Abstract
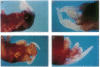
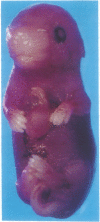
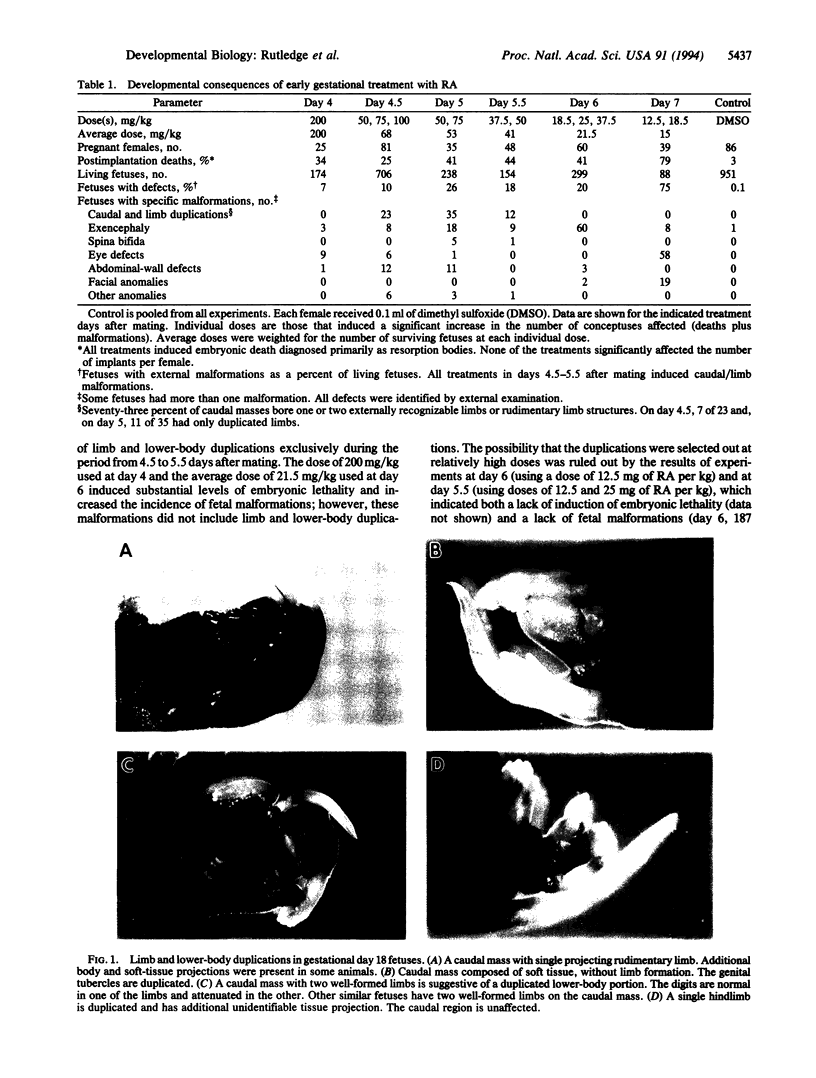
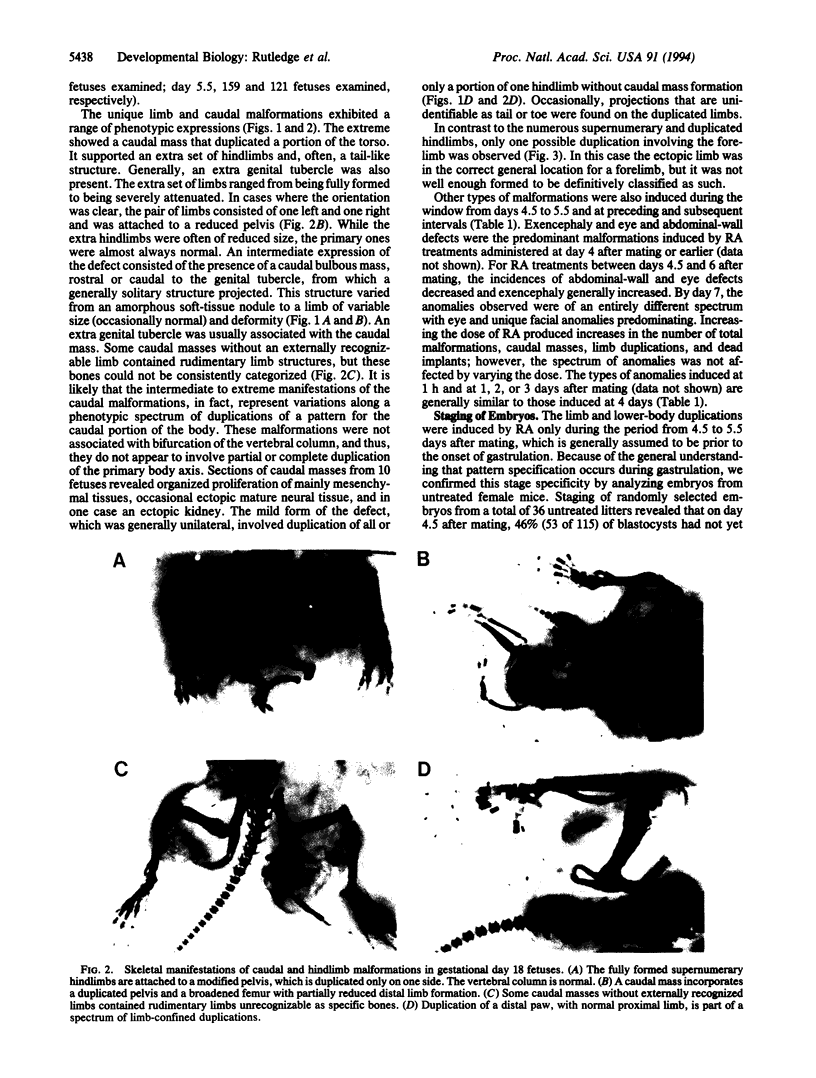
The zygote and subsequent preimplantation stages of early mammalian development are susceptible to certain chemical perturbations that cause abnormal development of the conceptus. In certain cases, disruption in patterns of gene expression could be a primary event leading to abnormal development. To investigate this hypothesis, we treated pregnant mice with trans-retinoic acid, a known modulator of gene expression. Treatments were administered at various times during pregastrulation stages and the presumed onset of gastrulation. trans-Retinoic acid induced a distinctive set of malformations, as manifest by supernumerary and ectopic limbs and duplication of portions of the lower body, but only when administered during the period of 4.5-5.5 days after mating. (Other malformations were induced at different stages.) The limb and lower-body duplications suggest that exogenous trans-retinoic acid may influence not only the pattern for the hindlimbs but also that for the entire lower body. Since it appears likely that the embryos were affected in the late blastocyst and proamniotic-embryo stages, the provocative possibility arises that aspects of pattern formation of limbs and lower body actually occur prior to gastrulation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryant S. V., Gardiner D. M. Retinoic acid, local cell-cell interactions, and pattern formation in vertebrate limbs. Dev Biol. 1992 Jul;152(1):1–25. doi: 10.1016/0012-1606(92)90152-7. [DOI] [PubMed] [Google Scholar]
- Crosby J. L., Varnum D. S., Nadeau J. H. Two-hit model for sporadic congenital anomalies in mice with the disorganization mutation. Am J Hum Genet. 1993 May;52(5):866–874. [PMC free article] [PubMed] [Google Scholar]
- Dominguez R., Rott J., Castillo M., Pittaluga R. R., Corriere J. N., Jr Caudal duplication syndrome. Am J Dis Child. 1993 Oct;147(10):1048–1052. doi: 10.1001/archpedi.1993.02160340034009. [DOI] [PubMed] [Google Scholar]
- Generoso W. M., Rutledge J. C., Cain K. T., Hughes L. A., Downing D. J. Mutagen-induced fetal anomalies and death following treatment of females within hours after mating. Mutat Res. 1988 May;199(1):175–181. doi: 10.1016/0027-5107(88)90243-6. [DOI] [PubMed] [Google Scholar]
- Iannaccone P. M., Bossert N. L., Connelly C. S. Disruption of embryonic and fetal development due to preimplantation chemical insults: a critical review. Am J Obstet Gynecol. 1987 Aug;157(2):476–484. doi: 10.1016/s0002-9378(87)80198-9. [DOI] [PubMed] [Google Scholar]
- Kimmel C. A., Generoso W. M., Thomas R. D., Bakshi K. S. A new frontier in understanding the mechanisms of developmental abnormalities. Toxicol Appl Pharmacol. 1993 Apr;119(2):159–165. doi: 10.1006/taap.1993.1056. [DOI] [PubMed] [Google Scholar]
- Maden M. The homeotic transformation of tails into limbs in Rana temporaria by retinoids. Dev Biol. 1993 Oct;159(2):379–391. doi: 10.1006/dbio.1993.1249. [DOI] [PubMed] [Google Scholar]
- Mohanty-Hejmadi P., Dutta S. K., Mahapatra P. Limbs generated at site of tail amputation in marbled balloon frog after vitamin A treatment. Nature. 1992 Jan 23;355(6358):352–353. doi: 10.1038/355352a0. [DOI] [PubMed] [Google Scholar]
- Petzel M. A., Erickson R. P. Disorganisation: a possible cause of apparent conjoint twinning. J Med Genet. 1991 Oct;28(10):712–714. doi: 10.1136/jmg.28.10.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijnappel W. W., Hendriks H. F., Folkers G. E., van den Brink C. E., Dekker E. J., Edelenbosch C., van der Saag P. T., Durston A. J. The retinoid ligand 4-oxo-retinoic acid is a highly active modulator of positional specification. Nature. 1993 Nov 25;366(6453):340–344. doi: 10.1038/366340a0. [DOI] [PubMed] [Google Scholar]
- Rizzo R., Lammer E. J., Parano E., Pavone L., Argyle J. C. Limb reduction defects in humans associated with prenatal isotretinoin exposure. Teratology. 1991 Dec;44(6):599–604. doi: 10.1002/tera.1420440602. [DOI] [PubMed] [Google Scholar]
- Rutledge J. C., Generoso W. M., Shourbaji A., Cain K. T., Gans M., Oliva J. Developmental anomalies derived from exposure of zygotes and first-cleavage embryos to mutagens. Mutat Res. 1992 Dec;296(1-2):167–177. doi: 10.1016/0165-1110(92)90040-g. [DOI] [PubMed] [Google Scholar]
- Sanders D. D., Stephens T. D. Review of drug-induced limb defects in mammals. Teratology. 1991 Sep;44(3):335–354. doi: 10.1002/tera.1420440310. [DOI] [PubMed] [Google Scholar]
- Shenefelt R. E. Morphogenesis of malformations in hamsters caused by retinoic acid: relation to dose and stage at treatment. Teratology. 1972 Feb;5(1):103–118. doi: 10.1002/tera.1420050115. [DOI] [PubMed] [Google Scholar]