Abstract
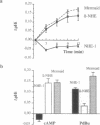
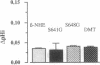
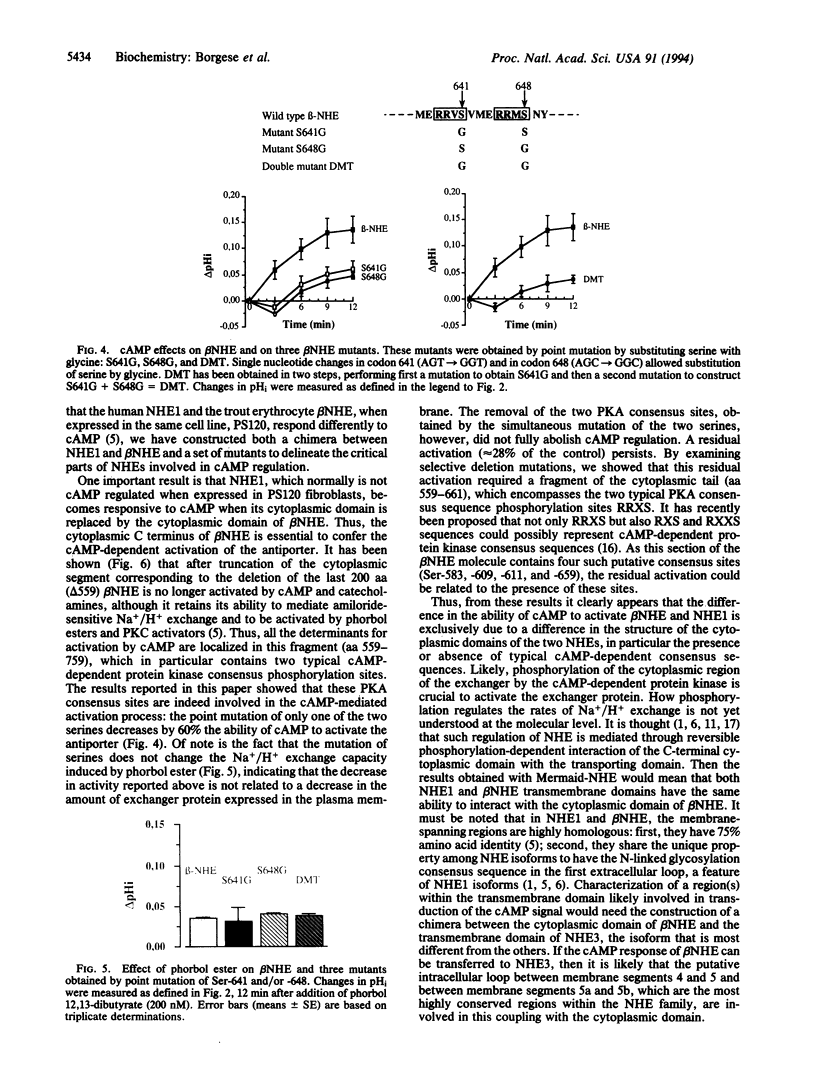
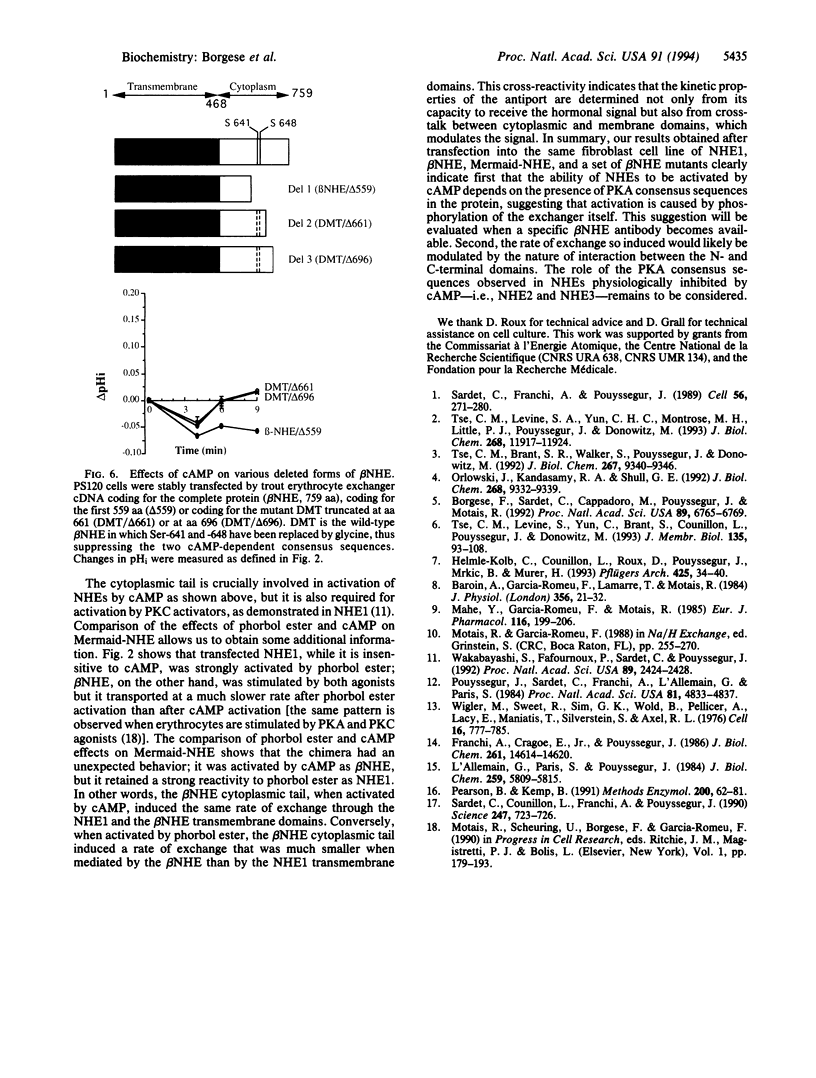
Studies of the effect of cAMP on the cloned Na+/H+ exchangers (NHEs) are difficult to interpret as variable results have been reported for the different isoforms when expressed in various cell types. We took advantage of the fact that the human NHE1 and the trout erythrocyte beta NHE, when expressed in the same cell line, PS120, respond differently to cAMP (NHE1 is insensitive, beta NHE is activated) to analyze the molecular mechanisms of cAMP activation. We constructed both a chimera between NHE1 and beta NHE and a set of beta NHE mutants to delineate the critical parts of the molecule involved in the activation process. NHE1 becomes cAMP stimulated when its cytoplasmic domain is replaced by the cytoplasmic domain of beta NHE; thus, the cytoplasmic C terminus of beta NHE, which contains two cAMP-dependent consensus sequences, is essential to confer cAMP dependence. Serine to glycine substitution of only one of the two protein kinase A (PKA) consensus sites decreased by 60% the ability of cAMP to activate Na+/H+ exchange. Simultaneous Ser to Gly substitution of the two PKA consensus sites decreased the cAMP-mediated activation by 72%. The residual activation required a cytoplasmic fragment (aa 559-661) that contains four sequences considered likely as putative PKA consensus sites. The results obtained with the chimeric NHE also demonstrated that if the cytoplasmic C terminus is crucially involved in the hormonal activation, the rate of Na+/H+ exchange so induced can be modulated by the nature of the interaction between the N- and C-terminal domains.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baroin A., Garcia-Romeu F., Lamarre T., Motais R. A transient sodium-hydrogen exchange system induced by catecholamines in erythrocytes of rainbow trout, Salmo gairdneri. J Physiol. 1984 Nov;356:21–31. doi: 10.1113/jphysiol.1984.sp015450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgese F., Sardet C., Cappadoro M., Pouyssegur J., Motais R. Cloning and expression of a cAMP-activated Na+/H+ exchanger: evidence that the cytoplasmic domain mediates hormonal regulation. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6765–6769. doi: 10.1073/pnas.89.15.6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi A., Cragoe E., Jr, Pouysségur J. Isolation and properties of fibroblast mutants overexpressing an altered Na+/H+ antiporter. J Biol Chem. 1986 Nov 5;261(31):14614–14620. [PubMed] [Google Scholar]
- Helmle-Kolb C., Counillon L., Roux D., Pouysségur J., Mrkic B., Murer H. Na/H exchange activities in NHE1-transfected OK-cells: cell polarity and regulation. Pflugers Arch. 1993 Oct;425(1-2):34–40. doi: 10.1007/BF00374501. [DOI] [PubMed] [Google Scholar]
- L'Allemain G., Paris S., Pouysségur J. Growth factor action and intracellular pH regulation in fibroblasts. Evidence for a major role of the Na+/H+ antiport. J Biol Chem. 1984 May 10;259(9):5809–5815. [PubMed] [Google Scholar]
- Mahé Y., Garcia-Romeu F., Motais R. Inhibition by amiloride of both adenylate cyclase activity and the Na+/H+ antiporter in fish erythrocytes. Eur J Pharmacol. 1985 Oct 22;116(3):199–206. doi: 10.1016/0014-2999(85)90154-2. [DOI] [PubMed] [Google Scholar]
- Orlowski J., Kandasamy R. A., Shull G. E. Molecular cloning of putative members of the Na/H exchanger gene family. cDNA cloning, deduced amino acid sequence, and mRNA tissue expression of the rat Na/H exchanger NHE-1 and two structurally related proteins. J Biol Chem. 1992 May 5;267(13):9331–9339. [PubMed] [Google Scholar]
- Pearson R. B., Kemp B. E. Protein kinase phosphorylation site sequences and consensus specificity motifs: tabulations. Methods Enzymol. 1991;200:62–81. doi: 10.1016/0076-6879(91)00127-i. [DOI] [PubMed] [Google Scholar]
- Pouysségur J., Sardet C., Franchi A., L'Allemain G., Paris S. A specific mutation abolishing Na+/H+ antiport activity in hamster fibroblasts precludes growth at neutral and acidic pH. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4833–4837. doi: 10.1073/pnas.81.15.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardet C., Counillon L., Franchi A., Pouysségur J. Growth factors induce phosphorylation of the Na+/H+ antiporter, glycoprotein of 110 kD. Science. 1990 Feb 9;247(4943):723–726. doi: 10.1126/science.2154036. [DOI] [PubMed] [Google Scholar]
- Sardet C., Franchi A., Pouysségur J. Molecular cloning, primary structure, and expression of the human growth factor-activatable Na+/H+ antiporter. Cell. 1989 Jan 27;56(2):271–280. doi: 10.1016/0092-8674(89)90901-x. [DOI] [PubMed] [Google Scholar]
- Tse C. M., Brant S. R., Walker M. S., Pouyssegur J., Donowitz M. Cloning and sequencing of a rabbit cDNA encoding an intestinal and kidney-specific Na+/H+ exchanger isoform (NHE-3). J Biol Chem. 1992 May 5;267(13):9340–9346. [PubMed] [Google Scholar]
- Tse C. M., Levine S. A., Yun C. H., Montrose M. H., Little P. J., Pouyssegur J., Donowitz M. Cloning and expression of a rabbit cDNA encoding a serum-activated ethylisopropylamiloride-resistant epithelial Na+/H+ exchanger isoform (NHE-2). J Biol Chem. 1993 Jun 5;268(16):11917–11924. [PubMed] [Google Scholar]
- Tse M., Levine S., Yun C., Brant S., Counillon L. T., Pouyssegur J., Donowitz M. Structure/function studies of the epithelial isoforms of the mammalian Na+/H+ exchanger gene family. J Membr Biol. 1993 Aug;135(2):93–108. doi: 10.1007/BF00231435. [DOI] [PubMed] [Google Scholar]
- Wakabayashi S., Fafournoux P., Sardet C., Pouysségur J. The Na+/H+ antiporter cytoplasmic domain mediates growth factor signals and controls "H(+)-sensing". Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2424–2428. doi: 10.1073/pnas.89.6.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]