Abstract
The authors evaluated the usefulness of intraoperative photodynamic eye (PDE) observation in patients with nonocclusive mesenteric ischemia (NOMI). Between February 2012 and July 2013, 6 patients who had undergone emergency surgery for NOMI were enrolled. Intraoperative PDE observation was performed to decide the adequate length of bowel resection including all skipped dark spots, which could not be detected as ongoing mucosal ischemic changes under visible light observation. All ongoing mucosal ischemic changes were easily detected as dark spots using PDE observation in all 6 patients. The mean length of adequate ileal resection (92 ± 48 cm) was significantly longer than that of ischemic ileum (85 ± 50 cm) (mean ± SD) (P = 0.043). After resection of an adequate length of bowel, all the patients had a good course until discharge without incidents due to residual bowel ischemia, except for 1patient who died. PDE observation is useful for deciding the adequate length of bowel to resect, including ongoing mucosal ischemic changes that cannot be detected under visible light observation. In patients with NOMI, resection of an adequate length of bowel is necessary to prevent postoperative incidents due to residual bowel ischemia.
Key words: Dark spot, Fluorescence, Indocyanine green, Nonocclusive mesenteric ischemia, Photodynamic eye
Numerous studies have provided evidence for the usefulness of imaging-guided surgery. The photodynamic eye (PDE), in particular, can clearly reveal bile flow1 and blood flow2,3 by highly sensitive fluorescence imaging after intravenous injection of indocyanine green (ICG). In fact, PDE has a wide range of surgical applications, such as sentinel lymph node navigation surgery in patients with breast cancer,4,5 detection of the biliary tract in hepato-biliary surgery,1 evaluation of blood flow through vascular anastomoses in cardiovascular surgery6 and detection of chyle fistula after esophagectomy,7
Nonocclusive mesenteric ischemia (NOMI) is a life-threatening condition that requires emergency surgery.8 In patients with NOMI, necrotic changes in the bowel may progress, even if mesenteric arterial blood flow is not obstructed. Therefore, the ongoing ischemic changes that spread from the mucosa to the serosa are difficult to detect by extra-luminal observation alone. In order to prevent postoperative incidents related to residual bowel ischemia, it is necessary to resect an adequate length of bowel that includes all ongoing mucosal ischemic changes.
Although there are several studies which reported the usefulness of fluorescein fluorescence for NOMI patients,9,10 a recent report has described the use of new color CCD camera system, known as the HyperEye Medical System (HEMS, Mizuho Co, Ltd, Sakura, Japan), which can simultaneously detect color and near-infrared rays under room light, allowing precise intraoperative evaluation of mesenteric and bowel circulation for a NOMI patient.11 And the report demonstrated that fluorescence was not observed in infarctions in several segments of the bowel wall. However, the ability of PDE for diagnosing NOMI remained unclarified.
Accordingly, we hypothesized that if all such infarctions could be included in the length of bowel resected, there would be no postoperative incidents associated with residual bowel ischemia. Therefore, PDE observation would be useful for determining the adequate length of bowel that should be resected.
In order to test this hypothesis, therefore, we performed intraoperative observation using PDE in patients with NOMI and investigated the resulting postoperative outcomes.
Materials and Methods
We prospectively collected data for 6 patients who underwent emergency surgery for NOMI at the Department of Gastroenterological Surgery, Dokkyo Medical University, between February 2012 and July 2013 under the same trained surgical team. All of the patients were diagnosed as having NOMI on the basis of intraoperative findings. NOMI was first described by Ende et al in 1958 as a disease affecting the vasculature of the intestine.12 NOMI is defined as “intestinal gangrene in the presence of a patent arterial tree.”13 Although patients with NOMI have good blood flow in mesenteric vessels, ischemic, or necrotic changes occur in the small or large bowel.
Intraoperative observation was performed using a PDE (Hamamatsu Photonics K.K., Hamamatsu, Japan; Fig. 1a). ICG is activated by emitted light (wavelength: 760 nm) and filters out light with a wavelength below 820 nm. The light source for activation of ICG is a bank of light-emitting diodes (LEDs), and the detector is a charge-coupled device (CCD) camera.
Fig. 1.
(a) The PDE system consists of a desktop computer and a CDC camera connected with a flexible line. The light handy-type instrument is very convenient for performing highly sensitive intraoperative observation. (b) This hand-piece instrument allows free observation of the abdominal cavity with high sensitivity.
The camera and its flexible line are covered with a sterile vinyl bag and can be easily handled in the operating area from any angle. Before observation, 1 mL of ICG (2.5 mg/mL) was injected intravenously into the patient. Then, about 40 seconds after the injection, blood flow in the mesentery, and the small or large bowel, was observed using the CCD camera during the operation. The images were recorded and viewed directly on a desktop computer. This hand-held instrument allows free observation of the abdominal cavity with high sensitivity (Fig. 1b).
None of the patients had an allergic reaction due to ICG injection. Informed consent was obtained from the patients or their representatives before surgery. This study was approved by the ethics committee of our institute.
Statistical Analysis
Data are presented as mean ± standard deviation (SD), with the median and range. Differences between values obtained before and after PDE assessment of the length of bowel ischemia were analyzed using Student's t test. Statistical analyses were performed using the SPSS statistical software package, version 16.0 (SPSS Inc, Chicago, Illinois) at a significance level of P < 0.05.
Results
Six patients (male:female = 5:1) underwent emergency surgery for acute abdomen and were diagnosed as having NOMI on the basis of intraoperative findings. Most patients had several clinical complications such as diabetes mellitus (DM; n = 4), chronic renal failure (CRF; n = 4), chronic respiratory dysfunction (CRD; n = 1) and old myocardial infarction (OMI; n =1). In fact, 3 patients had received hemodialysis (HD) preoperatively, and 1 patient received HD after surgery. In addition, 2 patients had hepatic portal venous gas (HPVG) and 1 patient was diagnosed as having pneumatosis cystoides intestinalis (PCI) before surgery (Table 1).
Table 1.
Clinical background characteristics of patients undergoing surgery for NOMI
The mean age of the patients was 73 ± 8 years (mean ± standard deviation) (median: 72.5; range, 62–82 years), and the mean operation time was 116 ± 39 minutes (median: 124.5; range, 50–157 minutes; Table 1).
All dark spots were easily detectable by PDE observation as areas lacking fluorescence (Fig. 2). Three patients underwent partial resection of the ileum, and 3 patients underwent extended ileocecal resection because dark spots were also detected in the cecum (Table 2).
Fig. 2.
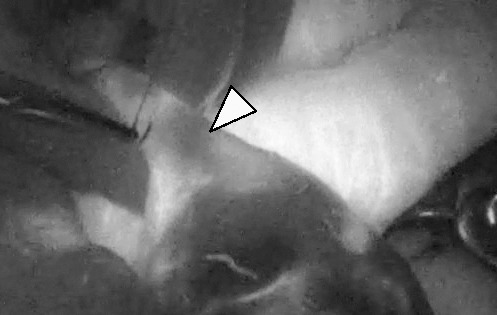
Ongoing mucosal ischemic change is easily detected by PDE as a skipped dark spot. The arrow indicates a dark spot, which is sutured for inclusion in adequate bowel resection.
Table 2.
Length of adequate resection required in patients undergoing surgery for NOMI
All resections were performed using an Echelon Flex 60 Endopath linear stapler (Ethicon Endo-Surgery, Inc, Cincinnati, Ohio) and all reconstructions were performed in a functional end-to-end manner using the same device. Although all anastomoses were observed using PDE to confirm whether blood flow was sufficient, no patients with dark spots had insufficient anastomotic blood flow, and evaluation was easily achieved on the basis of fluorescence. After reconstruction, additional observations using PDE demonstrated no dark spots in the residual small or large bowel.
The mean length of ischemic bowel was 85 ± 50 cm (median: 75; range, 30–180 cm), and the mean length of adequate resection required was 92 ± 48 cm (median: 82.5; range, 35–180 cm) (Table 2). The mean length of adequate ileal resection determined using PDE was significantly greater than that of the ischemic ileum decided under visible light observation (P = 0.043; Student's t test).
Among the 6 patients, only 2 showed compatibility between the length of ischemic bowel decided on the basis of visible light observation and the length of bowel resection considered adequate on the basis of PDE observation. Therefore, most patients (67%, 4/6) required resection of a longer length of bowel than the length of ischemic bowel evaluated by visible light observation. Most such patients (75%, 3/4) underwent extended ileocecal resection to include ischemic change in the cecum.
Table 3 shows the pathologic findings for the marginal area of resected bowels. All patients had ulceration or necrosis in the oral side of the resected bowel. Among 3 patients who had no ulceration or necrosis in the anal side of the resected bowel, 2 patients underwent extended ileocecal resection. When the ileocecal resection was performed, the anal side resection was performed in the normal area of the colon to prevent anastomotic leakage due to bowel ischemia.
Table 3.
Pathologic findings for the marginal areas of resected bowels
All patients had a good postoperative course until discharge without incidents due to residual bowel ischemia except for one patient, who died due to multiple organ failure (Table 2). No patients were readmitted due to bowel ischemia in the observation period. The mean observation period was 116 ± 190 days (median: 41; range, 20–503 days; Table 2).
Discussion
NOMI is usually caused by intense vasoconstriction in the setting of splanchnic hypoperfusion resulting from a variety of conditions. Among several risk factors for NOMI,14 hypotension is the most important and immediate precipitating factor in patients undergoing HD. The susceptibility of HD patients to NOMI can be explained by the common known risk factors in such patients, which may include widespread atherosclerosis, advanced age, long-standing hypertension, DM, congestive heart failure, arrhythmias and the use of mesenteric vasoconstrictor drugs.15,16
In fact, the patients in our present series included 4 elderly patients aged over 70 years (67%), 4 patients (67%) who received HD therapy, and 4 patients with DM (67%). Because NOMI is also associated with high mortality17 due to delayed diagnosis and treatment initiation,18,19 early recognition and emergency surgery are important in order to rescue such patients. In fact, 1 of the present patients died due to multiple organ dysfunction syndrome (MODS).
There are preoperative and intraoperative imaging examinations to estimate bowel ischemia. Computed tomography (CT) and magnetic resonance imaging (MRI) are well known as representative preoperative examinations. Especially, enhance CT using contrast agent is commonly used to estimate the bloodstream of the bowel, because there is a clear demarcation between bowel ischemia and nonischemia in patients with thrombosis or strangulation ileus. However, there is no clear demarcation in NOMI patients, because arterial flow of mesenterium is preserved in such patients. On the other hand, MRI is rarely used to estimate bowel ischemia of such disease. Because MRI takes a longer time to construct images than CT, MRI has a lesser advantage to estimate the bloodstream of the bowel than CT. On the same way, with regard to intraoperative imaging examinations, among several modalities reported to be useful for diagnosis of NOMI, Doppler ultrasound and fluorescein fluorescence are well known.9,10,20 Recently, PDE is regarded as a variable device to directly evaluate the bloodstream of bowel ischemia not only NOMI but also thrombosis or strangulation ileus. In fact, intraoperative PDE observation can easily demonstrate the dark spots which are not detected by preoperative imaging in NOMI patients. In fact, although preoperative enhance CT using contrast agent could detect the necrotic change of the bowel or pneumatosis cystoides intestinalis (PCI; Table 1), PDE could detect longer length of ischemic bowel than that of preoperative CT examination could (Table 2). Therefore, intraoperative PDE observation would be superior to preoperative CT examination, because PDE could detect ongoing ischemic change as dark spots, which could not be detected by preoperative CT examination.
The need for bowel resection in patients with bowel ischemia due to thrombosis is clearly decided, because the area of arterial obstruction is well correlated with bowel color change. However, in patients with NOMI, it is difficult to define the area of bowel resection because arterial flow is not obstructed and, particularly, the areas of ischemic change in the bowel are not located sequentially, sometimes showing skipping. In addition, ongoing mucosal ischemic changes cannot be detected by observation from the serosal side. We have found that even if intraoperative colonoscopy demonstrates necrotic changes in the mucosa of the terminal ileum, such changes cannot be recognized from the serosal side by observation under visible light, whereas PDE observation is able to reveal such mucosal necrotic changes as skipped dark spots.
In the present series of patients with NOMI, we found that use of the PDE allowed easy detection of ongoing mucosal ischemic changes as areas lacking fluorescence, as well as bowel ischemia when anastomotic blood flow was examined after bowel resection. In order to prevent postoperative incidents associated with bowel ischemia, we carried out additional bowel resection including such areas of ongoing ischemic change that were detected by PDE.
In fact, all patients in our present series had ulceration or necrosis in the oral side of the resected bowels. Therefore, skipped dark spots which were detected by PDE observation should be included in the surgical resection, because there were pathologically proved ongoing ischemic changes.
Intraoperative PDE observation has 3 merits:
First, it can clearly demonstrate blood flow in the mesentery. Therefore, in patients with strangulation ileus, PDE observation is very useful for evaluation of mesenteric blood flow or ischemia of the small bowel after resolution of the strangulation.
Second, PDE can easily determine the blood flow through anastomoses after reconstruction. Therefore, routine PDE observation should be performed to evaluate anastomotic blood flow after reconstruction of the small bowel, not only in NOMI patients but also patients with strangulation ileus.
Third, PDE can easily detect skipped ongoing mucosal ischemic changes from the serosal side as areas of fluorescence. This makes it unnecessary to reconfirm mucosal necrosis by intraoperative colonoscopy or direct observation by opening the small bowel.
However, intraoperative PDE observation has one demerit. Because PDE observation of fluorescence is qualitative, it cannot provide a quantitative estimate of blood flow. Therefore, there is a degree of risk in performing resection on the basis of an operator's subjective judgment, because it is not possible to determine quantitatively whether a dark spot represents a reversible or an irreversible change.
Although there is no evidence whether ongoing mucosal ischemic changes detectable as skipped dark spots will, in fact, result in tissue necrosis, we considered that additional resection would reduce the risk of postoperative incidents such as bowel ischemia. However, a prospective observational study of this issue will be required. However, 5 of the present 6 patients who underwent surgery for NOMI based on intraoperative PDE observation had a good postoperative course until discharge, without any incidents associated with bowel ischemia or symptoms attributable to their short bowel. Because most necrotic changes occurred in the ileum, most patients underwent partial ileal resection. Our initial experience suggests that this approach is indeed effective for rescue of such patients. Although PDE evaluation before and after surgery revealed a significant difference in the length of bowel ischemia, no patients suffered short bowel syndrome, even after extended ileocecal resection.
A recent report has described the use of a new color CCD camera system, known as the HyperEye Medical System (HEMS, Mizuho Co, Ltd), which can simultaneously detect color and near-infrared rays under room light, allowing precise intraoperative evaluation of mesenteric and bowel circulation.11 Although that report involved only 1 patient who underwent surgery for NOMI, the results lend strong support to our present study, as the authors noted infarction in several segments of the bowel, and concluded that evaluation of the bowel circulation is necessary for deciding the extent of resection.
Therefore, we conclude that intraoperative PDE observation is a promising procedure for detection of fine areas of residual ongoing ischemic change that are not evident under visible light during surgery for patients with NOMI, and we believe that this procedure would reduce the incidence of postoperative complications associated with bowel ischemia.
Acknowledgments
We have no conflicts of interest to declare. We received no funding/grant support for this study.
References
- 1.Tagaya N, Shimoda M, Kato M, Nakagawa A, Abe A, Iwasaki Y, et al. Intraoperative exploration of biliary anatomy using fluorescence imaging of indocyanine green in experimental and clinical cholecystectomies. J Hepatobiliary Pancreat Sci. 2010;17(5):595–600. doi: 10.1007/s00534-009-0195-2. [DOI] [PubMed] [Google Scholar]
- 2.Kubota K, Kita J, Shimoda M, Rokkaku K, Kato M, Iso Y, et al. Intraoperative assessment of reconstructed vessels in living-donor liver transplantation, using a novel fluorescence imaging technique. J Hepatobiliary Pancreat Surg. 2006;13(2):100–104. doi: 10.1007/s00534-005-1014-z. [DOI] [PubMed] [Google Scholar]
- 3.Sawada T, Solly M, Kita J, Shimoda M, Kubota K. An alternative tool for intraoperative assessment of renal vasculature after revascularization of a transplanted kidney. Am J Surg. 2010;199(6):e69–71. doi: 10.1016/j.amjsurg.2009.08.049. [DOI] [PubMed] [Google Scholar]
- 4.Tagaya N, Yamazaki R, Nakagawa A, Abe A, Hamada K, Kubota K, et al. Intraoperative identification of sentinel lymph nodes by near-infrared fluorescence imaging in patients with breast cancer. Am J Surg. 2008;195(6):850–853. doi: 10.1016/j.amjsurg.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 5.Tagaya N, Aoyagi H, Nakagawa A, Abe A, Iwasaki Y, Tachibana M, et al. A novel approach for sentinel lymph node identification using fluorescence imaging and image overlay navigation surgery in patients with breast cancer. World J Surg. 2011;35(1):154–158. doi: 10.1007/s00268-010-0811-y. [DOI] [PubMed] [Google Scholar]
- 6.Unno N, Suzuki M, Yamamoto N, Inuzuka K, Sagara D, Nishiyama M, et al. Indocyanine green fluorescence angiography for intraoperative assessment of blood flow: a feasibility study. Eur J Vasc Endovasc Surg. 2008;35(2):205–207. doi: 10.1016/j.ejvs.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Kamiya K, Unno N, Konno H. Intraoperative indocyanine green fluorescence lymphography, a novel imaging technique to detect a chyle fistula after an esophagectomy: report of a case. Surg Today. 2009;39(5):421–424. doi: 10.1007/s00595-008-3852-1. [DOI] [PubMed] [Google Scholar]
- 8.Trompeter M, Brazda T, Remy CT, Vestring T, Reimer P. Non-occlusive mesenteric ischemia: etiology, diagnosis, and interventional therapy. Eur Radiol. 2002;12(5):1179–1187. doi: 10.1007/s00330-001-1220-2. [DOI] [PubMed] [Google Scholar]
- 9.Mann A, Fazio VW, Lucas FV. A comparative study of the use of fluorescein and the Doppler device in the determination of intestinal viability. Surg Gynecol Obstet. 1982;154(1):53–55. [PubMed] [Google Scholar]
- 10.Carter MS, Fantini GA, Sammartano RJ, Mitsudo S, Silverman DG, Boley SJ. Qualitative and quantitative fluorescein fluorescence in determining intestinal viability. Am J Surg. 1984;147(1):117–123. doi: 10.1016/0002-9610(84)90044-8. [DOI] [PubMed] [Google Scholar]
- 11.Nitori N, Deguchi T, Kubota K, Yoshida M, Kato A, Kojima M, et al. Successful treatment of non-occlusive mesenteric ischemia (NOMI) using the HyperEye Medical System for intraoperative visualization of the mesenteric and bowel circulation: report of a case. Surg Today. 2014;44(2):359–362. doi: 10.1007/s00595-013-0503-y. [DOI] [PubMed] [Google Scholar]
- 12.Ende N. Infarction of the bowel in cardiac failure. N Engl J Med. 1958;258(18):879–881. doi: 10.1056/NEJM195805012581804. [DOI] [PubMed] [Google Scholar]
- 13.Archodovassilis F, Lagoudiannakis EE, Tsekouras DK, Vlachos K, Albanopoulos K, Fillis K, et al. Nonocclusive mesenteric ischemia: a lethal complication in peritoneal dialysis patients. Perit Dial Int. 2007;27(2):136–141. [PubMed] [Google Scholar]
- 14.Acosta S, Ogren M, Sternby NH, Bergqvist D, Bjorck M. Fatal nonocclusive mesenteric ischaemia: population-based incidence and risk factors. J Intern Med. 2006;259(3):305–313. doi: 10.1111/j.1365-2796.2006.01613.x. [DOI] [PubMed] [Google Scholar]
- 15.Bassilios N, Menoyo V, Berger A, Mamzer MF, Daniel F, Cluzel P, et al. Mesenteric ischaemia in haemodialysis patients: a case/control study. Nephrol Dial Transplant. 2003;18(5):911–917. doi: 10.1093/ndt/gfg004. [DOI] [PubMed] [Google Scholar]
- 16.Ori Y, Chagnac A, Schwartz A, Herman M, Weinstein T, Zevin D, et al. Non-occlusive mesenteric ischemia in chronically dialyzed patients: a disease with multiple risk factors. Nephron Clin Pract. 2005;101(2):c87–93. doi: 10.1159/000086346. [DOI] [PubMed] [Google Scholar]
- 17.Boley SJ, Sprayregan S, Siegelman SS, Veith FJ. Initial results from an aggressive roentgenological and surgical approach to acute mesenteric ischemia. Surgery. 1977;82(6):848–855. [PubMed] [Google Scholar]
- 18.Haglund U, Lundgren O. Non-occlusive acute intestinal vascular failure. Br J Surg. 1979;66(3):155–158. doi: 10.1002/bjs.1800660305. [DOI] [PubMed] [Google Scholar]
- 19.Stoney RJ, Cunningham CG. Acute mesenteric ischemia. Surgery. 1993;114(3):489–490. [PubMed] [Google Scholar]
- 20.Wright CB, Hobson RW., 2nd Prediction of intestinal viability using Doppler ultrasound technics. Am J Surg. 1975;129(6):642–645. doi: 10.1016/0002-9610(75)90337-2. [DOI] [PubMed] [Google Scholar]



