Abstract
Background
Drug abuse and addiction has become a major public health problem that impacts all societies. The use of heroin may cause spongiform leukoencephalopathy (SLE).
Material/Methods
Cerebellar granule cells were derived from 7-day-old Sprague-Dawley rat pups.
Neurons were dissociated from freshly dissected cerebella by mechanical disruption in the presence of 0.125% trypsin and DNaseI and then seeded at a density of 4×106 cells/ml in Dulbecco’s modified Eagle’s medium/nutrient mixture F-12 ham’s containing 10% fetal bovine serum and Arc-C(sigma) at concentrations to inhibit glial cell growth inoculated into 6-well plates and a small dish.
Results
We found that heroin induces the apoptosis of primary cultured cerebellar granule cells (CGCS) and that the c-Jun N-terminal kinase (JNK) pathway was activated under heroin treatment and stimulated obvious increases in the levels of C-jun, Cytc, and ATF3mRNA. CYTC and ATF3 were identified as candidate targets of the JNK/c-Jun pathway in this process because the specificity inhibitors SP600125 of JNK/C-jun pathways reduced the levels of C-jun, Cytc, and ATF3mRNA. The results suggested that SP600125 of JNK/C-jun can inhibit heroin-induced apoptosis of neurons.
Conclusions
The present study analyzes our understanding of the critical role of the JNK pathway in the process of neuronal apoptosis induced by heroin, and suggests a new and effective strategy to treat SLE.
Keywords: Activating Transcription Factor 3; Genes, jun; Heroin Dependence; Neuronal Apoptosis-Inhibitory Protein
Background
Drug abuse and addiction has become a major public health problem that impacts all societies [1]. Heroin, which remains the top drug of concern, affects the central nervous system by rapidly penetrating the blood-brain barrier. It changes neurotransmitters; depresses nerve transmission in sensory pathways of the spinal cord and brain that signal pain; and deregulates the level of MDM2, which leads to the brain edema, neuron loss, and other effects [2–5]. Oliveira et al. indicated that the activity of PC-12 cells decreases with increasing doses of heroin used. A previous study has also shown that heroin helps release the cysteinyl aspartate protease-dependent mitochondrial pathway and induces cortical neuronal apoptosis. Therefore, cell apoptosis is a focus in the study of heroin and neuronal function loss [6,7].
Heroin is reported to cause spongiform leukoencephalopathy (SLE) in heroin addicts and it damages many parts of brain tissues, especially the cerebellum. The typical clinical symptoms of cerebellar injury by heroin inhalation are ataxia, dysmetria, and dysarthria [8,9]. However, the precise mechanism of cerebellar neuronal death induced by heroin remains unclear. Neuron cell culture in vitro has been regarded as an ideal experimental model in which cell morphology and biochemical changes can be directly observed. Cerebellar granule neurons (cerebellar granule neurons, CGC) are the most commonly used model in the research of neuron apoptosis, of which the purity can reach more than 90% [10,11].
There are 2 major pathways to activate apoptosis: the “Extrinsic” and the ”Intrinsic” pathway. The extrinsic pathway usually begins outside a cell, when conditions in the extracellular environment determine that a cell must die (e.g., FAS and TNF-R). The intrinsic apoptosis pathway begins when an injury occurs within the cell and is determined by interactions between 3 factions of the Bcl-2 protein family [12]. Studies indicate that JNK is an important mediator of the neurotoxic effects of heroin; therefore, we believe that inhibiting JNK activity may prevent cell apoptosis [13,14]. Cytc, a water-soluble protein locate in the mitochondrial outer membrane lacuna, plays a vital role in the process of cell apoptosis ]19]. CytC releases into the cytoplasm from mitochondria, which can activate ammonia acyl aspartic enzyme-induced neuronal apoptosis under hypoxic conditions [20]. Mitochondrial structure and function undergoes a significant change includes respiratory electron transport chain interruption, energy synthesis blockage, the mitochondrial membrane potential declination; it releases the proapoptotic factors which leads to cell apoptosis [21]. ATF3 also plays a vital role in the signaling of neuron apoptosis [22] and is equipped with leucine zipper structure transcription factors in the ATF/CREB family. ATF3 transcription factor contains ATF2 and c-jun potential binding sites which can be phosphorylated and activated by the JNK/SPAK pathway, and ATF2 and c-jun over-expression increases the activity of ATF3 promoter in the process of instantaneous transfection [23–25]. In the present study, there was no evidence showing that ATF3 and CytC are involved in the processes of the JNK/c-Jun pathway and heroin-induced CGC apoptosis.
In the present study, we found that the level of Cytc and ATF3 mRNA was up-regulated under heroin treatment, and also found that up-regulated Cytc and ATF3mRNA activated the cell apoptosis. However, the levels of Cytc and ATF3 mRNA expression are reduced by inhibiting JNK pathways. It indicated that Cytc and ATF3 have a critical role in the JNK pathways in this apoptotic process. The present study proves that Cytc and ATF3 played a vital part in the process of heroin-induced CGC apoptosis and that they are also involved in the activation of the JNK pathway in mitochondrial pathways.
Material and Methods
Cell culture
Cerebellar granule cells were isolated as described above [14]: CGCs were derived from 7-day-old Sprague-Dawley rat pups. Neurons were dissociated from freshly dissected cerebella by mechanical disruption in the presence of 0.125% trypsin and DNaseI (100 ul/ml) and then seeded at a density of 4×106 cells/ml in Dulbecco’s modified Eagle’s medium/nutrient mixture F-12 ham’s containing 10% fetal bovine serum and Arc-C (sigma) at concentrations to inhibit glial cell growth inoculated into 6-well plates and a small dish. The fluid was later switched to Neurobasal containing 2% B27 and changed half of the fluid every 48 h. For the administration of inhibitors, after 7 days in vitro (DIV7), CGCs were incubated in the presence of the following reagents: heroin (Sigma-Aldrich, St. Louis, MO, USA), and SP600125(Sigma-Aldrich). Cells that did not receive drugs received a control vehicle. To avoid toxicity, the final concentration of DMSO remained <0.1%.
Immunofluorescence
Removing the culture medium at 5 days, neurons were washed 3 times (5 min/time) by 0.01 mol/l PBS, then moved into 4% paraformaldehyde container for 40 min, again washed by PBS 2 times (5 min/time) and later sealed in serum albumin antibody dilution 1:200 β-tubulin III for a night at 4°C. The next day, it was placed in a greenhouse for 40 min, washed with PBS 3 times (5 min/time), added FITC, waited for 40 min and washed with PBS for another 3 times, adding DAIP for 10 min, and washed again by PBS for 3 times. At the end, we conducted the purity identification of the neurons: we selected 5 samples and counted the numbers of positive cells. When the positive cells were greater than 90%, they were selected to do the follow-up experiments.
At the 7th day, when nerve cells were differentiated by maturation, 10 μg/mL, 40 μg/mL, 80 μg/mL, and 120 μg/mL concentrations of heroin were separately added into the container for 24 h. We kept adding 120 ug/ml heroin and 10 umol /L SP600125 and observing their intervention effect. After PBS clearance, we collected 2×105~5×105 cells. We mixed 100 ul binding buffer suspension cells with 5 ul Annexin V-FITC and 5 ul propidium iodide, incubated them in the dark at room temperature for 15 min, then mixed with 400 ul Binding Buffer, examined the flow cytometry within 1 h, observing the morphological changes of the cells and examined the cell apoptosis.
RT-PCR Reverse transcription-PCR
RNA isolation was performed as described [26], total RNA (2 μg) was incubated with RevertAid H Minus First Strand cDNA Synthesis Kit (Thermo company). P-c-jun: primer upstream: 5′GAGTCTCAGGAGCGGATCAA3′, downstream: 5′ CTGTTCCCTGAGCATGTTGG 3′. cytc primer upstream: 5′gGATTGACCAGGAAGCTGCAG3′, downstream: 5′CCACCAAAATCTCCTGCGTT 3′; ATF3 primer upstream: 5′GAGCGAAGACTGGAGCAA3′, downstream: 5′CTCTGCTAGCTCTGCAATC3′. β-actin: primer upstream: 5′CAACTGGGAGATATGGAGAAG3′, downstream: 5′ TCTTCCTTCTGATCCTGTCAG3, (Invitrogen design synthesis). P-c-jun annealing temperature of 55°C, Cytc annealing temperature is 58°C, ATF3 annealing temperature of 54.2°C, 35 cycles, through the RT-PCR Kit (Thermo company) to detect related RNA.
Statistical analysis
All parameters determined in this study represent the mean standard error. The groups were compared using Student’s t-test with control values. The significance is indicated as follows: * P<0.05 and ** P<0.01. The results were analyzed by SPSS17.0 statistical software.
Results
Immunofluorescence
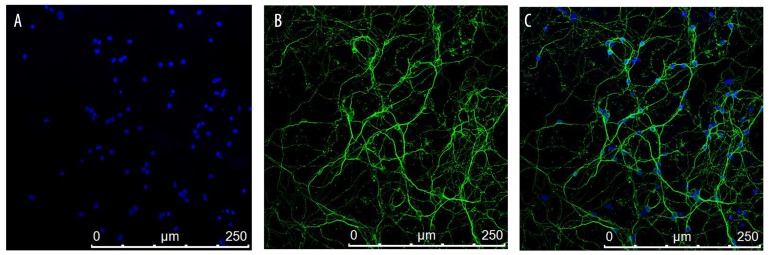
On Day 5, DIV5 cells were examined and identified by laser confocal: β-tubulin expressed in cerebellar granule cell cytoplasm (CGCs), blue showed DAPI staining nuclei that can excite the nucleus (Figure 1A), the second antibody with fluorescence-excited cytoplasm was green (Figure 1B). Figure 1C shows synthesis results for the morphological structure of integral CGCs. The purity of cultured cerebellar granule cells was more than 90% and can be used for further experiments.
Figure 1.
In vitro culture cerebellar granule cells after five days, By laser confocal detection and identification Neuronal cell (×400), DAPI nuclear staining is blue (A), β-tubulin III Cytoplasmic staining is green (B); A+B was condition of merge (C).
Heroin induces apoptosis of CGCs
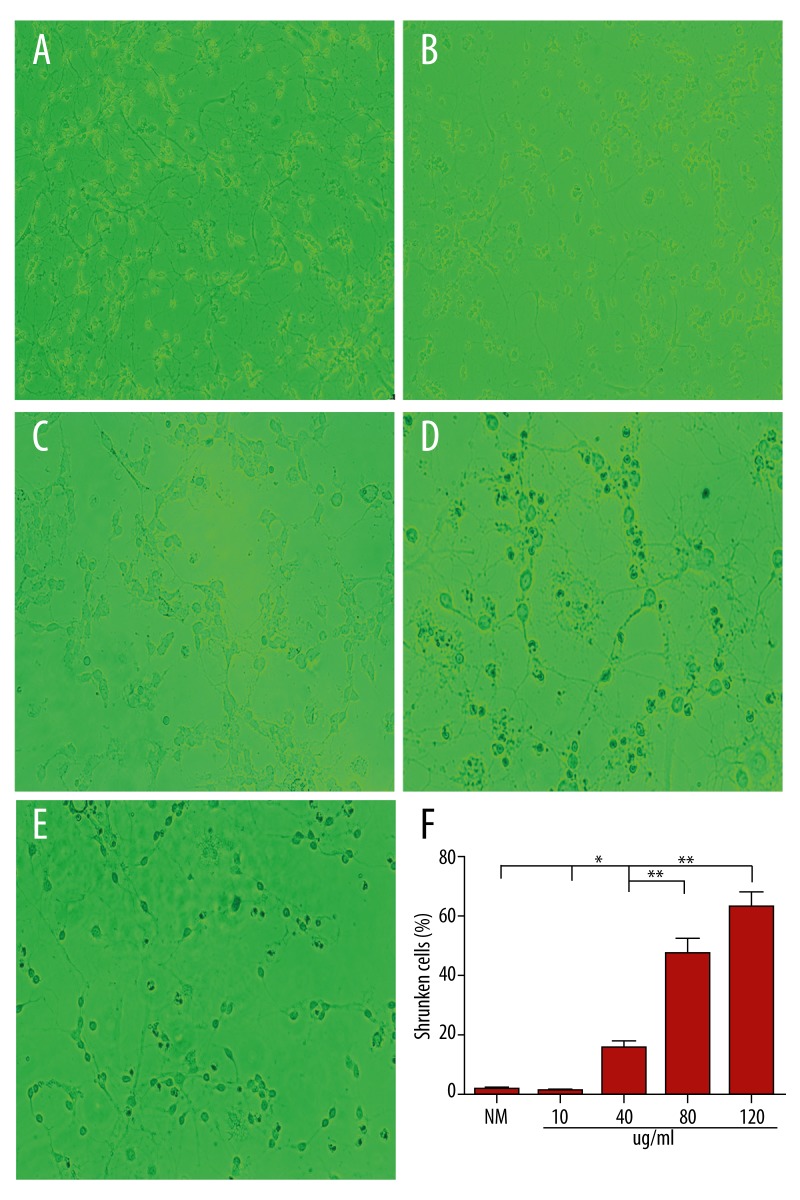
To investigate the effect of heroin, CGCs were cultured for 7 days in vitro. The mature neurons were then shifted to media containing 10 ug/ml (b), 40 ug/ml (c), 80 ug/ml (d), and 120 ug/ml (e) heroin for 24 h. In the medium without heroin, the neurons were healthy (Figure 2A). Within 24 h of switching to 10 ug/ml heroin-containing media, neurons underwent apoptosis and the apoptotic rate was higher than in the control group (Figure 2B). In 40-ug/ml heroin media, cell volume was shrunken (Figure 2C). With a dose of 80 ug/ml heroin, most of the CGCs were undergoing apoptosis (Figure 2D). However, more than 50% of cells were shrunken in 120 ug/ml heroin media with the disappearances of grid structures (Figure 2E). The morphological changes accompanying death of granule neurons are the characteristics of cells undergoing apoptosis.
Figure 2.
Cerebellar granule cells die when maintained in different of heroin (×200). DIV7 CGNs were then switched to medium containing 10 ug/ml, 40 ug/ml 80 ug/ml, 120 ug/ml heroin. Phase-contrast micrographs show neurons maintained in 10 ug/ml (B), 40 ug/ml (C), 80 ug/ml (D), 120 ug/ml (E) heroin for 24. Control cells (A) were maintained for 24 h in heroin-free medium. The percentages of death neurons under the treatments indicated were quantified (F). Scale bar=10 um. Data represent the means ±SEM of five independent experiments. * p<0.05,** p<0.01.
Detection of the apoptosis rates in CGCs
To define experimental conditions of moderate neurotoxicity, we performed a dose-response analysis of cell viability following flow cytometry: After 24 h, neuronal cells remained normal in the no-heroin medium (Figure 3A). Apoptosis rates reached 5.23% with 10 ug/ml heroin concentration (Figure 3B) and 11.5% in 40 ug/ml heroin medium (Figure 3C). However, the average apoptosis rates were 46.3% and 57% in 80 ug/mL and 120 ug/mL heroin concentrations, respectively (Figure 3D, 3E), and the model groups were significantly different compared with the control group (** P<0.01). We proved that these cells were undergoing apoptosis. With these criteria, the results consistently demonstrated that heroin in our model system triggered CGC apoptosis. Heroin-induced CGC apoptosis rates were almost the same as morphological changes, which indicated that the apoptosis rates rapidly increased when the concentration of heroin increased.
Figure 3.
Crebellar granule cells apoptosis when maintained in different of heroin. DIV7 CGNs were then switched to medium containing 10 ug/ml (B), 40 ug/ml (C), 80 ug/ml (D), 120 ug/ml (E) heroin. Flow cytometry show neurons apoptosis maintained in different of heroin dosage for 24h. Control cells (A) were maintained for 24 h in heroin-free medium. The percentages of neurons apoptosis under the treatments indicated were quantified (F). Scale bar=10 um. Data represent the means ±SEM of three independent experiments. * p<0.05,** p<0.01.
JNK pathway is involved in heroin-induced CGC apoptosis
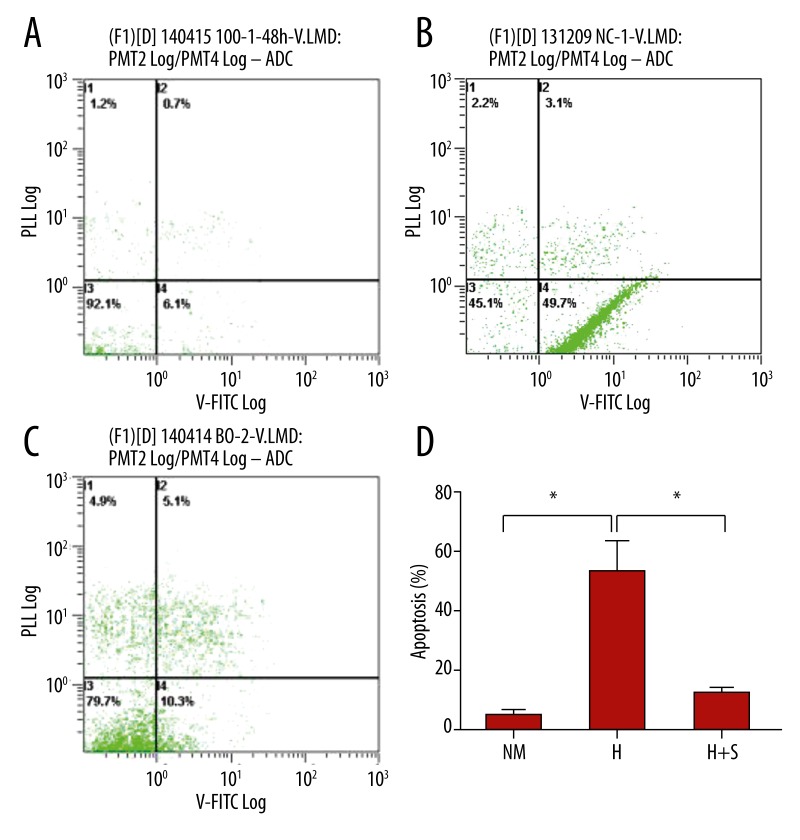
The present study showed that the JNK pathway is involved in the apoptosis process. It also indicated that the JNK/c-Jun pathway cascade was activated and amplified, leading to heroin-induced neuron apoptosis [14,27]. The rates of cell apoptosis were described by flow cytometry. We used 3 groups in our study: NM (control group), H (120 ug/ml heroin group), and H + S (120 ug/ml heroin + SP600125 inhibitor group). The results are shown in Figure 4, and indicate that the apoptosis rates in Group H were significantly higher than in group H+S (* p<0.05). The result suggests that the JNK pathway is involved in heroin-induced apoptosis.
Figure 4.
Cerebellar granule cells apoptosis when maintained in 120 ug/ml heroin (H) and SP600125+120ug/ml heroin (H+S). DIV7 CGNs were then switched to medium containing 120 ug/ml heroin (B), SP600125 +120 ug/ml heroin (C). Flow cytometry show neurons apoptosis maintained in 120ug/ml heroin and SP600125 +120 ug/ml heroin for 24 h. Control cells (A) were maintained for 24 h in normal medium. The percentages of neurons apoptosis under the treatments indicated were quantified (D). Scale bar=10 um. Data represent the means ±SEM of three independent experiments.*p <0.05.
Cytc and ATF3 mRNA is up-regulated under heroin treatment in CGCs
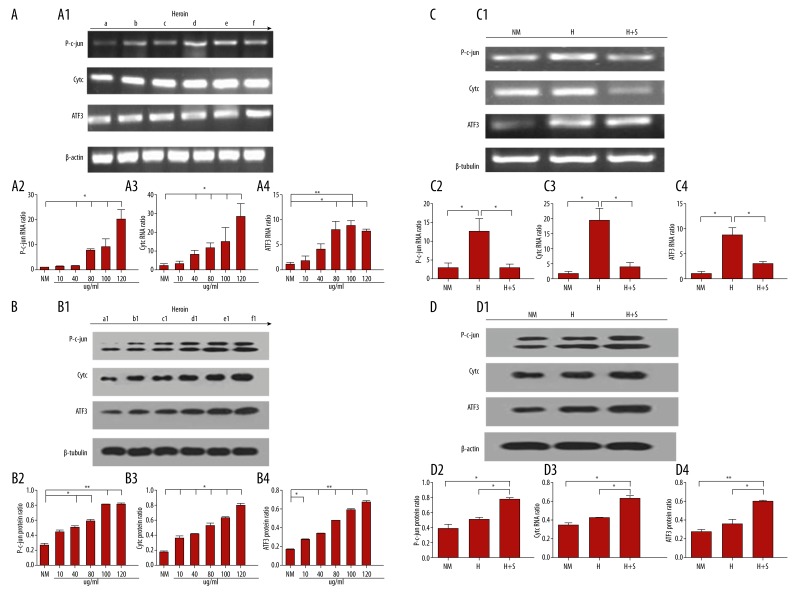
We wondered whether ATF3 and Cytc were closely related with heroin-induced apoptosis and how C-jun acted as a target gene in the JNK pathway. Therefore, we studied the mechanism by which Cytc and ATF3 are regulated under heroin intoxication through JNK pathway. We started with observing the expression level of p-c-jun, cytc, and ATF3 mRNA in different concentrations of heroin. As shown in Figure 5A, we found that expression levels of p-c-jun, cytc and ATF3 mRNA began to up-regulate with increasing concentrations of heroin medium (10 ug/ml, 40 ug/ml, 80 ug/ml, 100 ug/ml, and 120 ug/ml). In this process, the β-actin gene acted as an internal reference. By reverse transcription polymerase chain reaction (RT-PCR) detection, we compared the results with the control group, and found that the expression level of P-c-jun mRNA reached a peak at a heroin concentration of 120 ug/ml (Figure 5A2). The expression level of Cytc mRNA was far higher compared to the control group without heroin (Figure 5A3), and the expression level of ATF3 mRNA was also increased dramatically (Figure 5A4). The same result applies to the effect of the Western blotting. P-c-jun, cytc and ATF3 protein expression levels began to up-regulate with increasing doses of heroin (10 ug/ml, 40 ug/ml, 80 ug/ml, 100 ug/ml and 120 ug/ml) (Figure 5B). It was shown that P-c-jun, Cytc and ATF3 factors involved in the process of neuronal apoptosis by heroin when P-c-jun, cytc and ATF3 mRNA and protein level were shown dose-denpendent with heroin. Then, we observed the expression level of p-c-jun, cytc and ATF3 mRNA in H group (120 ug/ml heroin), H+S group (120 ug/ml heroin +10 uM SP600125) and NM group(control group) with medium. As shown in Figure 5C, we found that expression levels of p-c-jun, cytc and ATF3 mRNA reduced obviously in H+S group through ordinary polymerase chain reaction(PCR) detecting (Figure 5C1). By reverse transcription polymerase chain reaction (RT-PCR) detection, The results compared with the H group, expression level of P-c-jun mRNA was reaching a lower peak in 120 ug/ml heroin and 10 uM SP600125 (H+S group) (Figure 5C2), expression level of Cytc mRNA was decreased compared to H group (Figure 5C3), expression level of ATF3 mRNA was also decreased dramatically (Figure 5C4). Meanwhile, P-c-jun, cytc and ATF3 protein expression levels were similarity to their mRNA expression in three groups (NM group, H group and H+S group) by western blotting detected (Figure 5D1). We also found the expression level of P-c-jun, cytc and ATF3 protein were decreased obviously in H+S group compared with H group (*P<0.05) (Figure 5D2–D4]. With applying of JNK pathway inhibitor SP600125, P-c-jun, Cytc and ATF3 mRNA and protein expression levels had downward trend compared the single factor of heroin (120 ug/ml) which intervened in CGNs (Figure 5C, D1). The expression levels of P-c-jun, Cytc and ATF3 mRNA and proteins were significantly decreased which also indicated Cytc and ATF3 played a vital role in promoting apoptosis as C-jun candidate genes through JNK pathway. All these datas indicated that P-c-jun, Cytc and ATF3 factors were involved in the process of heroin-induced CGN apoptosis.
Figure 5.
JNK/c-Jun pathway regulates Cytc and ATF3 induction in heroin-induced neuronal apoptosis. (A) DIV7 CGNs were placed in media with or without different dosage of heroin for 24 h (a=normal medium, b=10 ug/ml, c=40 ug/ml, d=80 ug/ml, e=120 ug/ml), then neurons were assessed by PCR using the indicated the adopt of p-c-jun, cytc, ATF3 and β-actin primer (A1) and RT-PCR using the p-c-jun, cytc, ATF3 and β-actin were quantified (A2–A4). (B) DIV7 CGNs were placed in media with or without different dosage of heroin for 24 h (a=normal medium, b=10 ug/ml, c=40 ug/ml, d=80 ug/ml, e=120 ug/ml), then neurons were assessed by Western Blotting using the indicated the adopt of p-c-jun, cytc, ATF3 and β-tubulin protein (B2–B4). (C) DIV7 CGNs were transferred to media containing 120 ug/ml heroin in the absence or presence of 10 um SP600125.After 24 h, neurons were assessed by PCR using the indicated Semi-quantitative (C1) and RT-PCR using the p-c-jun, cytc and β-actin were quantified (C2–C4). (D) DIV7 CGNs were transferred to media containing 120 ug/ml heroin in the absence or presence of 10 um SP600125. After 24 h, neurons were assessed by Western Blotting using the p-c-jun, cytc and β-tubulin protein were quantified (D2–D4).* p<0.05, **p<0.01.
Discussion
Apoptosis is a complex process in which eukaryotic cells receive stimuli. Its genetic mechanisms start to activate and release the DNA endogenous endonucleases, which causes the cell death. Heroin can induce apoptosis of many kinds of cells, especially neurons. However, no significant work has been done in this filed. In the present study we try to take a further step in looking into the relationship between different dosages of heroin and cell apoptosis. We found that more cells experienced apoptosis at higher dosages of heroin, and the apoptosis rates reached a peak. We also found that the JNK pathway has a role in the process of heroin-induced neuronal apoptosis. Cytc and ATF3 mRNA had an obvious upward trend with increasing dosage of heroin, while both of expression levels can be reduced by JNK/c-jun inhibitor SP600125 (Figure 5A). This study proves that Cytc and ATF3 are involved in the process of heroin-induced neuronal apoptosis as the candidate target genes of the JNK signaling pathway. The results provide a platform for further study on the mechanism of by which heroin induces neuronal apoptosis.
Heroin induces apoptosis through release of cytochrome, activates caspase-3, and changes the Bax/Bcl-2 ratio in cortical neurons and CGCs [29]. During the apoptosis, CytC is a soluble protein loosely bound to the outer face of the inner mitochondrial membrane, and its release is associated with an interruption of the normal electron flow at the complex III site of the respiratory chain, which can divert electron transfer to the generation of superoxide [20]. ROS is a main element of mitochondrial dysfunction-induced cell death, and heroin can directly induce cells to produce ROS oxidative stress reactions in neuronal cells [30,31]. Cytc has a close relationship with the process of cell apoptosis under pathological conditions. We also found expression of ATF3, a member of the ATF subfamily of basic leucine zipper (B-ZIP) [32]. It is a highly versatile stress sensor for a wide range of conditions including hypoxia, malnutrition, oxidative stresses, ER stresses, and various genotoxic stresses, as well as inflammatory reactions. Francis et al. [33] found that ATF3 has dual functions in promoting either call death or cell survival in different conditions [34]. However, the role of ATF3 in JNK signaling pathway inhibition of cell death is unclear [35].
Generally, ATF3 is expressed at low levels in normal cells, but can be rapidly induced in response to diverse stress signals and is probably involved in controlling a wide variety of cellular activities. ATF3 and cell apoptosis are closely related. Studies show that ATF3 can either promote cell apoptosis or protect cells from damage. A growing number of studies suggest that ATF3 is an essential element in cell apoptosis, transcriptional regulation, and tumor formation. Controversy remains about the role of ATF3 in cell apoptosis. Thus, to study ATF3 in heroin-induced apoptosis and its expression in the JNK signaling pathway is essential.
There are different views on whether heroin-induced neuronal apoptosis can activate the related receptors (e.g., opioid receptors and glutamate receptors). Therefore, in-depth research on heroin-induced apoptosis mechanisms is needed. We hypothesize from our study that heroin may interact with apoptosis factors to form complexes that can activate the intrinsic pathway of apoptosis.
Conclusions
The present study shows the JNK/c-Jun pathway as a critical cascade, and CYTC and ATF3 are the candidate targets in the process of heroin-induced neuronal apoptosis. Therefore, inhibition of the JNK/c-Jun pathway and reducing the candidate target gene ATF3/Cytc activity may provide a new and effective strategy to treat heroin addiction and heroin-induced SLE.
Footnotes
Source of support: The study was supported by the National Science Foundation of China (NSFC), China (No. 81260464)
Reference
- 1.Jordan MT, Bryant SM, Aks SE, et al. A five-year review of the medical outcome of heroin body stuffers. J Emerg Med. 2009;36(3):250–56. doi: 10.1016/j.jemermed.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 2.Nutt D, King LA, Saulsbury W, et al. Development of a rational scale to assess the harm of drugs of potential misuse. Lancet. 2007;369(9566):1047–53. doi: 10.1016/S0140-6736(07)60464-4. [DOI] [PubMed] [Google Scholar]
- 3.Di Chiara G, Bassareo V. Reward system and addiction: what dopamine does and doesn’t do. Curr Opin Pharmacol. 2007;7(1):69–76. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Büttner A, Mall G, Penning R, et al. The neuropathology of heroin abuse. Forensic Sci Int. 2000;113(1–3):435–42. doi: 10.1016/s0379-0738(00)00204-8. [DOI] [PubMed] [Google Scholar]
- 5.Vassilev LT, Vu BT, Graves B, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303(5659):844–48. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 6.Cunha-Oliveira T, Rego AC, Garrido J, et al. Street heroin induces mitochondrial dysfunction and apoptosis in rat cortical neurons. J Neurochem. 2007;101(2):543–54. doi: 10.1111/j.1471-4159.2006.04406.x. [DOI] [PubMed] [Google Scholar]
- 7.Oliveira MT, Rego AC, Macedo TR, et al. Drugs of abuse induce apoptosic features in PC12 cells. Ann NY Acad Sci. 2003;1010:667–70. doi: 10.1196/annals.1299.121. [DOI] [PubMed] [Google Scholar]
- 8.Cordova JP, Balan S, Romero J, et al. ‘Chasing the Dragon’: new knowledge for an old practice. Am J Ther. 2014;21(1):52–55. doi: 10.1097/MJT.0b013e31820b8856. [DOI] [PubMed] [Google Scholar]
- 9.Kass-Hout T, Kass-Hout O, Darkhabani MZ, et al. “Chasing the dragon” – heroin-associated spongiform leukoencephalopathy. J Med Toxicol. 2012;7:240–42. doi: 10.1007/s13181-011-0139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W, Shi L, Xie Y, et al. SP600125, a new JNK inhibitor, protects dopaminergic neurons in the MPTP model of Parkinson’s disease. Neurosci Res. 2004;48(2):195–202. doi: 10.1016/j.neures.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Contestabile A. Cerebellar granule cells as a model to study mechanisms of neuronal apoptosis or survival in vivo and in vitro. Cerebellum. 2002;1(1):41–55. doi: 10.1080/147342202753203087. [DOI] [PubMed] [Google Scholar]
- 12.Scorrano L, Korsmeyer SJ. Mechanisms of cytochrome c release by proapoptosic BCL-2 family members. Biochem Biophys Res Commun. 2003;304(3):437–44. doi: 10.1016/s0006-291x(03)00615-6. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Zhang Y, Wei Z, et al. JNK inhibitor protects dopaminergic neurons by reducing COX-2 expression in the MPTP mouse model ofsubacute Parkinson’s disease. J Neurol. 2009;285(1–2):172–77. doi: 10.1016/j.jns.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 14.Lai B, Pu H, Cao Q, et al. Activation of caspase-3 and c-Jun NH2-terminal kinase signalinging pathways involving heroin-induced neuronalapoptosis. Neurosci Lett. 2011;502(3):209–13. doi: 10.1016/j.neulet.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 15.Morishima Y, Gotoh Y, Zieg J, et al. Beta-amyloid induces neuronal apoptosis via a mechanism that involves the c-Jun N-terminal kinase pathway and the induction of Fas ligand. J Neurosci. 2001;21(19):7551–60. doi: 10.1523/JNEUROSCI.21-19-07551.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le-Niculescu H, Bonfoco E, Kasuya Y, et al. Withdrawal of survival factors results in activation of the JNK pathway in neuronal cells leading to Fas ligandinduction and cell death. Mol Cell Biol. 1999;9(1):751–63. doi: 10.1128/mcb.19.1.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuan CY, Whitmarsh AJ, Yang DD, et al. A critical role of neural-specific JNK3 for ischemic apoptosis. Proc Natl Acad Sci USA. 2003;100(25):15184–89. doi: 10.1073/pnas.2336254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan XL, Zhang JS, Zhang XQ, et al. Chronic morphine treatment and withdrawal induce up-regulation of c-Jun N-terminal kinase 3 gene expression inrat brain. J Neurosci Res. 2003;122(4):997–1002. doi: 10.1016/j.neuroscience.2003.08.062. [DOI] [PubMed] [Google Scholar]
- 19.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281(5381):1309–12. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 20.Hirai K, Sugawara T, Chan PH, et al. Cytochrome c associated apoptosis during ATP recovery after hypoxia in neonatal rat cerebrocortical slices. J Neurochem. 2002;83(2):309–19. doi: 10.1046/j.1471-4159.2002.01130.x. [DOI] [PubMed] [Google Scholar]
- 21.Chen L, Xue Z, Jiang H. Effect of propofol on pathologic time-course and apoptosis after cerebral ischemia-reperfusion injury. Acta Anaesthesiol Scand. 2008;52(3):413–19. doi: 10.1111/j.1399-6576.2007.01560.x. [DOI] [PubMed] [Google Scholar]
- 22.Mei Y, Yuan Z, Song B, et al. Activating transcription factor 3 up-regulated by c-Jun NH(2)-terminal kinase/c-Jun contributes to apoptosisinduced by potassium deprivation in cerebellar granule neurons. J Neurosci Res. 2008;151(3):771–79. doi: 10.1016/j.neuroscience.2007.10.057. [DOI] [PubMed] [Google Scholar]
- 23.Cai Y, Zhang C, Nawa T, et al. Responsive ATF3 gene expression in human vascular endothelial cells: activation of c-Jun NH(2)-terminal kinase and promoter res19onse element. Blood. 2000;96(6):2140–48. [PubMed] [Google Scholar]
- 24.Lu D, Chen J, Hai T. The regulation of ATF3 gene expression by mitogenactivated protein kinases. J Biochem. 2007;401(2):559–67. doi: 10.1042/BJ20061081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SH, Min KW, Zhang X, et al. 3,3′-diindolylmethane induces activating transcription factor 3 (ATF3) via ATF4 in human colorectal cancer cells. J Nutr Biochem. 2013;24(4):664–71. doi: 10.1016/j.jnutbio.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu JS, Cheung WM, Tsai YS, et al. Ligand-activated peroxisome proliferator-activated receptor-gamma protects against ischemic cerebral infarction and neuronal apoptosis by 14-3-3 epsilon upregulation. Circulation. 2009;119(8):1124–34. doi: 10.1161/CIRCULATIONAHA.108.812537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorini A, Canosi U, Devecchi E, et al. ATPases enzyme activities during ageing in different types of somatic and synaptic plasma membranes from rat frontal cerebral cortex. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(1):81–90. doi: 10.1016/s0278-5846(01)00233-0. [DOI] [PubMed] [Google Scholar]
- 28.Cunha-Oliveira T, Rego AC, Garrido J, et al. Neurotoxicity of heroin-cocaine combinations in rat cortical neurons. Toxicology. 2010;276(1):11–17. doi: 10.1016/j.tox.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 29.Tan M, Li Z, Ma S, et al. Heroin activates Bim via c-Jun N-terminal kinase/c-Jun pathway to mediate neuronal apoptosis. J Neurosci. 2013;233:1–8. doi: 10.1016/j.neuroscience.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Pan J, Zhang Q, Zhang Y, et al. Oxidative stress in heroin administered mice and natural antioxidants protection. Life Sci. 2005;77(2):183–93. doi: 10.1016/j.lfs.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 31.Qiusheng Z, Yuntao Z, Rongliang Z, et al. Effects of verbascoside and luteolin on oxidative damage in brain of heroin treated mice. Pharmazie. 2005;60(7):539–43. [PubMed] [Google Scholar]
- 32.Hai T, Hartman MG. The molecular biology and nomenclature of the activating transcription factor/cAMP responsive element binding family of transcription factors: activating transcription factor proteins and homeostasis. Gene. 2001;273(1):1–11. doi: 10.1016/s0378-1119(01)00551-0. [DOI] [PubMed] [Google Scholar]
- 33.Francis JS, Dragunow M, During MJ. Over expression of ATF-3 protects rat hippocampal neurons from in vivo injection of kainic acid. Brain Res Mol Brain Res. 2004;124(2):199–203. doi: 10.1016/j.molbrainres.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 34.Khuu CH, Barrozo RM, Hai T, et al. Activating transcription factor 3(ATF3)represses the expression of CCIA in murine macmphages. Mol Immunol. 2007;44(7):1598–605. doi: 10.1016/j.molimm.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Park GH, Park JH, Song HM, et al. Anti-cancer activity of Ginger (Zingiber officinale)leaf through the expression of activating transcription factor 3 in human colorectal cancer cells. BMC Complement Altern Med. 2014;14(1):408. doi: 10.1186/1472-6882-14-408. [DOI] [PMC free article] [PubMed] [Google Scholar]