Abstract
In this study, we aimed to compare the effects of dexpanthenol and N-acetylcysteine on wound healing. The wound healing process is a multifaceted sequence of activities associated with tissue restoration process. A number of investigations and clinical studies have been performed to determine new approaches for the improvement of wound healing. A total of 30 rats were divided into 3 equal groups. A linear 2-cm incision was made in the rats' skin. No treatment was administered in the first (control) group. Dexpanthenol cream was administered to the rats in the second group and 3% N-acetylcysteine cream was administered to the rats in the third group. The wound areas of all of the rats were measured on certain days. On the 21st day, all wounds were excised and histologically evaluated. The epithelialization and granulation rates between the groups were revealed to be similar in microscopic evaluations. Although the fibrosis was remarkable in the control group as compared with the other groups, it was similar in N-acetylcysteine and dexpanthenol groups. Angiogenesis rate was remarkable in the N-acetylcysteine group compared with the others. In multiple-comparison analysis, Dexpanthenol and N-acetylcysteine groups had similar results in terms of wound healing rates (P < 0.05), which were both higher than in the control group (P > 0.05). The efficacy of N-acetylcysteine in wound healing is comparable to dexpanthenol, and both substances can be used to improve wound healing.
Key words: N-acetylcysteine, Dexpanthenol, Wound healing
Wound healing is a multifaceted sequence of activities associated with the tissue restoration process.1 This activity takes place in 4 main steps: inflammation, proliferation, matrix deposition, and remodeling.2,3 Moreover, there are many factors that might have harmful effects on the wound healing process. In this regard, there have been numerous analyses and clinical studies aiming to find new methods for the improvement of wound healing processes.4
Dexpanthenol is the biologically-active alcohol of pantothenic acid, which leads to an elevation in the amount of coenzyme A in the cell. Dexpanthenol is extensively used in topical form, since it can easily penetrate the skin even at large local concentrations. When used in formulations, dexpanthenol is most effective for the stimulation of epithelialization, granulation, and the mitigation of itching.5
N-acetylcysteine (NAC) is a prodrug that supplies bioavailable cysteine for glutathione replenishment and prevents oxidative damage as well as inflammation. It also leads to glutathione (GSH) formation in the body. Besides fostering angiogenesis, it is used to scavenge free radicals. NAC has a number of functions in the stages of repair process, including cell proliferation, migration, and scratch wound healing. Moreover, NAC has also been reported to promote wound healing in diabetic rats.6
In this study, we aimed to compare the effects of dexpanthenol, a molecule that is widely used to improve wound healing, and NAC, a molecule that reduces oxidative stress and inflammation, on wound healing.
Materials and Methods
Animals
Thirty albino Wistar rats weighing 250–300 g were used in this study. The animals were kept under 12-hour light/dark cycle at 21 ± 2°C with a humidity of 45 to 55%. The rats were fed standard rat chow and allowed ad libitum access to water. This study was approved by the Ethics Committee for Animal Use at Dicle University.
Dexpanthenol cream
A commercially available mixture containing a 5% dexpanthenol water-in-oil emulsion was used (Bepanthol cream, 5% dexpanthenol, Bayer, Germany).
NAC cream
After weighing each component for the mixture, a 3% NAC cream was prepared, and then the necessary number of NAC (Asist 200-mg capsule, Husnu Arsan, Istanbul, Turkey) was crushed into tiny pieces and added to water, producing a paste. The targeted amount of this mixture was added into cold cream and mixed until a thorough mixture was achieved.
Surgical procedure and application
The anesthesia of the rats was achieved with administration of ketamine hydrochloride (25 mg/kg) through the muscles. Once the back hair was shaved, the target field was marked using a pen, and the back was opened via a 2-cm linear full-depth incision on the skin. Three groups were formed with 10 rats each. The control group undertook no treatment. The second group received dexpanthenol cream, and the third group was administered with 3% NAC cream. Daily cleaning of the wounds was performed with saline. Following the cleaning process, the whole wound area was covered with adequate amount of cream. On the 21st day, the rats were anesthetized using intra muscular ketamine hydrochloride (25 mg/kg), the wound and surrounding tissues were removed, and the defects on the skin were closed with nonabsorbable suture.
Evaluation and measurement of wound healing
The wound areas in all 3 groups were measured on the first day and on the third, fifth, seventh, 10th, 14th, and 21st days after the wound. The measurements were performed using transparencies and a permanent marker. Graph paper was used to record and measure the wound areas, and the Walker formula was utilized for assessing the ratio of wound healing.7
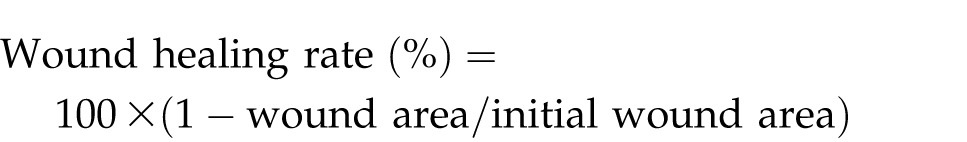 |
Histology
The samples obtained from the skin were put into 10% neutral formalin, processed via the classical histologic preparation technique, and then embedded in paraffin. The blocks were used to obtain 5-μm serial sections, and the general appearance of the tissues was evaluated by staining the sections with hematoxylin-eosin. The slides were examined using a light microscope (Axiophot, Zeiss, Germany). The scoring of morphologic findings, including inflammation, fibrosis, and angiogenesis was as follows: score 0, “none”; score 1, “few”; score 2, “moderate”; and score 3, “many.” The assessment of epithelialization and granulation was determined as either present or absent. The histologic analyses and the scoring process were conducted in a blinded fashion.
Statistical method
Data analyses were performed using SPSS for Windows Version 13.0 (SPSS, Chicago, Illinois). Descriptive statistics were presented as mean ± SD. ANOVA was used to evaluate the group differences on the continuous variables specified at baseline, and the wound closure rate method was employed as the grouping variable. Group differences on categoric variables were assessed using the chi-square test. Repeated-measures ANOVA were performed to evaluate the group differences across time in overall percent wound closure ratio (preliminary wound closure ratio and maintenance). In repeated-measures analyses, the sphericity was controlled by Greenhouse-Geisser correction, as appropriate. Both main effect and simple effect post hoc contrasts were managed with the Bonferroni adjustment. A P value of <0.05 was considered statistically significant.
Results
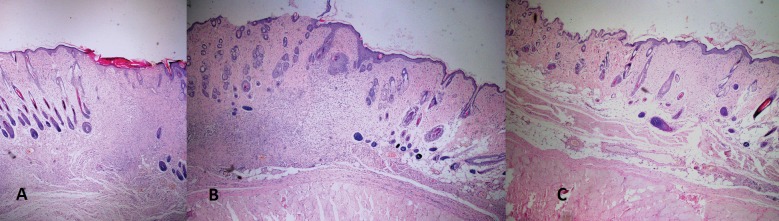
The epithelialization and granulation rates between the groups were revealed similar in microscopic evaluations (P > 0.05). Less inflammation was revealed in the control group (P = 0.027). Although there was more fibrosis in control group than in the other groups, it was similar in the NAC and dexpanthenol groups (P = 0.002). Angiogenesis rate was more remarkable in the NAC group than in the others (P = 0.04; Table 1; Fig. 1).
Table 1.
Histopathologic findings of the groups
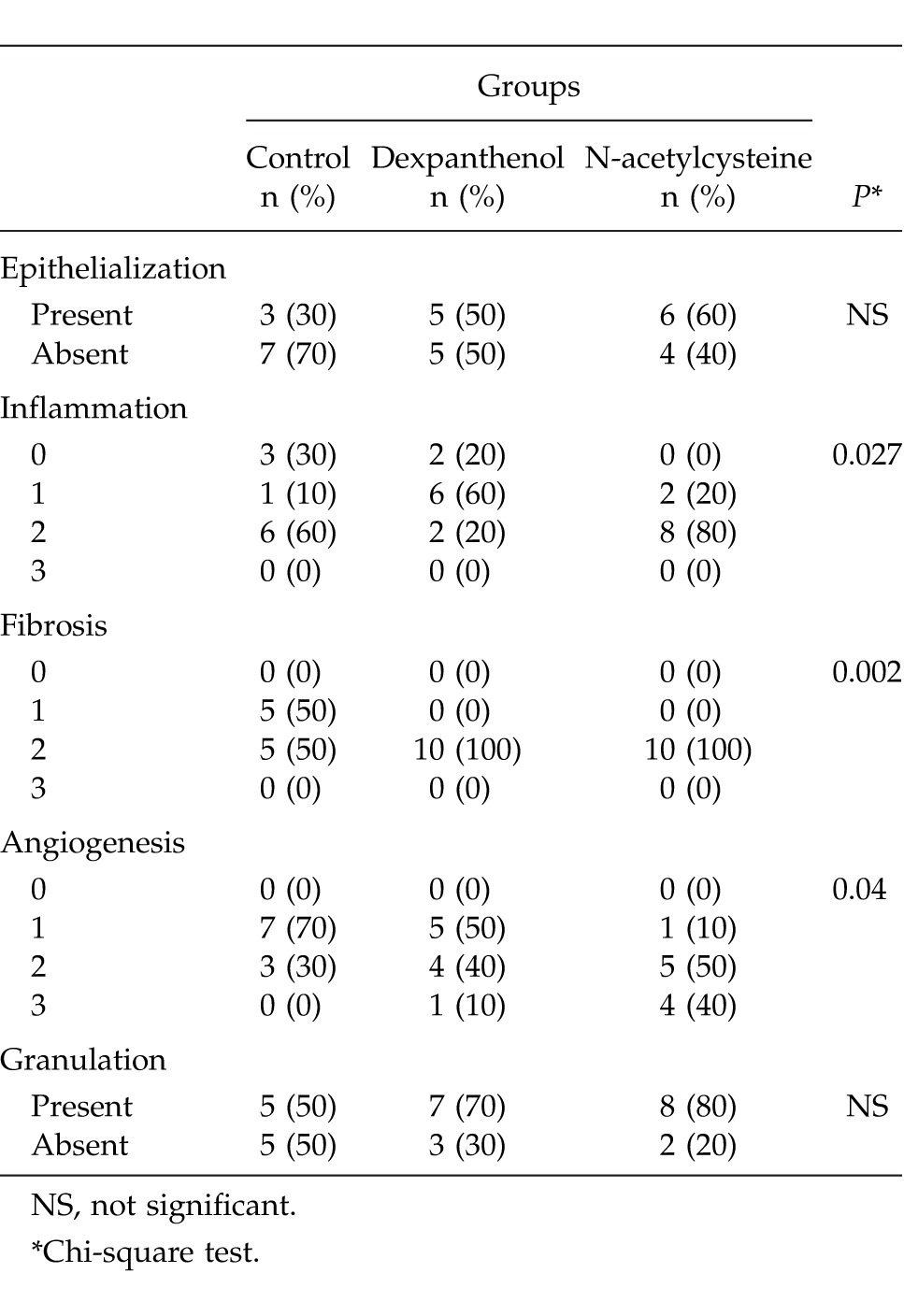
Fig. 1.
Microscopic images of the (A) Control group, (B) Dexpanthenol group, (C) N-acetylcysteine group (hematoxylin-eosin × 25).
Wound closure rates of dexpanthenol and NAC groups were similar and were significantly higher than the control group's rates (P < 0.001; Table 2; Fig. 2).
Table 2.
Wound closure rates of the groups
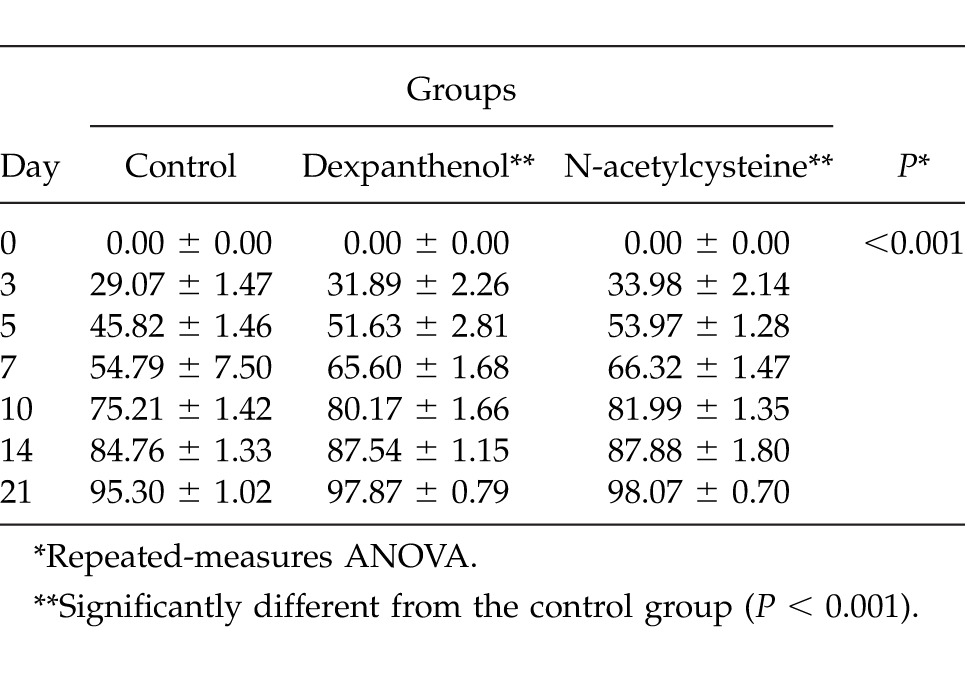
Fig. 2.
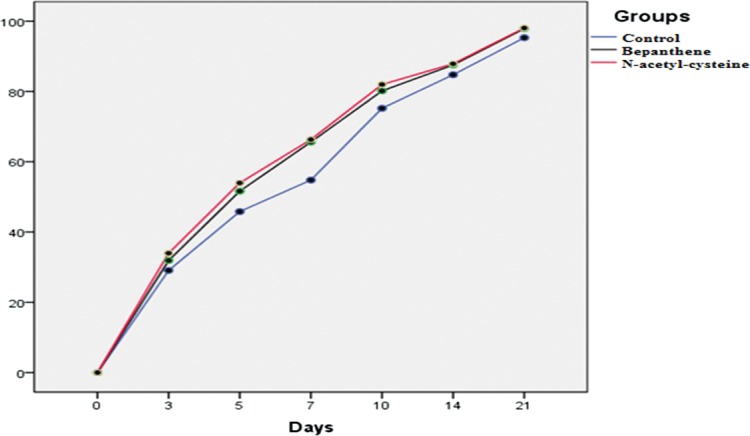
Wound healing rates of the groups.
In multiple comparison analysis, dexpanthenol and NAC groups had similar results in terms of wound healing rates (P < 0.05), which were both higher than in the control group (P > 0.05).
Discussion
As the largest and the most essential part of the body, skin plays a vital role in maintaining body homeostasis. In terms of skin health, the wound healing process is a principal activity, since it is the key element for the restoration and regeneration of tissues and functions that have been impaired or injured as a result of physical, chemical, bacterial, or viral attacks.8 The wound-healing process is comprised of 4 stages: hemostasis, inflammation, proliferation, and tissue remodeling.2,3 An ideal wound healing process is a multi-element activity involving the collaboration of the inflammatory cells, biochemical mediators, extracellular matrix molecules, the microenvironment of the cell, and remodeling. This process can be facilitated by a number of natural or artificial agents.9 However, the wound healing process can be interrupted by a number of factors, which may impair 1 or more stages of the process and thus lead to inappropriate or failed tissue repair. Failed processes of wound healing lead to large amounts of health care expenses, which are estimated to exceed $3 billion per year.10 Because of this high cost, finding new methods to facilitate wound healing process has been studied by many researchers and scholars.4
When administered in tissues, dexpanthenol is transformed to pantothenic acid, which is crucial for the epithelia to sustain their normal function. Following the topical administration of dexpanthenol, both skin regeneration and wound healing process are facilitated. This is the main reason why dexpanthenol is commonly used in skin care and also in the treatment of a number of skin diseases.5 As Biro et al and Proksch et al reported, the topical administration of dexpanthenol provides effective results in the treatment of and protection against skin irritation and inflammation.11,12 Weimann et al documented that the need for pantothenic acid in wound healing processes increases since the turnover of coenzyme A in the skin wound is elevated. For this reason, the level of local pantothenic acid could be raised to enhance most of the cellular activities associated with wound healing.13 Wiederholt et al reported that the gene expression in proliferating human dermal fibroblasts is altered by calcium pantothenate.14 In a study by Ebner et al, dexpanthenol is reported to activate fibroblast proliferation and speed up re-epithelialization in wound healing.5 It is shown that dexpanthenol is effective for promoting epidermal renewal and speeding up the wound healing process in in vivo models of skin damages. It also revealed that dexpanthenol promotes epithelialization and granulation.5 Another study argues that the fibroblast proliferation during the wound healing process is influenced by dexpanthenol through its anti-inflammatory and positive effects.15 Similar to these studies, in our study, the dexpanthenol group had higher values in wound healing ratios, epithelialization ratios, and the mean fibrosis scores than the control group. Based on these data, we hypothesize that the improvement in wound healing ratios is a result of increased fibroblast proliferation as well as accelerated epithelialization. In addition, we observed significantly less inflammation in the dexpanthenol group than in the NAC group and the control group, which we believe was due to the anti-inflammatory effects of dexpanthenol.
N-acetylcysteine (NAC) supplies bioavailable cysteine for glutathione replenishment and prevents oxidative damage and inflammation. In the wound healing process, reactive oxygen species (ROS) are generated by a number of inflammatory cells, including neutrophils, macrophages, endothelial cells, and fibroblasts.16 With the generation of ROS in cutaneous wounds, the wound healing process is constrained since they damage the cellular membranes, lipids, proteins, and DNA. GSH is an antioxidant that prevents the damage to important cellular components caused by ROS.17 Angiogenesis plays a key role in the proliferation of wound healing.1 Aktunç et al reported that NAC facilitates the wound healing process by stimulating angiogenesis and also by scavenging free oxygen radicals.6 Yunsook Lim et al demonstrated that the early inflammatory reactions during cutaneous wound healing in protein-malnourished mice are increased by dietary administration of NAC.18 Rosato et al reported that the healing of ulcers in patients with bullous morphea is facilitated by the administration of NAC.19 Murat et al described NAC as a potent antioxidant since it prevents the necrosis of zone of stasis.20 Demir et al reported that the healing of the wounds in irradiated rats was improved by intraperitoneal or oral administration of NAC.21 Min-Ling Tsai et al reported that the topical administration of 3% NAC was significantly effective on the cell proliferation, migration, scratch wound healing, and collagenous expression of MMP–1 via the PKC/Stat3 signaling pathway.9 In addition, topical NAC 8% eye drops have also been reported to improve the initial treatment of experimental alkali corneal burns.22 In our study, better wound healing ratios and epithelialization ratios were obtained in the NAC group than in the control group, and the mean fibrosis score was significantly higher in the NAC group than in the control group.
Although the dexpanthenol and NAC groups had better wound healing ratios and epithelialization ratios than the control group, there were no statistically significant differences between the dexpanthenol and NAC groups.
In light of these findings, we believe that NAC and dexpanthenol have positive effects on fibroblast proliferation and fibroplasia and thus improve wound healing.
This study is limited by the small number of animals used. More experimental and clinical studies should be performed in order to evaluate the clinical benefits of topical NAC administration on wound healing.
Conclusion
The efficacy of NAC in wound healing is comparable to dexpanthenol, which is widely used in the treatment of wounds, and both substances can be used to improve wound healing. However, some clinical trials are needed to assess the use of NAC as a standard treatment in wound healing.
References
- 1.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366(9498):1736–1743. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 2.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83(3):835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 3.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341(10):738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 4.Gál P, Toporcer T, Grendel T, Vidová Z, Smetana K, Jr, Dvoránková B, et al. Effect of Atropa belladonna L. on skin wound healing: biomechanical and histological study in rats and in vitro study in keratinocytes, 3T3 fibroblasts, and human umbilical vein endothelial cells. Wound Repair Regen. 2009;17(3):378–386. doi: 10.1111/j.1524-475X.2009.00475.x. [DOI] [PubMed] [Google Scholar]
- 5.Ebner F, Heller A, Rippke F, Tausch I. Topical use of dexpanthenol in skin disorders. Am J Clin Dermatol. 2002;3(6):427–433. doi: 10.2165/00128071-200203060-00005. [DOI] [PubMed] [Google Scholar]
- 6.Aktunc E, Ozacmak VH, Ozacmak HS, Barut F, Buyukates M, Kandemir O, et al. N-acetyl cysteine promotes angiogenesis and clearance of free oxygen radicals, thus improving wound healing in an alloxan-induced diabetic mouse model of incisional wound. Clin Exp Dermatol. 2010;35(8):902–909. doi: 10.1111/j.1365-2230.2010.03823.x. [DOI] [PubMed] [Google Scholar]
- 7.Jarrahi M, Vafaei AA, Taherian AA, Miladi H. Rashidi Pour A. Evaluation of topical Matricaria chamomilla extract activity on linear incisional wound healing in albino rats. Nat Prod Res. 2010;24(8):697–702. doi: 10.1080/14786410701654875. [DOI] [PubMed] [Google Scholar]
- 8.Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai ML, Huang HP, Hsu JD, Lai YR, Hsiao YP, Lu FJ, et al. Topical N-acetylcysteine accelerates wound healing in vitro and in vivo via the PKC/Stat3 pathway. Int J Mol Sci. 2014;15(5):7563–7578. doi: 10.3390/ijms15057563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menke NB, Ward KR, Witten TM, Bonchev DG, Diegelmann RF. Impaired wound healing. Clin Dermatol. 2007;25(1):19–25. doi: 10.1016/j.clindermatol.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Proksch E, Nissen HP. Dexpanthenol enhances skin barrier repair and reduces inflammation after sodium lauryl sulphate-induced irritation. J Dermatolog Treat. 2002;13(4):173–178. doi: 10.1080/09546630212345674. [DOI] [PubMed] [Google Scholar]
- 12.Biro K, Thaçi D, Ochsendorf FR, Kaufmann R, Boehncke WH. Efficacy of dexpanthenol in skin protection against irritation: a double-blind, placebo-controlled study. Contact Dermatitis. 2003;49(2):80–84. doi: 10.1111/j.0105-1873.2003.00184.x. [DOI] [PubMed] [Google Scholar]
- 13.Weimann BI, Hermann D. Studies on wound healing: effects of calcium D-pantothenate on the migration, proliferation and protein synthesis of human dermal fibroblasts in culture. Int J Vitam Nutr Res. 1999;69(2):113–119. doi: 10.1024/0300-9831.69.2.113. [DOI] [PubMed] [Google Scholar]
- 14.Wiederholt T, Heise R, Skazik C, Marquardt Y, Joussen S, Erdmann K, et al. Calcium pantothenate modulates gene expression in proliferating human dermal fibroblasts. Exp Dermatol. 2009;18(11):969–978. doi: 10.1111/j.1600-0625.2009.00884.x. [DOI] [PubMed] [Google Scholar]
- 15.Oztürk N, Korkmaz S, Oztürk Y. Wound-healing activity of St. John's Wort (Hypericum perforatum L.) on chicken embryonic fibroblasts. J Ethnopharmacol. 2007;111(1):33–39. doi: 10.1016/j.jep.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 16.Parihar A, Parihar MS, Milner S, Bhat S. Oxidative stress and anti-oxidative mobilization in burn injury. Burns. 2008;34(1):6–17. doi: 10.1016/j.burns.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Pompella A, Visvikis A, Paolicchi A, De Tata V, Casini AF. The changing faces of glutathione, a cellular protagonist. Biochem Pharmacol. 2003;66(8):1499–1503. doi: 10.1016/s0006-2952(03)00504-5. [DOI] [PubMed] [Google Scholar]
- 18.Lim Y, Levy MA, Bray TM. Dietary supplementation of N-acetylcysteine enhances early inflammatory responses during cutaneous wound healing in protein malnourished mice. J Nutr Biochem. 2006;17(5):328–336. doi: 10.1016/j.jnutbio.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Rosato E, Veneziano ML, Di Mario A, Molinaro I, Pisarri S, Salsano F. Ulcers caused by bullous morphea: successful therapy with N-acetylcysteine and topical wound care. Int J Immunopathol Pharmacol. 2013;26(1):259–262. doi: 10.1177/039463201302600128. [DOI] [PubMed] [Google Scholar]
- 20.Deniz M, Borman H, Seyhan T, Haberal M. An effective antioxidant drug on prevention of the necrosis of zone of stasis: N-acetylcysteine. Burns. 2013;39(2):320–325. doi: 10.1016/j.burns.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 21.Demir EO, Cakmak GK, Bakkal H, Turkcu UO, Kandemir N, Demir AS, et al. N-acetyl-cysteine improves anastomotic wound healing after radiotherapy in rats. J Invest Surg. 2011;24(4):151–158. doi: 10.3109/08941939.2011.560237. [DOI] [PubMed] [Google Scholar]
- 22.Sekundo W, Augustin AJ, Strempel I. Topical allopurinol or corticosteroids and acetylcysteine in the early treatment of experimental corneal alkali burns: a pilot study. Eur J Ophthalmol. 2002;12(5):366–372. doi: 10.1177/112067210201200504. [DOI] [PubMed] [Google Scholar]