoproteolytic processing through a ZU5-like domain. PloS one. 2011;6:e27396. doi: 10.1371/journal.pone.0027396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ewald SE, Chavarria-Smith J, Boothroyd JC. NLRP1 is an inflammasome sensor for Toxoplasma gondii. Infection and immunity. 2014;82:460–468. doi: 10.1128/IAI.01170-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cirelli KM, et al. Inflammasome sensor NLRP1 controls rat macrophage susceptibility to Toxoplasma gondii. PLoS pathogens. 2014;10:e1003927. doi: 10.1371/journal.ppat.1003927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Witola WH, et al. NALP1 influences susceptibility to human congenital toxoplasmosis, proinflammatory cytokine response, and fate of Toxoplasma gondii-infected monocytic cells. Infection and immunity. 2011;79:756–766. doi: 10.1128/IAI.00898-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Faustin B, et al. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Molecular cell. 2007;25:713–724. doi: 10.1016/j.molcel.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 76.Gregory SM, et al. Discovery of a viral NLR homolog that inhibits the inflammasome. Science. 2011;331:330–334. doi: 10.1126/science.1199478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gorfu G, et al. Dual role for inflammasome sensors NLRP1 and NLRP3 in murine resistance to Toxoplasma gondii. mBio. 2014;5 doi: 10.1128/mBio.01117-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cavailles P, et al. A highly conserved Toxo1 haplotype directs resistance to toxoplasmosis and its associated caspase-1 dependent killing of parasite and host macrophage. PLoS pathogens. 2014;10:e1004005. doi: 10.1371/journal.ppat.1004005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Quintiliani R, Jr, Quintiliani R. Inhalational anthrax and bioterrorism. Current opinion in pulmonary medicine. 2003;9:221–226. doi: 10.1097/00063198-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 80.Mordue DG, Monroy F, La Regina M, Dinarello CA, Sibley LD. Acute toxoplasmosis leads to lethal overproduction of Th1 cytokines. Journal of immunology. 2001;167:4574–4584. doi: 10.4049/jimmunol.167.8.4574. [DOI] [PubMed] [Google Scholar]
- 81.Gavrilescu LC, Denkers EY. IFN-gamma overproduction and high level apoptosis are associated with high but not low virulence Toxoplasma gondii infection. Journal of immunology. 2001;167:902–909. doi: 10.4049/jimmunol.167.2.902. [DOI] [PubMed] [Google Scholar]
- 82.Masters SL, et al. NLRP1 inflammasome activation induces pyroptosis of hematopoietic progenitor cells. Immunity. 2012;37:1009–1023. doi: 10.1016/j.immuni.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Magitta NF, et al. A coding polymorphism in NALP1 confers risk for autoimmune Addison's disease and type 1 diabetes. Genes and immunity. 2009;10:120–124. doi: 10.1038/gene.2008.85. [DOI] [PubMed] [Google Scholar]
- 84.Li P, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 85.Wang S, Miura M, Jung YK, Zhu H, Li E, Yuan J. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell. 1998;92:501–509. doi: 10.1016/s0092-8674(00)80943-5. [DOI] [PubMed] [Google Scholar]
- 86.Saleh M, et al. Enhanced bacterial clearance and sepsis resistance in caspase-12-deficient mice. Nature. 2006;440:1064–1068. doi: 10.1038/nature04656. [DOI] [PubMed] [Google Scholar]
- 87.Kuida K, et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 88.Kang SJ, et al. Dual role of caspase-11 in mediating activation of caspase-1 and caspase-3 under pathological conditions. The Journal of cell biology. 2000;149:613–622. doi: 10.1083/jcb.149.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science. 2013;341:1250–1253. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kayagaki N, et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341:1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 91.Gurung P, et al. Toll or interleukin-1 receptor (TIR) domain-containing adaptor inducing interferon-beta (TRIF)-mediated caspase-11 protease production integrates Toll-like receptor 4 (TLR4) protein- and Nlrp3 inflammasome-mediated host defense against enteropathogens. The Journal of biological chemistry. 2012;287:34474–34483. doi: 10.1074/jbc.M112.401406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Broz P, et al. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature. 2012;490:288–291. doi: 10.1038/nature11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rathinam VA, et al. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell. 2012;150:606–619. doi: 10.1016/j.cell.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Case CL, et al. Caspase-11 stimulates rapid flagellin-independent pyroptosis in response to Legionella pneumophila. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:1851–1856. doi: 10.1073/pnas.1211521110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aachoui Y, et al. Caspase-11 protects against bacteria that escape the vacuole. Science. 2013;339:975–978. doi: 10.1126/science.1230751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schauvliege R, Vanrobaeys J, Schotte P, Beyaert R. Caspase-11 gene expression in response to lipopolysaccharide and interferon-gamma requires nuclear factor-kappa B and signal transducer and activator of transcription (STAT) 1. The Journal of biological chemistry. 2002;277:41624–41630. doi: 10.1074/jbc.M207852200. [DOI] [PubMed] [Google Scholar]
- 97.Hur J, Kim SY, Kim H, Cha S, Lee MS, Suk K. Induction of caspase-11 by inflammatory stimuli in rat astrocytes: lipopolysaccharide induction through p38 mitogen-activated protein kinase pathway. FEBS letters. 2001;507:157–162. doi: 10.1016/s0014-5793(01)02975-1. [DOI] [PubMed] [Google Scholar]
- 98.Shi J, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 99.Kang SJ, et al. Caspase-11 is not necessary for chemotherapy-induced intestinal mucositis. DNA and cell biology. 2004;23:490–495. doi: 10.1089/1044549041562302. [DOI] [PubMed] [Google Scholar]
- 100.Lin XY, Choi MS, Porter AG. Expression analysis of the human caspase-1 subfamily reveals specific regulation of the CASP5 gene by lipopolysaccharide and interferon-gamma. The Journal of biological chemistry. 2000;275:39920–39926. doi: 10.1074/jbc.M007255200. [DOI] [PubMed] [Google Scholar]
- 101.Raymond AA, et al. Nine procaspases are expressed in normal human epidermis, but only caspase-14 is fully processed. The British journal of dermatology. 2007;156:420–427. doi: 10.1111/j.1365-2133.2006.07656.x. [DOI] [PubMed] [Google Scholar]
- 102.Akhter A, et al. Caspase-11 promotes the fusion of phagosomes harboring pathogenic bacteria with lysosomes by modulating actin polymerization. Immunity. 2012;37:35–47. doi: 10.1016/j.immuni.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pilla DM, et al. Guanylate binding proteins promote caspase-11-dependent pyroptosis in response to cytoplasmic LPS. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:6046–6051. doi: 10.1073/pnas.1321700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Meunier E, Broz P. Interferon-Induced Guanylate-Binding Proteins Promote Cytosolic Lipopolysaccharide Detection by Caspase-11. DNA and cell biology. 2014 doi: 10.1089/dna.2014.2701. [DOI] [PubMed] [Google Scholar]
- 105.Demon D, Kuchmiy A, Fossoul A, Zhu Q, Kanneganti TD, Lamkanfi M. Caspase-11 is expressed in the colonic mucosa and protects against dextran sodium sulfate-induced colitis. Mucosal immunology. 2014;7:1504. doi: 10.1038/mi.2014.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Knodler LA, et al. Noncanonical inflammasome activation of caspase-4/caspase-11 mediates epithelial defenses against enteric bacterial pathogens. Cell host & microbe. 2014;16:249–256. doi: 10.1016/j.chom.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Takeuchi O, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 108.Kayagaki N, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 109.von Moltke J, et al. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature. 2012;490:107–111. doi: 10.1038/nature11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Leemans JC, Cassel SL, Sutterwala FS. Sensing damage by the NLRP3 inflammasome. Immunological reviews. 2011;243:152–162. doi: 10.1111/j.1600-065X.2011.01043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Romberg N, et al. Mutation of NLRC4 causes a syndrome of enterocolitis and autoinflammation. Nature genetics. 2014;46:1135–1139. doi: 10.1038/ng.3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Canna SW, et al. An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nature genetics. 2014;46:1140–1146. doi: 10.1038/ng.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kitamura A, Sasaki Y, Abe T, Kano H, Yasutomo K. An inherited mutation in NLRC4 causes autoinflammation in human and mice. The Journal of experimental medicine. 2014;211:2385–2396. doi: 10.1084/jem.20141091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Peel M, Donachie W, Shaw A. Physical and antigenic heterogeneity in the flagellins of Listeria monocytogenes and L. ivanovii. Journal of general microbiology. 1988;134:2593–2598. doi: 10.1099/00221287-134-9-2593. [DOI] [PubMed] [Google Scholar]
- 115.Tsuji NM, et al. Roles of caspase-1 in Listeria infection in mice. International immunology. 2004;16:335–343. doi: 10.1093/intimm/dxh041. [DOI] [PubMed] [Google Scholar]
- 116.Ge J, Gong YN, Xu Y, Shao F. Preventing bacterial DNA release and absent in melanoma 2 inflammasome activation by a Legionella effector functioning in membrane trafficking. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6193–6198. doi: 10.1073/pnas.1117490109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sampson TR, et al. A CRISPR-Cas system enhances envelope integrity mediating antibiotic resistance and inflammasome evasion. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:11163–11168. doi: 10.1073/pnas.1323025111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.LaRock CN, Cookson BT. The Yersinia virulence effector YopM binds caspase-1 to arrest inflammasome assembly and processing. Cell host & microbe. 2012;12:799–805. doi: 10.1016/j.chom.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brodsky IE, et al. A Yersinia effector protein promotes virulence by preventing inflammasome recognition of the type III secretion system. Cell host & microbe. 2010;7:376–387. doi: 10.1016/j.chom.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kobayashi T, et al. The Shigella OspC3 effector inhibits caspase-4, antagonizes inflammatory cell death, and promotes epithelial infection. Cell host & microbe. 2013;13:570–583. doi: 10.1016/j.chom.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 121.Johnston JB, et al. A poxvirus-encoded pyrin domain protein interacts with ASC-1 to inhibit host inflammatory and apoptotic responses to infection. Immunity. 2005;23:587–598. doi: 10.1016/j.immuni.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 122.Kanneganti TD. Central roles of NLRs and inflammasomes in viral infection. Nature reviews Immunology. 2010;10:688–698. doi: 10.1038/nri2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gerlic M, et al. Vaccinia virus F1L protein promotes virulence by inhibiting inflammasome activation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:7808–7813. doi: 10.1073/pnas.1215995110. [DOI] [PMC free article] [PubMed] [Google Scholar]