Abstract
We aimed to explore the optimal follow-up time for benign gastric Schwannoma. Benign gastric Schwannoma is an uncommon type of gastric neoplasias. Most of the studies are case reports and case series. Although it is generally considered to be benign, the optimal follow-up time and the chance of recurrence have not yet been investigated fully. We presented a case of benign gastric Schwannoma and systematically reviewed published case series with follow-up data. Eight studies were included, totaling 137 patients (44 male and 93 female) with the median follow-up time ranging from 22–132 months across different studies. No recurrence had been recorded during the follow-up period. Benign gastric Schwannoma rarely recurs after complete surgical resection. Long-term survival will be expected in most patients.
Key words: Schwannoma, Stomach, Survival, Follow-up
Gastrointestinal mesenchymal tumors are spindle-shaped tumors, originating from the gastrointestinal mesenchymal stem cells. Three major types are gastrointestinal stromal tumors (GIST), smooth muscle tumor (leiomyomas or leiomyosarcomas), and nerve sheath tumor (Schwannomas).1 Gastric Schwannoma is a type of nerve sheath tumor that originates from the Schwann cells of peripheral nerves. The definite diagnosis of Schwannoma is based on postoperative immunohistochemical stain, which is usually S–100 positive. Surgical interventions are the mainstay for gastric Schwannoma and complete removal of the tumors could prevent recurrence. Here we present a case of gastric Schwannoma located in the lower segment of stomach and we discuss the issue of postoperative recurrence.
Case Report
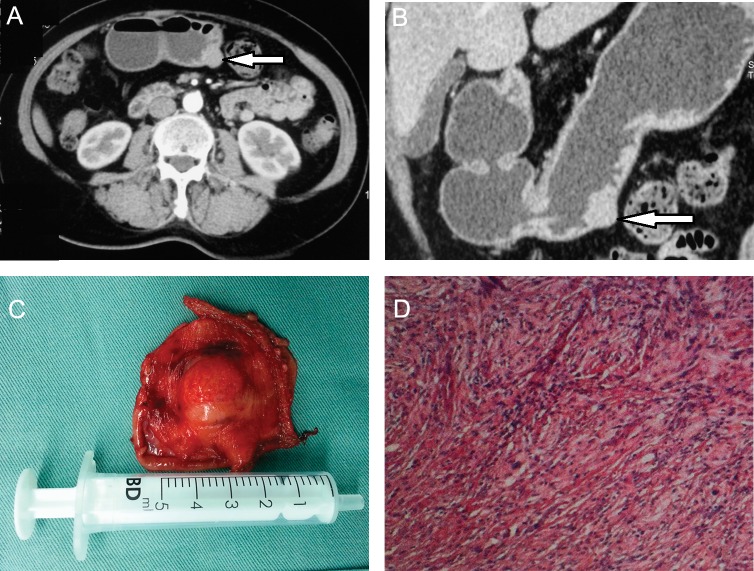
A 66-year-old female presented with episodes of upper gastric discomfort heart burn and nausea for 10 years, which aggregated after meals. The computed tomography scan and gastric reconstruction image showed a 1.5-cm, well-demarcated, round mass in the muscular layer of the greater curvatures of stomach, with median degree of enhance in the arterial phase (Fig. 1A, B). The past medical history was remarkable for cataract surgery 5 years ago. Preoperative diagnosis was stomach mesenchymal tumor. The patient then underwent a laparoscopic partial gastrectomy with 1 cm of tumor-free margins (Fig. 1C). Pathologic examination showed spindle cell by hematoxylin and eosin stain (Fig. 1D). The immunohistochemistry was positive for S–100 and negative for CD117, CD34, DOG–1 and desmin. Thus gastric Schwannoma was diagnosed. No postoperative complications were observed and the patient was discharged from the hospital on postoperative day 10.
Fig. 1.
(A) Abdominal CT scan showing a 1.5-cm mass in the right side of the stomach, arterial phase; white arrow showing pointing at the mass. (B) Abdominal CT scan with stomach reconstruction showing a 1.5-cm mass in the greater curvatures of the stomach, white arrow showing pointing at the mass. (C) Surgical specimen showing a mass with surrounding noncancerous tissue. (D) Pathologic evaluation showing spindle cells in hematoxylin and eosin stain.
Discussion
Schwannoma originates from Schwann cells, which compose the peripheral nerve sheath. Patients with Schwannoma are usually asymptomatic, but occasionally the tumor would compress the nearby nerves and cause symptoms. Gastric Schwannoma originates from the gastrointestinal neural plexus, only constituting 0.2% of all gastric neoplasms.2 The peak incidence is within the middle ages with female predominance.3 It usually resides in the submucosa and muscularis propria area of the stomach, which should be differentially diagnosed with other submucosal mesenchymal origin-stromal tumors, such as leiomyomas and gastrointestinal stromal tumors (GIST).4,5 Histologically, Schwannoma consists of spindle cells. S–100 protein, which is present in cells of neural crest origin, is a distinctive marker for Schwannoma by immunohistochemistry stain and may help to differentially diagnose from other spindle cell tumors. Gastric Schwannoma is usually positive for positive for S–100, while leiomyomas and GIST are more frequently positive for muscle cell markers (desmin and smooth muscle actin) and CD34, respectively. Although the postoperative diagnosis is reliable based on immunohistochemistry stain, the preoperative differential diagnosis is still challenging. Endoscopic ultrasound (EUS)-guided biopsies are needed in some cases to yield reliable diagnosis.6
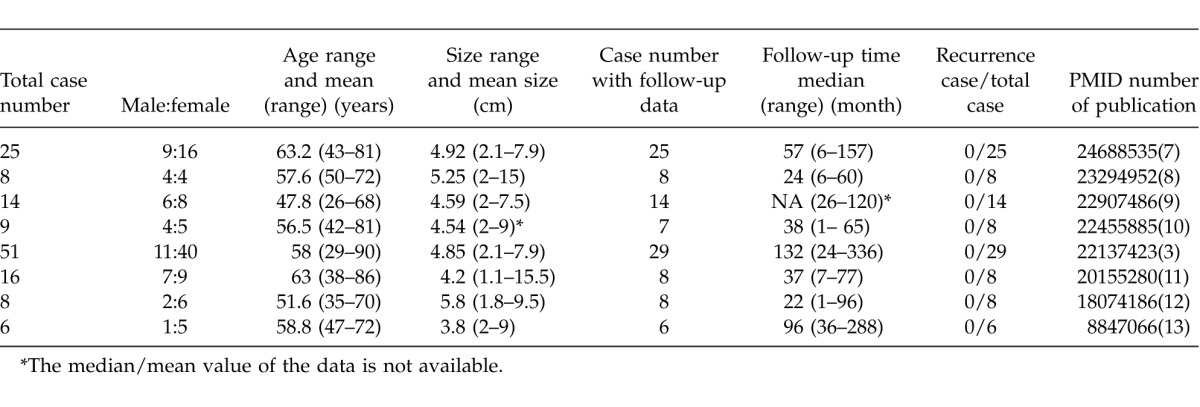
Benign gastric Schwannoma usually carries good prognosis. The growth rate of benign Schwannoma is slower than GIST. Choi et al calculated the growth rate based on computed tomography (CT) images of gastric Schwannoma patients with a series of follow-ups. The mean doubling time of Schwannoma was nearly 5 years.4 Surgical intervention is the mainstay of this disease and R0 resection is recommended, if possible, to minimize the chance of in-situ recurrence. However, the optimal follow-up schedule for benign gastric Schwannoma needs to be carefully investigated. Due to the rarity of this disease and low recurrence rate, it is necessary to carefully review the previous reports to address this issue and to explore the chance of occurrence and optimized follow-up interval. We conducted comprehensive literature review on this disease. We searched Medline with the key word [(“Schwannoma” AND “stomach”) OR (“Gastric” AND “Schwannoma”)], without language and publication year restriction up to March 2014. A total of 593 abstracts were retrieved and manually reviewed by authors. We included studies with patients that numbered 5 or more and with adequate follow-up data. Eight articles reported the follow-up information with adequate same size, with 137 patients in total (44 male and 93 female; Table 1).3,7−13 The mean age of diagnosis ranged from 51.6 to 63 years and patients' ages ranged from 29 to 90 years old. The average size of tumor ranged from 4.2 to 5.8 cm and tumor size ranged from 1.1 to 15 cm. The follow-up data of 105 patients had clear statements whether or not the disease had recurrence. For these patients, the median follow-up time ranged from 22 to 132 months across different studies, while the follow-up time for individual patients ranged from 1 to 336 months. No recurrence had been recorded during the follow-up period.
Table 1.
Clinical and follow-up information for publications of benign gastric Schwannoma
Thus, we hold that benign gastric Schwannoma usually will not recur. It is not recommended to frequently conduct follow-up with CT scan due to the cost and harm of the CT scan for patients. Long-term survival will be expected in most cases.
Acknowledgments
There was no support in the form of grants, equipment, or drugs. All authors declared no conflict of interest. Xiafei Hong and Wenming Wu contributed equally to this article.
References
- 1.Miettinen M, Majidi M, Lasota J. Pathology and diagnostic criteria of gastrointestinal stromal tumors (GISTs): a review. Eur J Cancer. 2002;38:S39–S51. doi: 10.1016/s0959-8049(02)80602-5. [DOI] [PubMed] [Google Scholar]
- 2.Melvin W, Wilkinson M. Gastric Schwannoma. Clinical and pathologic considerations. Am Surg. 1993;59(5):293–296. [PubMed] [Google Scholar]
- 3.Voltaggio L, Murray R, Lasota J, Miettinen M. Gastric Schwannoma: a clinicopathologic study of 51 cases and critical review of the literature. Hum Pathol. 2012;43(5):650–659. doi: 10.1016/j.humpath.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi JW, Choi D, Kim KM, Sohn TS, Lee JH, Kim HJ, et al. Small submucosal tumors of the stomach: differentiation of gastric Schwannoma from gastrointestinal stromal tumor with CT. Kor J Radiol. 2012;13(4):425–433. doi: 10.3348/kjr.2012.13.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miettinen M, Virolainen M. Gastrointestinal stromal tumors-value of CD34 antigen in their identification and separation from true leiomyomas and Schwannomas. Am J Surg Pathol. 1995;19(2):207–216. doi: 10.1097/00000478-199502000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Hong SW, Cho WY, Kim JO, Chun CG, Shim KY, Bok GH, et al. Gastric Schwannoma diagnosed by endoscopic ultrasonography-guided trucut biopsy. Clinical endoscopy. 2013;46(3):284–287. doi: 10.5946/ce.2013.46.3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng L, Wu X, Kreis ME, Yu Z, Feng L, Chen C, et al. Clinicopathological and immunohistochemical characterisation of gastric Schwannomas in 29 cases. Gastroenterol Res Pract. 2014;2014:202960. doi: 10.1155/2014/202960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaconescu MR, Diaconescu S. Mesenchymal (non-epithelial) “non-GIST” tumors of the digestive tract. Chirurgia (Bucur) 2012;107(6):742–750. [PubMed] [Google Scholar]
- 9.Fujiwara S, Nakajima K, Nishida T, Takahashi T, Kurokawa Y, Yamasaki M, et al. Gastric Schwannomas revisited: has precise preoperative diagnosis become feasible? Gastric Cancer. 2013;16(3):318–323. doi: 10.1007/s10120-012-0186-x. [DOI] [PubMed] [Google Scholar]
- 10.Wang ZB, Shi HY, Yuan J, Chen W, Wei LX. Clinical and pathologic features of gastric Schwannoma [in Chinese] Zhonghua Bing Li Xue Za Zhi. 2012;41(2):97–101. [PubMed] [Google Scholar]
- 11.Agaimy A, Märkl B, Kitz J, Wünsch PH, Arnholdt H, Füzesi L, et al. Peripheral nerve sheath tumors of the gastrointestinal tract: a multicenter study of 58 patients including NF1-associated gastric Schwannoma and unusual morphologic variants. Virchows Arch. 2010;456(4):411–422. doi: 10.1007/s00428-010-0886-8. [DOI] [PubMed] [Google Scholar]
- 12.Goh BK, Chow PK, Kesavan S, Yap WM, Ong HS, Song IC, et al. Intraabdominal Schwannomas: a single institution experience. J Gastrointest Surg. 2008;12(4):756–760. doi: 10.1007/s11605-007-0441-3. [DOI] [PubMed] [Google Scholar]
- 13.Sarlomo-Rikala M, Miettinen M. Gastric Schwannoma–a clinicopathological analysis of six cases. Histopathology. 1995;27(4):355–360. doi: 10.1111/j.1365-2559.1995.tb01526.x. [DOI] [PubMed] [Google Scholar]