Abstract
The regulation of endothelial NO synthase (eNOS) employs multiple different cellular control mechanisms impinging on level and activity of the enzyme. This review aims at summarizing the current knowledge on the posttranslational modifications of eNOS, including acylation, nitrosylation, phosphorylation, acetylation, glycosylation and glutathionylation. Sites, mediators and impact on enzyme localization and activity of the single modifications will be discussed. Moreover, interdependence, cooperativity and competition between the different posttranslational modifications will be elaborated with special emphasis on the susceptibility of eNOS to metabolic cues.
Keywords: Endothelial NO synthase, regulation of enzyme activity, posttranslational modification
1. INTRODUCTION
In line with the crucial homeostatic function of endothelial NO synthase (eNOS) and bioavailable nitric oxide (NO) for the vasculature, eNOS enzyme activity is subject of a delicate and interconnected system of various control mechanisms. This network impinges on hubs influencing the eNOS system on sites as diverse as the level and stability of eNOS mRNA and interaction of the eNOS protein with distinct binding partners. Like this, a wide spectrum of kinetics and amplitudes in the modulation of eNOS enzyme activity can be achieved. This article will focus on known posttranslational modifications which can rapidly and intimately regulate and fine-tune the subcellular localization and/or activity of the eNOS enzyme. So far acylation, nitrosylation, phosphorylation, acetylation, glycosylation, and glutathionylation of eNOS have been reported and found to enable a dynamic control of eNOS activity and NO bioavailability in response to a variety of physiologic and pathophysiologic cues.
In the following chapters, a snapshot on these posttranslational modifications, concentrating on site of modification, respective impact on eNOS enzyme activity and potential susceptibility to exogenous modulation, will be presented with all sites numbered according to the complete 1203 amino acid human sequence (protein accession number P29474.3).
2. ACYLATION
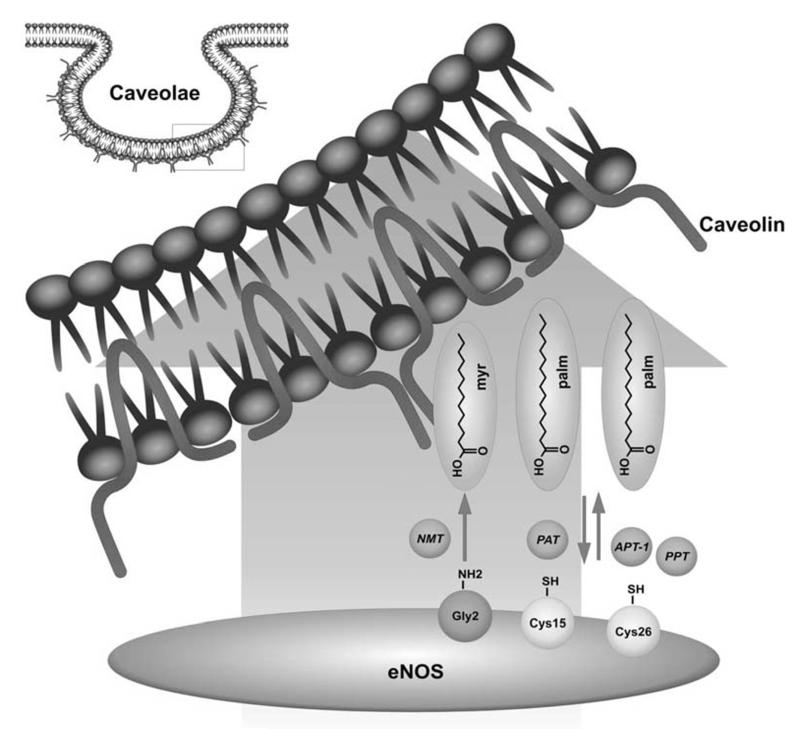
ENOS is a membrane protein mainly found in plasma membrane caveolae or on the Golgi apparatus. As all dually acylated proteins including Src kinase family members or some G-protein alpha subunits, eNOS is co-translationally N-myristoylated on cytoplasmic ribosomes by N-myristoyltransferases (NMT) and post-translationally palmitoylated by palmitoyl-acyl-transferases (PAT) on cysteine residues. The de- or attachment of these lipid anchors to the eNOS protein determines its subcellular localization. The cellular situation of eNOS, in turn, is crucial for optimized signal integration from upstream signalling pathways, for interaction with binding partners or for communication with downstream effectors and thus an important parameter for the actual enzymatic activity. In line, acylation-defective mutants of eNOS cannot be properly targeted and display impaired basal and agonist-stimulated NO release [1,2].
In 1996, Shaul et al. revealed that eNOS is targeted to plas-malemmal caveolae, i.e. plasmamembrane invaginations defined by the presence of the scaffolding protein caveolin [3]. Those authors showed by biochemical subfractionation that eNOS activity was 7-fold greater in the plasma membrane fraction than in the cytosol. Within the plasma membrane, eNOS activity was undetectable in the noncaveolar fraction, whereas it was 9.4-fold enriched in caveolar membranes compared with whole plasma membrane. More precisely, 57-100% of the total eNOS activity in the plasma membrane was recovered in the caveolar fraction, indicating that plasmalemmal NOS is primarily localized to caveolae. Caveolae are relatively rich in cholesterol and sphingolipids which lend these membrane compartments a distinct fluidity and create a unique environment for an efficient control of protein-protein interactions and signal transduction. In resting endothelial cells, eNOS strongly and directly interacts with caveolin-1 in the caveolae, a protein-protein interaction which tonically inhibits eNOS activity by masking the calmodulin binding site. Interestingly, Feron et al. showed that interaction of eNOS with caveolin is favoured by but not necessarily dependent on acylation [4]. Upon stimulus-induced increases in intracellular calcium levels caveolin-1 is displaced by calcium-calmodulin (CaM) and this results in elevated enzymatic activity (for a detailed overview over modulation of eNOS activity by protein-protein interaction please refer to an accompanying article in this issue).
General membrane localization of eNOS is dependent on irreversible co-translational myristoylation of the amino group at glycine 2 (after removal of the N-terminal methionine 1) [3,5,6]. The myristoyl group anchors eNOS to membranes via hydrophobic interactions with membrane lipids. From a thermodynamic point of view, however, the free energy of membrane binding for myristoylated proteins is rather low rendering those interactions readily reversible and unstable [7]. Palmitoylation of eNOS therefore is thought to provide additional hydrophobic interactions that stabilize the weak membrane association brought about by myristoylation alone. Posttranslational thiopalmitoylation at cysteine 15 and cysteine 26 is mediated by palmitoyltransferases at the Golgi apparatus and fixates eNOS at specific biological membranes [6,8,9,10]. The enzymes confering palmitoylation to eNOS have been elusive for a long time. In 2006 Fernandez-Hernando et al. screened 23 known Asp-His-His-Cys-motif palmitoyl-acyl-transferases DHHC-PATs and identified five cDNAs of DHHC-PAT that were able to palmitoylate eNOS in vivo [11]. Those authors found that these five palmitoyltransferases, namely DHHC-2, -3, -7, -8, and -21, are expressed in endothelial cells, and colocalize with the Golgi matrix protein GM-130, indicating that palmitoylation occurs on the cytoplasmic face of the Golgi complex. The DHHC-PATs further colocalized and associated with eNOS mainly on the Golgi apparatus. The interaction eNOS /DHHC and thus palmitoylation were further favoured by N-myristoylation. Particularly, DHHC-21 reduction in endothelial cells resulted in decreased eNOS palmitoylation, mislocalization of eNOS and impairment of agonist-stimulated NO release. Thiopalmitoyl bonds are labile and the calveolar targeting of eNOS therefore remains a reversible process allowing a high degree of dynamic control [12]. There is indeed a constant cellular shuttling of the enzyme, mainly between plasma membrane and Golgi, which is also reflected by a constant turnover of palmitate residues attached to the eNOS protein [8]. Upon prolonged agonist activation, eNOS is depalmitoylated and translocated from the plasma membrane to interior cell compartments or the cytosol [12,13]. Palmitoyl-protein thioesterase (PPT) and acyl-protein thioesterase 1 (APT-1) have been implicated in eNOS depalmitoylation [12,14]. There is evidence that calmodulin may promote cytosolic translocation of eNOS by favouring APT 1-mediated depalmitoylation of eNOS [13]. Translocation to the cytosol may thus serve as a mean to terminate eNOS activity after prolonged agonist stimulation. Repalmitoylation at the Golgi then closes the acylation cycle and restores the basal-caveolin bound state of eNOS at the plasma membrane. Independent of stimulation, a continous basal (de-)-acylation cycle is moreover enabling constant access to the sorting machinery of the Golgi from where eNOS can be shuttled according to cellular requirements [15].
Overall, acylation of eNOS primarily mediates caveolar localization of eNOS and compartimentilization of NO production. Several signaling molecules which play an important role in regulation of eNOS enzyme activity, such as G-protein coupled receptors, modulators of calcium flux or AKT kinase, are also compartimentalized in caveolae or recruited to calveolae or nearby membrane regions upon activation. Therefore caveolar localization of eNOS appears handy for regulation of the enzyme by hormonal cues, such as estrogen or insulin. Accordingly, caveolar localization influences the site-specific phosphorylation pattern of eNOS (see below). Agonist-stimulated phosphorylation at Ser 1177, which increases enzyme activity, was nearly abrogated in eNOS mutants deficient in myristoylation but could fully be restored by membrane targeting of these mutants. A reciprocal regulation is observed for Ser 114 with membrane localization impairing, and cytosolic localization favoring phosphorylation at this site [7,16]. Moreover, as caveolae interact with the cytoskeleton (through actin and microtubules) they are prone to deformation upon reorganization of the cytoskeleton due to stretching or compression of the endothelial cell. Thereby caveolar membrane channels and carriers may be opened, a mechanism important for susceptibility of eNOS function to physical stimuli such as shear stress [17-20]. Figure 1 schematically illustrates the acylation sites of eNOS.
Fig. (1). Acylation sites of eNOS.
NMT: N-myristoyltransferases; PAT: palmitoyl-acyl-transferases; APT-1: acyl-protein thioesterase 1; PPT: palmitoyl-protein thioesterase
Seen from a strictly chemical point of view, acetylation is another form of acylating posttranslational modification. Due to its distinguished impact on eNOS activity it will, however, be dealt with in a separate chapter (see 5.).
3. S-NITROSYLATION
Reversible S-nitrosylation of cysteine residues, i.e. the formal replacement of the hydrogen atom in the thiol group of cysteine residues by a NO moiety, is another dynamic posttranslational modification of eNOS [21,22]. S-nitrosylation is closely linked to the subcellular localization of eNOS. It requires membrane targeting evident by the fact that acylation deficient eNOS mutants do not undergo nitrosylation [21]. It seems that the lipid environment of caveolae supports the formation of S-nitrosothiols whereas the reducing cytosolic environment favours denitrosylation [21-23]. S-Nitrosylation inhibits eNOS enzyme activity and upon agonist stimulation eNOS is rapidly denitrosylated with kinetics mirroring the observed increase in eNOS enzyme activity. Receptor-mediated decrease in nitrosylation was hereby found to be inversely related to enzyme phosphorylation at Ser 1177, a site associated with eNOS activation (see below under point 4) [21]. Following prolonged agonist exposure, eNOS undergoes depalmitoylation (see above under point 1) and translocation to the cytoplasmic compartment where the de-nitroso form of eNOS is further favoured. As eNOS returns to resting activity levels it is progressively renitrosylated and reshutteled to caveolae [22,24].
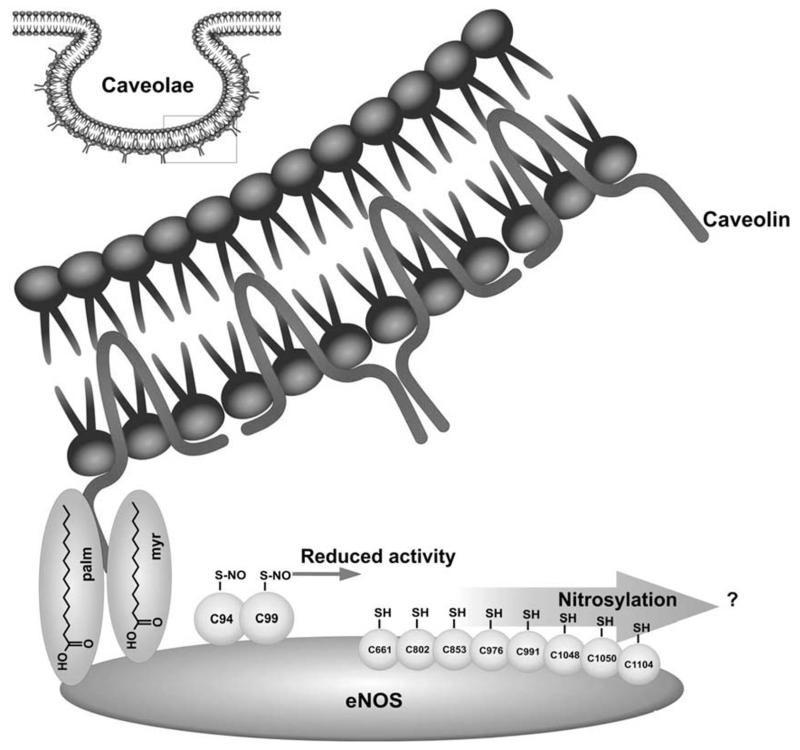
Cys 94 and Cys 99 are intensively investigated and discussed S-nitrosylation sites of eNOS and form the zinc tetrathiolate cluster at the eNOS homodimer interface which is responsible for dimer formation of the active enzyme [21,25]. As eNOS itself seems to provide the NO required for its own S-nitrosylation, this implies a mechanism securing some degree of spatial selectivity given the overall 29 cysteine residues in the eNOS enzyme [22]. However, Tummala et al. employed a modified biotin switch assay coupled to mass spectrometric analysis of tryptic peptides and identified next to Cys 94 and Cys 99 further cysteine residues capable of undergoing S-nitrosylation [26]. These included cysteine residue 661, 802 and 1114 and are located in regions known to bind flavin mononucleotide (FMN), flavin adenine dinucleotide (FAD) and nicotinamide adenine dinucleotide (NADPH), but their regulatory role remains speculative. Additional cysteines susceptible to S-nitrosy-lation are Cys 853, 976/991, and 1048/1050, respectively, which reside in regions of the eNOS enzyme that have not been assigned any biochemical function or significance in eNOS regulation. Thus, S-nitrosylation may occur at multiple sites other than Cys 94 and 99, however, with so far undisclosed effect on eNOS activity [26]. Concerning the cause underlying eNOS inhibition by nitrosylation of Cys 94 and 99 there is discord in the published literature whether S-nitrosylation promotes dimer collapse [25-27] or rather disturbs electron transfer between eNOS monomers or substrate / cofactor binding [21,22]. Taking into account that S-nitrosylation involves the zinc cluster cysteine that is involved in dimerization of eNOS and that zinc could be shown to be released upon eNOS S-nitrosylation, Ravi et al. suggested a mechanism that is based on destruction of the zinc cluster. Subsequently deduced dissociation of the eNOS homodimer could then account for the observed decline in activity [25]. Erwin et al. mutated the zinc-tetrathiolate cysteines and observed elimination of eNOS S-nitrosylation but not NO synthase activity which questions if disruption of the zinc-tetrathiolate necessarily has to result in eNOS monomerization [21].
Further work is still needed to mechanistically delineate how S-nitrosylation impacts eNOS activity. Particular emphasis may be put on i) the role of cellular redox state as thioredoxin and thioredoxin reductase have been found to counteract nitrosylation of eNOS [25], ii) the mechanism of denitrosylation or cytosolic trans-nitrosylation and not to forget iii) a detailed analysis of the mutual cross-talk and interdependence between eNOS nitrosylation, acylation, and phosphorylation reactions. Figure 2 gives a snapshot over the currently identified S-nitrosylation sites of eNOS.
Fig. (2).
Overview over reported S-nitroyslation sites of eNOS with known and so far unrevealed effect on eNOS enzyme activity.
4. PHOSPHORYLATION
Phosphorylation is a major and so far presumably the best studied posttranslational modification influencing eNOS enzyme activity. Changes in the phosphorylation state allow rapid integrative responses to different mechanical, humoral, metabolic, or pharmacological stimuli. The pleiotropy of cues known to merge into altered eNOS enzyme activity is reflected by multiple identified kinases and phosphatases involved in the site-selective regulation of eNOS phosphorylation. Up to now, eight phosphorylation sites have been linked to changes in eNOS activity. In the following, these phosphorylation sites, separated according to serine/threonine or tyrosine phosphorylations, shall be discussed in their numerical order.
Serine/ Threonine Phosphorylation
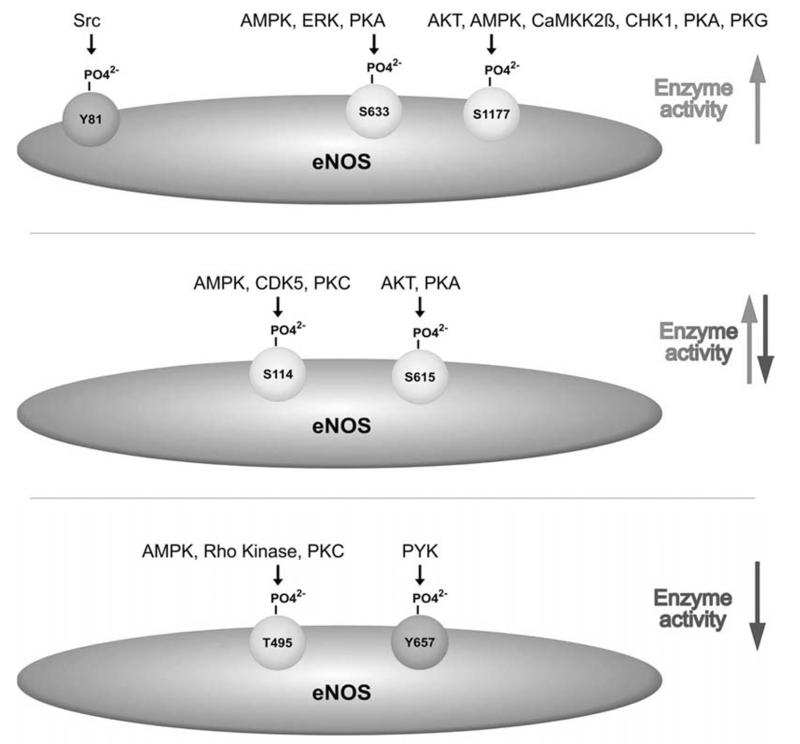
Phosphorylation at Serine 114 in the oxygenase domain of eNOS, is reduced by calphostin, a protein kinase C (PKC) inhibitor, indicating PKC as upstream kinase [28]. Also AMP-activated kinase (AMPK) was considered as possible upstream kinase [29]. Recent studies additionally show involvement of cyclin-dependent kinase 5 (CDK5) in Ser 114 phosphorylation with a concomitantly decreased NO production [30,31]. Phosphorylation at Ser 114 is commonly assumed to inhibit eNOS enzyme activity although not all data support this notion. Dephosphorylation at this site has been observed after stimulation with agonists, such as vascular endothelial growth factor (VEGF) [28] or the antidiabetic drug troglitazone [32]. The dephosphorylation mutant (S114A) displayed increased enzyme activity [28]. However, in [33] the authors found increased activity with the phosphomimetic SI 14D mutant of eNOS. Moreover, shear stress and high density lipoprotein (HDL) have led to an increased Ser 114 phosphorylation proposing rather an activating phosphorylation site [24, 34]. These discrepancies need to be solved by further studies and possibly suggest that phosphorylation at this site may have different functions in the context of distinct stimuli. Calcineurin seems to be involved in the dephosphorylation of eNOS at Ser 114 [28,35].
Threonine 495, located in the Ca++/CaM binding domain, represents the major negative regulatory site of eNOS, which is constitutively phosphorylated in most cultured endothelial cells. Kinases involved in phosphorylation of this site are PKC, although with no certainty about the involved isoform, Rho kinase [34,36,37] or, based on in vitro results, AMPK [38]. Phosphorylation at Thr 495 attenuates eNOS activity by interfering with CaM binding due to repulsive steric or charge effects. In response to certain agonists – mainly those increasing intracellular calcium – Thr 495 is dephosphorylated by the protein phosphatases PP1, PP2A or PP2B [39-41]. Moreover, there is evidence for coordination between dephosphorylation of eNOS at Thr 495 and activating phosphorylation of eNOS at Ser 1177 [39-43]. However, dephosphorylation of Thr 495 without concomitant increased Ser 1177 phosphorylation has also been reported [44]. Another implication for eNOS Thr 495 phosphorylation came from Lin et al. who found that mimicking Thr 495 dephosphorylation by mutation of the site to alanin resulted in eNOS uncoupling, i.e. increased production of superoxide rather than of NO [42]. These findings suggest that phosphorylation at Thr 495 may tune the product mix of NO and superoxide output.
Phosphorylation at Serine 615, also located in the CaM autoinhibitory sequence within the FMN binding domain, is catalyzed by AKT and PKA [45]. The role of Ser 615 phosphorylation is still not entirely clear [34]. Whereas a phosphomimetic S615D mutant of eNOS increased maximal enzyme activity [33] and several eNOS agonists transiently increase phosphorylation at this site [33, 45-47], the S625A dephosphorylation mimic mutant also increased basal and agonist stimulated NO release and enhanced the association of eNOS with the kinase AKT and heat shock protein 90 (Hsp90) [33]. Ser 615 furthermore corresponds to Ser 852 of neuronal NOS [45] which, when phosphorylated by CaMKK alpha, inhibits enzyme activity. Given these partly inconsistent findings, one could speculate that the phosphorylated Ser 615 rather modulates interactions between eNOS and other proteins and hereby influences phosphorylation of eNOS at other sites with direct impact on eNOS activity [33]. In this line, Ser 615 phosphorylation has been claimed to sensitize eNOS for CaM binding by displacement of the autoinhibitory loop [45].
Serine 633 is a positive regulatory site. It is located in the CaM autoinhibitory sequence of eNOS within the FMN binding domain and appears to be under control of PKA [34,48], AMPK [49] and extracellular signal regulated kinase (ERK) 1/2 [50]. Ser 633 and Ser 1177 (see below) share some of their activating stimuli, such as VEGF [33,51], shear stress [51], or 8-bromo cyclic AMP (cAMP) [51], but phosphorylation at Ser 633 occurs generally slower than that of Ser 1177 and is believed to contribute to the maintenance of NO synthesis following the NO burst occurring after activation at Ser 1177. Phosphorylation of Ser 633 may thus play an important role in chronic regulation of eNOS in response to mechanical and humoral stimuli [51]. Moreover, phosphorylation at Ser 633 renders NO production by eNOS independent from changes in intracellular calcium [48]. Phosphatases acting on Ser 633 have not been identified.
Phosphorylation at Serine 1177 confers the most important positive modulation of eNOS activity. Phosphorylation of Ser 1177 is catalyzed by a number of distinct kinases including AKT, PKA [33,38,52], AMPK [38], calcium-calmodulin kinase kinase (CaMKK) II beta [39,53] and checkpoint kinase (CHK)l [54], This stresses Ser 1177 phosphorylation as highly integrative and central posttranslational modification since the different individual kinases themselves can in turn be regulated by very diverse stimuli. To name a few: AKT can be activated by shear stress [55], growth factors including VEGF and insulin [57], or reactive oxygen species [58]. AMPK activity can be elevated by increased intracellular calcium levels or a relative increase in AMP. The latter can be caused by nutrient starvation, inhibition of the mitochondrial respiratory chain, such as after treatment with the antidiabetic drugs troglitazone [59], metformin [60] or multiple plant-derived compounds [61], or impaired ATP synthase activity. AMPK activation is also observed upon exposure to adiponectin [62] or salicylate [63]. Overall, most stimuli promoting eNOS activation finally lead to increased eNOS phosphorylation at Ser 1177. Ser 1177 phosphorylation enhances eNOS enzyme activity by increasing the electron flux at the reductase domain. The carboxyterminal tail of eNOS, containing Ser 1177, is believed to be wedged between two eNOS monomers and thus act as an autoinhibitory domain by blocking the electron transfer between them [64]. Upon phosphorylation of eNOS at Ser 1177 and a subsequent conformational change the wedge is removed, and eNOS activity is thereby increased. eNOS Serll77 phosphorylation further contributes to elevated eNOS enzyme activity by reducing CaM dissociation from activated eNOS [34,65] and allowing activation of eNOS at resting levels of calcium [45,66]. Dephosphorylation at Ser 1177 is predominantly mediated by the phosphatase PP2A [40].
Tyrosine Phosphorylation
Phosphorylation of eNOS at tyrosines is less investigated although indications for it have been existing for more than 15 years [67,68]. It is still unresolved whether tyrosine phosphorylation is involved in modulating enzyme activity or rather in providing binding sites for proteins with a Src homology 2 (SH2) or a phospho tyrosine binding (PTB) domain. Two tyrosines have been investigated in more detail: phosphorylation at Tyr 81 was reported to be mediated by Src kinase in response to H2O2 and to enhance basal eNOS activity [69]. Tyr 657 has been found to be phosphorylated upon insulin or angiotensin stimulation and fluid shear stress by proline rich tyrosine kinase 2 (PYK2). Mutation analyses revealed that Tyr 657 phosphorylation attenuates eNOS enzyme activity, presumably in order to limit the detrimental consequences of maintained high NO output in situations of redox stress [70,71].
Figure 3 summarizes the known phosphorylation sites of eNOS with positive, ambiguous or negative impact on eNOS enzyme activity.
Fig. (3). Overview of known phosphorylation sites of eNOS with positive, ambiguous or negative impact on eNOS activity.
AKT: protein kinase B/ Akt kinase; AMPK: AMP-activated kinase; CDK5: cyclin-dependent kinase 5; CHK1: checkpoint kinase 1; ERK: extracellular stimuli regulated kinase; PKA: protein kinase A, PKC: protein kinase C; PKG: protein kinase G; PYK: proline-rich tyrosine kinase; Src: Src kinase;
5. ACETYLATION
Acetylation of proteins at the epsilon amino group of lysines constitutes another important posttranslational modification with considerable impact on protein functionality. By inferring an acetyl group to a lysine, there is an increase in the amide dipol and electrostatic field compared with the primary amino group of the lysine precursor which influences cell signalling [72]. Lysine acetyltrans-ferases (KATs) usually catalyse the transfer of acetyl groups from acetyl-CoA to epsilon amino groups of lysine. KATs depend on the availability of acetyl-CoA, a common key metabolite within the catabolism of sugars, fatty acids and proteins, which renders acetylation a protein modification highly indicative of and susceptible to the current availability of nutrients [73]. Deacetylation is executed by deacetylases, mainly of the family of NAD+-dependent SIRT histone deacetylases (HDAC). Having in mind that the ratio of NAD+/ NADH increases with nutrient deprivation, it becomes once more underlined that acetylation is favoured under conditions of high nutrient availability and counteracted under starving, calorie-restricted conditions [74].
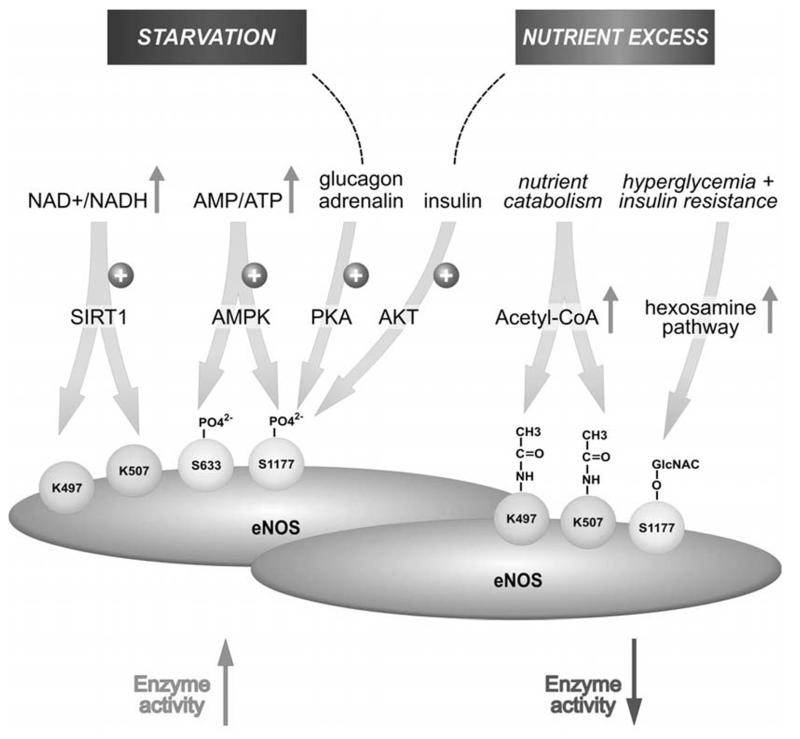
eNOS has been reported to be constitutively acetylated at Lys 497 and Lys 507 in its calmodulin (CaM) binding domain. Acetylation may have similar effects as a phosphate group on Thr 495 impeding CaM binding and thereby inhibiting eNOS activity. eNOS is deacetylated by SIRT1, a class III NAD+-dependent histone deacetylase (HDAC). SIRT1 colocalizes with eNOS, increases eNOS activity and promotes endothelium-dependent vasodilation [75]. In concert with their respective impact on eNOS activity, oxidants, cigarette smoke [76] or increasing age [77] decrease, whereas laminar flow [78], caloric restriction, the phosphodiesterase 3 inhibitor cilostazol [79] or resveratrol [76] increase SIRT1 levels or activity. Of note, deaeetylation of eNOS by SIRT1 succeeds AMPK-mediated phosphorylation at Ser 633 and Ser 1177. Since AMP-activated kinase is a fine sensor for the cellular AMP/ATP ratio and is activated by a low energy state of the cell, this suggests a close cooperativity between the two metabolic master hubs in the cell in regulation of eNOS activity [78]. A similar cooperativity between AMPK and SIRT1 has also been observed for the transcriptional modulator peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1 α) [80].
Aspirin, acetyl-salicylic acid, was found to lead to acetylation of eNOS and increased eNOS activity [81,82]. Jung et al. identified Lys 610 as the targeted lysine under chronic administration of low dose aspirin for vasoprotection [81]. This acetylation is counteracted by HDAC3 which abrogates aspirin-stimulated lysine acetylation of eNOS, eNOS enzyme activity and thus eNOS-derived NO. Unlike the acetylated lysines discussed above, acetyl-K610 seems to promote the binding of calmodulin to eNOS [81]. Figure 4 depicts the so far identified acetylation sites of the eNOS protein.
Fig. (4). Overview over the known acetylation sites of eNOS enzyme.
KAT: Lysine acetyltransferase; SIRT1: NAD-dependent deacetylase sirtuin-1; HDAC3: histone deacetylase 3
6. O-GLYCOSYLATION
Another nutrient sensitive posttranslational modification is O-glycosylation of proteins.
Intracellular proteins are hereby modified by attachment of N-acetylglucosamine (GlcNAc) to serine or threonine residues (O-glycosylation) [83]. This form of posttranslational modification is to be discerned from the complex N-or O-linked glycosylation pattern usually conferred to transmembrane or secretory proteins at ER or the Golgi. Activated GlcNAc, i.e. uridin diphosphate (UDP)-N-acetylglucosamine (UDP-GlcNAc), is formed through the hexosa-mine biosynthetic pathway and serves subsequently as substrate in the glycosylation reaction. The hexosamine pathway is a branch of glucose metabolism (3-5% of glucose carbon is presented to this pathway) that is sensitive to the availability of glucose, glutamine, and UTP [84]. Accordingly, UDP-GlcNAc pools are reduced when glucose uptake is limited [85].
O-GlcNAcylation, as found on eNOS, is a post-translational modification influencing stability, activity or subcellular localization of target proteins. In many ways, O-GlcNAcylation resembles phosphorylation since the sugar can be attached or removed upon changes in the cellular environment such as stress, hormones or nutrients [86]. O-GlcNAcylation is brought about by a highly conserved enzyme, which is uridine diphospho-N-acetylglucosamin: polypeptide β-N-acetylglucosaminyltransferase or shortly O-linked N-acetylglucosamine (O-GlcNAc) transferase (OGT). In cells, the OGT catalytic subunit dynamically forms many specific holoenzyme protein complexes that regulate its specific activity toward its myriad of target protein substrates. By contrast, phosphorylation involves many individual kinases, each with their own unique activity. Similarly, whereas there are several protein phosphatases that remove phosphorylation, there is only a single cytosolic or nuclear β-N-acetylglucosaminidase (O-GlcNAcase, OGA) that is also targeted to substrates by forming many transient holoenzyme complexes to remove sugar moieties [86]. Therefore, O-GlcNAcylations can hardly organize in hierarchic signalling cascades as observed for phosphorylation reactions. GlcNAcylations should rather be considered as a “rheostat” that controls the intensity of the signals travelling through different pathways according to the nutritional status of the cell [87]. Since O-GlcNAc is attached to serine/ threonine residues, the sugar can act in direct competition with phosphorylation at these sites. Moreover, O-GlcNAcylation or phosphorylation residues can be in close proximity to each other and sterically impair the attachment of the other modification [86].
In light of the above, it is not surprising that hyperglycemia and insulin resistance-induced excess fatty acid oxidation increases endothelial concentrations of GlcNAc by diverting the flux of fructoses-phosphate from glycolysis to the hexosamine pathway [88, 89]. This, in turn, has been shown to lead to attachment of GlcNAc to Ser 1117 of eNOS, thereby reducing Ser 1177 phosphorylation and eNOS activity [90-92]. Likewise, Du et al. observed that hyperglycemia and treatment with glucosamine abolished 67% of eNOS activity in bovine aortic endothelial cells. Hyperglycemia-associated inhibition of eNOS was accompanied by a twofold increase in O-linked GlcNAc modification in eNOS and a reciprocal decrease in phosphorylation at serine residue 1177. Both the inhibition of eNOS and the changes in its post-translational modifications were reversed by antisense inhibition of glutamine:fructose-6-phosphate amidotransferase, the rate-limiting enzyme of the hexosamine pathway. Similar changes in eNOS activity and covalent modification were found in aortae from diabetic animals [90]. Hence, chronic impairment of eNOS activity by this mechanism may partly provide the explanation for the micro-and macrovascular complications often observed in diabetic patients [87,93].
7. GLUTATHIONYLATION
Only recently, Chen et al. showed that eNOS is subject to reversible S-glutathionylation within its reductase domain at Cys 689 and 908 [94]. Protein-S-glutathionylation occurs through reversible formation of a mixed disulphide bond between a protein cysteine thiol and glutathione, the most abundant low molecular mass thiol in cells. S-glutathionylation of protein thiols can occur under redox stress through thiol-disulphide exchange with oxidized glutathione (GSSG). This mechanism is thought to mainly come into play when the cellular GSSG/GSH ratio is high [95-97]. Alternatively, superoxide, either from endogenous reactions, such as by uncoupled eNOS, or an exogenous source, can lead to eNOS protein thiyl radical formation, a formal one-electron oxidation. The thiyl radical, in turn, reacts with a vicinal SH- group of glutathione, and forms the mixed disulphide bond [98].
Glutathionylated eNOS is uncoupled, producing increased levels of superoxide and reduced levels of NO. Accordingly, eNOS S-glutathionylation in endothelial cells is associated with impaired endothelium-dependent vasodilation. Moreover, it was reported that in hypertensive vessels eNOS S-glutathionylation is increased. This is accompanied by an impaired endothelium-dependent vasodilation and can be restored by thiol-specific reducing agents, able to reverse this S-glutathionylation [94]. Limited availability of the pivotal cofactor tetrahydrobiopterin, depletion of L-arginine, or accumulation of assymmetric dimethylarginines have so far been blamed for eNOS uncoupling under redox stress [99]. All these mechanisms occur primarily at the heme of the oxygenase domain and are blocked by heme blockers or the NOS inhibitor N-nitro-L-arginine methylester. Thus, S-glutathionylation of eNOS in the reductase domain constitutes an alternative novel redox sensitive switch regulating cellular signalling, endothelial function and vascular tone.
In this line, it may be speculated that reduced S-glutathionylation partly accounts for the beneficial impact of antioxidants to endothelial function. Activated transcription factor nuclear factor E2 related factor 2 (Nrf2) which is involved in expression of antioxidant defense genes including glutathione synthase and glutathione peroxidase and/or other antioxidant mediators has been shown to prevent eNOS uncoupling [100-102]. Of note, telmisartan, an angiotensin receptor I blocker, was reported to normalize eNOS glutahionylation induced by chronic administration of nitroglycerine [103].
8. SUMMARY AND OUTLOOK
Posttranslational modifications of eNOS allow a fast integration of many cues to adapt eNOS enzyme activity in a reversible and fine-tuned manner to physiological needs. There is evident cross-talk between the individual modifications. This can occur in a collaborative way as in the case of acylation and nitrosylation which determine and depend on membrane localization of eNOS, respectively. Different cellular localization spots of eNOS are moreover reflected in eNOS phosphorylation at different sites which also suggests an interrelationship between eNOS acylation and phosphorylation in the dynamics of eNOS regulation [7]. A mutually exclusive pattern of posttranslational modification is observed for Ser 1177. This residue is either phosphorylated with an activating impact on eNOS enzyme activity or it is GlcNAcylated resulting in enzyme inhibition. Notably, both modifications follow a reciprocal metabolic control. Under nutrient starvation, Ser 1177 phosphorylation is favoured by activated AMPK whereas in situations of glucose excess (and concomitant insulin resistance) O-GlcNAcylation preferably occurs due to increased flux of sugars through the hexosamine pathway and a hampered signal relay between insulin and the AKT/ eNOS 1177 axis. Of note, transiently elevated blood glucose levels usually lead to increased insulin signalling, which in turn triggers activating phosphorylation of eNOS Ser 1177 via the IRS/ PI3K/ AKT axis. Thus, it seems that an intact insulin signalling may act as safeguard mechanism for eNOS function under high glucose conditions by still tilting the balance towards phosphorylation instead of GlcNAcylation. The tilt towards GlcNAcylation obviously only occurs when insulin resistance sets on. Interestingly, insulin resistance is favoured by elevated levels of GlcNAc closing the vicious cycle of GlcNAc, insulin resistance and eNOS dysfunction [104,105]. Notably, the two glucose-responsive, but reciprocally active hormones, glucagon and insulin, consistently lead to activation of eNOS by increased Ser 1177 phosphorylation via activation of PKA and AKT, respectively. Apparently, on the organismal level activation of eNOS and, thus elevated NO levels, are desirable both under high and low glucose conditions. One may speculate that the resulting vasodilation facilitates blood flow to the periphery where glucose is disposed in order to a) provide a fuel for energy production or replenishment of cellular stores and b) to reduce glucose levels in the blood and prevent formation of advanced glycation end products (AGE) or related glucotoxic events. These findings underline the susceptibility of eNOS activity to metabolic cues (summarized in Fig. 5) which may again not be a one-way street: NO, the product of NOS, reversibly inhibits oxidative phosphorylation by competing with oxygen for cytochrome C oxidase. This can lead to a less efficient OXPHOS-mediated ATP production and a potential compensatory shift to ATP production from glycolysis (Warburg effect) [106,107]. Although compartimentalization of NO production must here be kept in mind, eNOS certainly is a central player in the communication between cellular signalling and metabolism, and corroborates the increasingly appreciated “holistic” concept that metabolism is not a stable bystander, but is actively involved in the coordination of signal transduction in mammalian cells.
Fig. (5). Susceptibility of eNOS enzyme activity to the metabolic state and corresponding posttranslational modifications.
AMPK: AMP-activated protein kinase; SIRT1: NAD-dependent deacetylase sirtuin-1; KAT: Lysine acetyltransferase; OGT: O-linked N-acetylglucosamine (O-GlcNAc) transferase
Overall, the network of posttranslational modifications of eNOS is a fascinating and by far not exhausted field of research. It still leaves many mechanistic questions to answer, still hosts unravelled mutual cross talks which need to be understood and then could possibly be tackled by exogenous stimuli and – with help of sophisticated mass spectrometric methods – may lead to the discovery of so far unknown modifications of the eNOS protein.
ACKNOWLEDGEMENTS
We apologise to all investigators whose work could not be cited due to space limitations. The authors thank Andrea Szabo for preparation of the illustrations. EHH is funded by the Austrian Science Fund FWF (P23317).
ABBREVATIONS
- AGE
Advanced glycation endproduct
- AKT
Akt kinase aka protein kinase B
- AMPK
AMP-activated kinase
- APT1
Acyl-protein thioesterase 1
- ATP
Adenosin triphosphate
- Ca++/CaM
Calcium/ calmodulin
- CaMKK
Calcium calmodulin dependent kinase kinase
- cAMP
Cyclic AMP (adenosin monophosphate)
- CDK5
Cyclin dependent kinase 5
- CHK1
Checkpoint kinase 1
- CoA
Coenzyme A
- eNOS
Endothelial NO synthase
- ERK
Extracellular signal regulated kinase
- FAD
Flavin adenine dinucleotide
- FMN
Flavin mononucleotide
- GlcNAc
N-acetylglucosamine
- GSH
Glutathione
- GSSG
Oxidized glutathione
- HDAC
Histone deacetylase
- HDL
High density lipoprotein
- Hsp 90
Heat shock protein 90
- KAT
Lysine acetyltransferases
- NAD
Nicotinamide adenine dinucleotide
- NMT
N-myristoyltransferase
- NO
Nitric oxide
- OGA
β-N-acetylglucosaminidase
- OGT
O-linked N-acetylglucosamine transferase
- OXPHOS
Oxidative phosphorylation
- PAT
Palmitoyl-acyl-transferases
- PGC1 α
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- PKA
Protein kinase A
- PKC
Protein kinase C
- PP
Protein phosphatase
- PPT
Palmitoyl-protein thioesterase
- PTB
Phospho tyrosine binding
- PYK2
Proline rich tyrosine kinase 2
- SH2
Src homology 2
- UDP
Uridindiphosphate
- VEGF
Vascular endothelial growth factor
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
REFERENCES
- [1].Liu J, Garcia-Cardena G, Sessa WC. Biosynthesis and palmitoylation of endothelial nitric oxide synthase: mutagenesis of palmitoylation sites, cysteines-15 and/or -26, argues against depalmitoylation-induced translocation of the enzyme. Biochemistry. 1995;34:12333–40. doi: 10.1021/bi00038a029. [DOI] [PubMed] [Google Scholar]
- [2].Liu J, Garcia-Cardena G, Sessa WC. Palmitoylation of endothelial nitric oxide synthase is necessary for optimal stimulated release of nitric oxide: implications for caveolae localization. Biochemistry. 1996;35:13277–81. doi: 10.1021/bi961720e. [DOI] [PubMed] [Google Scholar]
- [3].Shaul PW, Smart EJ, Robinson LJ, et al. Acylation targets emdothelial nitric-oxide synthase to plasmalemmal caveolae. J Biol Chem. 1996;271:6518–22. doi: 10.1074/jbc.271.11.6518. [DOI] [PubMed] [Google Scholar]
- [4].Feron O, Michel JB, Sase K, Michel T. Dynamic regulation of endothelial nitric oxide synthase: complementary roles of dual acylation and caveolin interactions. Biochemistry. 1998;37:193–200. doi: 10.1021/bi972307p. [DOI] [PubMed] [Google Scholar]
- [5].Dudzinski DM, Igarashi J, Greif D, Michel T. The regulation and pharmacology of endothelial nitric oxide synthase. Annu Rev Pharmacol Toxicol. 2006;46:235–76. doi: 10.1146/annurev.pharmtox.44.101802.121844. [DOI] [PubMed] [Google Scholar]
- [6].Liu J, Hughes TE, Sessa WC. The first 35 amino acids and fatty acylation sites determine the molecular targeting of endothelial nitric oxide synthase into the Golgi region of cells: a green fluorescent protein study. J Cell Biol. 1997;137:1525–35. doi: 10.1083/jcb.137.7.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gonzalez E, Kou R, Lin AJ, Golan DE, Michel T. Subcellular targeting and agonist-induced site-specific phosphorylation of endothelial nitric-oxide synthase. J Biol Chem. 2002;277:39554–60. doi: 10.1074/jbc.M207299200. [DOI] [PubMed] [Google Scholar]
- [8].Garcia-Cardena G, Oh P, Liu J, Schnitzer JE, Sessa WC. Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: implications for nitric oxide signaling. Proc Natl Acad Sci U S A. 1996;93:6448–53. doi: 10.1073/pnas.93.13.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Shaul PW. Regulation of endothelial nitric oxide synthase: location, location, location. Annu Rev Physiol. 2002;64:749–74. doi: 10.1146/annurev.physiol.64.081501.155952. [DOI] [PubMed] [Google Scholar]
- [10].Prabhakar P, Cheng V, Michel T. A chimeric transmembrane domain directs endothelial nitric-oxide synthase palmitoylation and targeting to plasmalemmal caveolae. J Biol Chem. 2000;275:19416–21. doi: 10.1074/jbc.M001952200. [DOI] [PubMed] [Google Scholar]
- [11].Fernandez-Hernando C, Fukata M, Bernatchez PN, et al. Identification of Golgi-localized acyl transferases that palmitoylate and regulate endothelial nitric oxide synthase. J Cell Biol. 2006;174:69–77. doi: 10.1083/jcb.200601051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yeh DC, Duncan JA, Yamashita S, Michel T. Depalmitoylation of endothelial nitric-oxide synthase by acyl-protein thioesterase 1 is potentiated by Ca(2+)-calmodulin. J Biol Chem. 1999;274:33148–54. doi: 10.1074/jbc.274.46.33148. [DOI] [PubMed] [Google Scholar]
- [13].Michel JB, Feron O, Sase K, Prabhakar P, Michel T. Caveolin versus calmodulin. Counterbalancing allosteric modulators of endothelial nitric oxide synthase. J Biol Chem. 1997;272:25907–12. doi: 10.1074/jbc.272.41.25907. [DOI] [PubMed] [Google Scholar]
- [14].Michel JB, Michel T. The role of palmitoyl-protein thioesterase in the palmitoylation of endothelial nitric oxide synthase. FEBS Lett. 1997;405:356–62. doi: 10.1016/s0014-5793(97)00222-6. [DOI] [PubMed] [Google Scholar]
- [15].Oess S, Icking A, Fulton D, Govers R, Muller-Esterl W. Subcellular targeting and trafficking of nitric oxide synthases. Biochem J. 2006;396:401–9. doi: 10.1042/BJ20060321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Church JE, Fulton D. Differences in eNOS activity because of subcellular localization are dictated by phosphorylation state rather than the local calcium environment. J Biol Chem. 2006;281:1477–88. doi: 10.1074/jbc.M505968200. [DOI] [PubMed] [Google Scholar]
- [17].Anderson RG. Caveolae: where incoming and outgoing messengers meet. Proc Natl Acad Sci U S A. 1993;90:10909–13. doi: 10.1073/pnas.90.23.10909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lisanti MP, Scherer PE, Tang Z, Sargiacomo M. Caveolae, caveolin and caveolin-rich membrane domains: a signalling hypothesis. Trends Cell Biol. 1994;4:231–5. doi: 10.1016/0962-8924(94)90114-7. [DOI] [PubMed] [Google Scholar]
- [19].Smart EJ, Ying YS, Mineo C, Anderson RG. A detergent-free method for purifying caveolae membrane from tissue culture cells. Proc Natl Acad Sci U S A. 1995;92:10104–8. doi: 10.1073/pnas.92.22.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Smart EJ, Ying YS, Conrad PA, Anderson RG. Caveolin moves from caveolae to the Golgi apparatus in response to cholesterol oxidation. J Cell Biol. 1994;127:1185–97. doi: 10.1083/jcb.127.5.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Erwin PA, Lin AJ, Golan DE, Michel T. Receptor-regulated dynamic S-nitrosylation of endothelial nitric-oxide synthase in vascular endothelial cells. J Biol Chem. 2005;280:19888–94. doi: 10.1074/jbc.M413058200. [DOI] [PubMed] [Google Scholar]
- [22].Erwin PA, Mitchell DA, Sartoretto J, Marietta MA, Michel T. Subcellular targeting and differential S-nitrosylation of endothelial nitric-oxide synthase. J Biol Chem. 2006;281:151–7. doi: 10.1074/jbc.M510421200. [DOI] [PubMed] [Google Scholar]
- [23].Liu X, Miller MJ, Joshi MS, Thomas DD, Lancaster JR., Jr. Accelerated reaction of nitric oxide with 02 within the hydrophobic interior of biological membranes. Proc Natl Acad Sci US A. 1998;95:2175–9. doi: 10.1073/pnas.95.5.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dudzinski DM, Michel T. Life history of eNOS: partners and pathways. Cardiovasc Res. 2007;75:247–60. doi: 10.1016/j.cardiores.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ravi K, Brennan LA, Levic S, Ross PA, Black SM. S-nitrosylation of endothelial nitric oxide synthase is associated with monomerization and decreased enzyme activity. Proc Natl Acad Sci U S A. 2004;101:2619–24. doi: 10.1073/pnas.0300464101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tummala M, Ryzhov V, Ravi K, Black SM. Identification of the cysteine nitrosylation sites in human endothelial nitric oxide synthase. DNA Cell Biol. 2008;27:25–33. doi: 10.1089/dna.2007.0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Taldone FS, Tummala M, Goldstein EJ, Ryzhov V, Ravi K, Black SM. Studying the S-nitrosylation of model peptides and eNOS protein by mass spectrometry. Nitric Oxide. 2005;13:176–87. doi: 10.1016/j.niox.2005.06.004. [DOI] [PubMed] [Google Scholar]
- [28].Kou R, Greif D, Michel T. Dephosphorylation of endothelial nitric-oxide synthase by vascular endothelial growth factor. Implications for the vascular responses to cyclosporin A. J Biol Chem. 2002;277:29669–73. doi: 10.1074/jbc.M204519200. [DOI] [PubMed] [Google Scholar]
- [29].Drew BG, Fidge NH, Gallon-Beaumier G, Kemp BE, Kingwell BA. High-density lipoprotein and apolipoprotein AI increase endothelial NO synthase activity by protein association and multisite phosphorylation. Proc Natl Acad Sci U S A. 2004;101:6999–7004. doi: 10.1073/pnas.0306266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cho DH, Seo J, Park JH, et al. Cyclin-dependent kinase 5 phosphorylates endothelial nitric oxide synthase at serine 116. Hypertension. 2010;55:345–52. doi: 10.1161/HYPERTENSIONAHA.109.140210. [DOI] [PubMed] [Google Scholar]
- [31].Lee CH, Wei YW, Huang YT, et al. CDK5 phosphorylates eNOS at Ser-113 and regulates NO production. J Cell Biochem. 2010;110:112–7. doi: 10.1002/jcb.22515. [DOI] [PubMed] [Google Scholar]
- [32].Cho DH, Choi YJ, Jo SA, Jo I. Nitric oxide production and regulation of endothelial nitric-oxide synthase phosphorylation by prolonged treatment with troglitazone: evidence for involvement of peroxisome proliferator-activated receptor (PPAR) gamma-dependent and PPARgamma-independent signaling pathways. J Biol Chem. 2004;279:2499–506. doi: 10.1074/jbc.M309451200. [DOI] [PubMed] [Google Scholar]
- [33].Bauer PM, Fulton D, Boo YC, et al. Compensatory phosphorylation and protein-protein interactions revealed by loss of function and gain of function mutants of multiple serine phosphorylation sites in endothelial nitric-oxide synthase. J Biol Chem. 2003;278:14841–9. doi: 10.1074/jbc.M211926200. [DOI] [PubMed] [Google Scholar]
- [34].Mount PF, Kemp BE, Power DA. Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol. 2007;42:271–9. doi: 10.1016/j.yjmcc.2006.05.023. [DOI] [PubMed] [Google Scholar]
- [35].Szabo C, Farago M, Dora E. Effect of small changes in extracellular magnesium concentration on the tone of feline mesenteric arteries: involvement of endothelium. Acta Physiol Hung. 1992;79:295–303. [PubMed] [Google Scholar]
- [36].Forstermann U. Janus-faced role of endothelial NO synthase in vascular disease: uncoupling of oxygen reduction from NO synthesis and its pharmacological reversal. Biol Chem. 2006;387:1521–33. doi: 10.1515/BC.2006.190. [DOI] [PubMed] [Google Scholar]
- [37].Sugimoto M, Nakayama M, Goto TM, Amano M, Komori K, Kaibuchi K. Rho-kinase phosphorylates eNOS at threonine 495 in endothelial cells. Biochem Biophys Res Commun. 2007;361:462–7. doi: 10.1016/j.bbrc.2007.07.030. [DOI] [PubMed] [Google Scholar]
- [38].Chen ZP, Mitchelhill KI, Michell BJ, et al. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 1999;443:285–9. doi: 10.1016/s0014-5793(98)01705-0. [DOI] [PubMed] [Google Scholar]
- [39].Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of Thr(495) regulates Ca(2+)/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res. 2001;88:E68–75. doi: 10.1161/hh1101.092677. [DOI] [PubMed] [Google Scholar]
- [40].Michell BJ, Chen Z, Tiganis T, et al. Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase C and the cAMP-dependent protein kinase. J Biol Chem. 2001;276:17625–8. doi: 10.1074/jbc.C100122200. [DOI] [PubMed] [Google Scholar]
- [41].Harris MB, Ju H, Venema VJ, et al. Reciprocal phosphorylation and regulation of endothelial nitric-oxide synthase in response to bradykinin stimulation. J Biol Chem. 2001;276:16587–91. doi: 10.1074/jbc.M100229200. [DOI] [PubMed] [Google Scholar]
- [42].Lin MI, Fulton D, Babbitt R, et al. Phosphorylation of threonine 497 in endothelial nitric-oxide synthase coordinates the coupling of L-arginine metabolism to efficient nitric oxide production. J Biol Chem. 2003;278:44719–26. doi: 10.1074/jbc.M302836200. [DOI] [PubMed] [Google Scholar]
- [43].Thomas SR, Chen K, Keaney JF., Jr. Hydrogen peroxide activates endothelial nitric-oxide synthase through coordinated phosphorylation and dephosphorylation via a phosphoinositide 3-kinase-dependent signaling pathway. J Biol Chem. 2002;277:6017–24. doi: 10.1074/jbc.M109107200. [DOI] [PubMed] [Google Scholar]
- [44].Schmitt CA, Heiss EH, Aristei Y, Severin T, Dirsch VM. Norfuraneol dephosphorylates eNOS at threonine 495 and enhances eNOS activity in human endothelial cells. Cardiovasc Res. 2009;81:750–7. doi: 10.1093/cvr/cvn326. [DOI] [PubMed] [Google Scholar]
- [45].Michell BJ, Harris MB, Chen ZP, et al. Identification of regulatory sites of phosphorylation of the bovine endothelial nitric-oxide synthase at serine 617 and serine 635. J Biol Chem. 2002;277:42344–51. doi: 10.1074/jbc.M205144200. [DOI] [PubMed] [Google Scholar]
- [46].Harris MB, Blackstone MA, Sood SG, et al. Acute activation and phosphorylation of endothelial nitric oxide synthase by HMG-CoA reductase inhibitors. Am J Physiol Heart Circ Physiol. 2004;287:H560–6. doi: 10.1152/ajpheart.00214.2004. [DOI] [PubMed] [Google Scholar]
- [47].Ritchie SA, Kohlhaas CF, Boyd AR, et al. Insulin-stimulated phosphorylation of endothelial nitric oxide synthase at serine-615 contributes to nitric oxide synthesis. Biochem J. 2010;426:85–90. doi: 10.1042/BJ20091580. [DOI] [PubMed] [Google Scholar]
- [48].Boo YC, Sorescu GP, Bauer PM, et al. Endothelial NO synthase phosphorylated at SER635 produces NO without requiring intracellular calcium increase. Free Radic Biol Med. 2003;35:729–41. doi: 10.1016/s0891-5849(03)00397-6. [DOI] [PubMed] [Google Scholar]
- [49].Chen Z, Peng IC, Sun W, et al. AMP-activated protein kinase functionally phosphorylates endothelial nitric oxide synthase Ser633. Circ Res. 2009;104:496–505. doi: 10.1161/CIRCRESAHA.108.187567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Xiao Z, Wang T, Qin H, Huang C, Feng Y, Xia Y. Endoplasmic reticulum Ca2+ release modulates endothelial nitric-oxide synthase via extracellular signal-regulated kinase (ERK) 1/2-mediated serine 635 phosphorylation. J Biol Chem. 2011;286:20100–8. doi: 10.1074/jbc.M111.220236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Boo YC, Hwang J, Sykes M, et al. Shear stress stimulates phosphorylation of eNOS at Ser(635) by a protein kinase A-dependent mechanism. Am J Physiol Heart Circ Physiol. 2002;283:H1819–28. doi: 10.1152/ajpheart.00214.2002. [DOI] [PubMed] [Google Scholar]
- [52].Boo YC, Sorescu G, Boyd N, et al. Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Serll79 by Akt-independent mechanisms: role of protein kinase A. J Biol Chem. 2002;277:3388–96. doi: 10.1074/jbc.M108789200. [DOI] [PubMed] [Google Scholar]
- [53].Butt E, Bernhardt M, Smolenski A, et al. Endothelial nitric-oxide synthase (type III) is activated and becomes calcium independent upon phosphorylation by cyclic nucleotide-dependent protein kinases. J Biol Chem. 2000;275:5179–87. doi: 10.1074/jbc.275.7.5179. [DOI] [PubMed] [Google Scholar]
- [54].Park JH, Kim WS, Kim JY, et al. Chkl and Hsp90 cooperatively regulate phosphorylation of endothelial nitric oxide synthase at serine 1179. Free Radic Biol Med. 2011;51:2217–26. doi: 10.1016/j.freeradbiomed.2011.09.021. [DOI] [PubMed] [Google Scholar]
- [55].Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–5. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- [56].Gerber HP, McMurtrey A, Kowalski J, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-l/KDR activation. J Biol Chem. 1998;273:30336–43. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- [57].Burgering BM, Coffer PJ. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- [58].Shaw M, Cohen P, Alessi DR. The activation of protein kinase B by H202 or heat shock is mediated by phosphoinositide 3-kinase and not by mitogen-activated protein kinase-activated protein kinase-2. Biochem J. 1998;336:241–6. doi: 10.1042/bj3360241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].LeBrasseur NK, Kelly M, Tsao TS, et al. Thiazolidinediones can rapidly activate AMP-activated protein kinase in mammalian tissues. Am J Physiol Endocrinol Metab. 2006;291:El75–81. doi: 10.1152/ajpendo.00453.2005. [DOI] [PubMed] [Google Scholar]
- [60].Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–62. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278:45021–6. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- [63].Hawley SA, Fullerton MD, Ross FA, et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336:918–22. doi: 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Lane P, Gross SS. Disabling a C-terminal autoinhibitory control element in endothelial nitric-oxide synthase by phosphorylation provides a molecular explanation for activation of vascular NO synthesis by diverse physiological stimuli. J Biol Chem. 2002;277:19087–94. doi: 10.1074/jbc.M200258200. [DOI] [PubMed] [Google Scholar]
- [65].McCabe TJ, Fulton D, Roman LJ, Sessa WC. Enhanced electron flux and reduced calmodulin dissociation may explain “calcium-independent” eNOS activation by phosphorylation. J Biol Chem. 2000;275:6123–8. doi: 10.1074/jbc.275.9.6123. [DOI] [PubMed] [Google Scholar]
- [66].Montagnani M, Chen H, Barr VA, Quon MJ. Insulin-stimulated activation of eNOS is independent of Ca2+ but requires phosphorylation by Akt at Ser(1179) J Biol Chem. 2001;276:30392–8. doi: 10.1074/jbc.M103702200. [DOI] [PubMed] [Google Scholar]
- [67].Garcia-Cardena G, Fan R, Stern DF, Liu J, Sessa WC. Endothelial nitric oxide synthase is regulated by tyrosine phosphorylation and interacts with caveolin-1. J Biol Chem. 1996;271:27237–40. doi: 10.1074/jbc.271.44.27237. [DOI] [PubMed] [Google Scholar]
- [68].Fleming I, Bauersachs J, Fisslthaler B, Busse R. Ca2+-independent activation of the endothelial nitric oxide synthase in response to tyrosine phosphatase inhibitors and fluid shear stress. Circ Res. 1998;82:686–95. doi: 10.1161/01.res.82.6.686. [DOI] [PubMed] [Google Scholar]
- [69].Fulton D, Church JE, Ruan L, et al. Src kinase activates endothelial nitric-oxide synthase by phosphorylating Tyr-83. J Biol Chem. 2005;280:35943–52. doi: 10.1074/jbc.M504606200. [DOI] [PubMed] [Google Scholar]
- [70].Fisslthaler B, Loot AE, Mohamed A, Busse R, Fleming I. Inhibition of endothelial nitric oxide synthase activity by proline-rich tyrosine kinase 2 in response to fluid shear stress and insulin. Circ Res. 2008;102:1520–8. doi: 10.1161/CIRCRESAHA.108.172072. [DOI] [PubMed] [Google Scholar]
- [71].Loot AE, Schreiber JG, Fisslthaler B, Fleming I. Angiotensin II impairs endothelial function via tyrosine phosphorylation of the endothelial nitric oxide synthase. J Exp Med. 2009;206:2889–96. doi: 10.1084/jem.20090449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Kovacic P. Novel electrostatic mechanism for mode of action by N-acetylated proteins: cell signaling and phosphorylation. J Recept Signal Transduct Res. 2011;31:193–8. doi: 10.3109/10799893.2011.577784. [DOI] [PubMed] [Google Scholar]
- [73].Albaugh BN, Arnold KM, Denu JM. KAT(ching) metabolism by the tail: insight into the links between lysine acetyltransferases and metabolism. Chembiochem. 2011;12:290–8. doi: 10.1002/cbic.201000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Wellen KE, Thompson CB. A two-way street: reciprocal regulation of metabolism and signalling. Nat Rev Mol Cell Biol. 2012;13:270–6. doi: 10.1038/nrm3305. [DOI] [PubMed] [Google Scholar]
- [75].Mattagajasingh I, Kim CS, Naqvi A, et al. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2007;10:14855–60. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Arunachalam G, Yao H, Sundar IK, Caito S, Rahman I. SIRT1 regulates oxidant- and cigarette smoke-induced eNOS acetylation in endothelial cells: Role of resveratrol. Biochem Biophys Res Commun. 2010;393:66–72. doi: 10.1016/j.bbrc.2010.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Donato AJ, Magerko KA, Lawson BR, Durrant JR, Lesniewski LA, Seals DR. SIRT-1 and vascular endothelial dysfunction with ageing in mice and humans. J Physiol. 2011;589:4545–54. doi: 10.1113/jphysiol.2011.211219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Chen Z, Peng IC, Cui X, Li YS, Chien S, Shyy JY. Shear stress, SIRT1, and vascular homeostasis. Proc Natl Acad Sci U S A. 2010;107:10268–73. doi: 10.1073/pnas.1003833107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Ota H, Eto M, Kano MR, et al. Cilostazol inhibits oxidative stress-induced premature senescence via upregulation of Sirtl in human endothelial cells. Arterioscler Thromb Vase Biol. 2008;28:1634–9. doi: 10.1161/ATVBAHA.108.164368. [DOI] [PubMed] [Google Scholar]
- [80].Canto C, Auwerx J. PGC-lalpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20:98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Jung SB, Kim CS, Naqvi A, et al. Histone deacetylase 3 antagonizes aspirin-stimulated endothelial nitric oxide production by reversing aspirin-induced lysine acetylation of endothelial nitric oxide synthase. Circ Res. 2010;107:877–87. doi: 10.1161/CIRCRESAHA.110.222968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Taubert D, Berkels R, Grosser N, Schroder H, Grundemann D, Schomig E. Aspirin induces nitric oxide release from vascular endothelium: a novel mechanism of action. Br J Pharmacol. 2004;143:159–65. doi: 10.1038/sj.bjp.0705907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Hart GW. Dynamic O-linked glycosylation of nuclear and cytoskeletal proteins. Annu Rev Biochem. 1997;66:315–35. doi: 10.1146/annurev.biochem.66.1.315. [DOI] [PubMed] [Google Scholar]
- [84].Love DC, Hanover JA. The hexosamine signaling pathway: deciphering the “O-GlcNAc code”. Sci STKE. 2005;312:rel3. doi: 10.1126/stke.3122005re13. [DOI] [PubMed] [Google Scholar]
- [85].Wellen KE, Lu C, Mancuso A, et al. The hexosamine biosynthetic pathway couples growth factor-induced glutamine uptake to glucose metabolism. Genes Dev. 2010;24:2784–99. doi: 10.1101/gad.1985910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Zeidan Q, Hart GW. The intersections between O-GlcNAcylation and phosphorylation: implications for multiple signaling pathways. J Cell Sci. 2010;123:13–22. doi: 10.1242/jcs.053678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Issad T, Masson E, Pagesy P. O-GlcNAc modification, insulin signaling and diabetic complications. Diabetes Metab. 2010;36:423–35. doi: 10.1016/j.diabet.2010.09.001. [DOI] [PubMed] [Google Scholar]
- [88].Sayeski PP, Kudlow JE. Glucose metabolism to glucosamine is necessary for glucose stimulation of transforming growth factor-alpha gene transcription. J Biol Chem. 1996;271:15237–43. doi: 10.1074/jbc.271.25.15237. [DOI] [PubMed] [Google Scholar]
- [89].Du XL, Edelstein D, Rossetti L, et al. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Spl glycosylation. Proc Natl Acad Sci U S A. 2000;97:12222–6. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest. 2001;108:1341–8. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Musicki B, Kramer MF, Becker RE, Burnett AL. Inactivation of phosphorylated endothelial nitric oxide synthase (Ser-1177) by O-GlcNAc in diabetes-associated erectile dysfunction. Proc Natl Acad Sci US A. 2005;102:11870–5. doi: 10.1073/pnas.0502488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Federici M, Menghini R, Mauriello A, et al. Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation. 2002;106:466–72. doi: 10.1161/01.cir.0000023043.02648.51. [DOI] [PubMed] [Google Scholar]
- [93].Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–70. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Chen CA, Wang TY, Varadharaj S, et al. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature. 2010;468:1115–8. doi: 10.1038/nature09599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Winterbourn CC, Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic Biol Med. 2008;45:549–61. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- [96].Gallogly MM, Mieyal JJ. Mechanisms of reversible protein glutathionylation in redox signaling and oxidative stress. Curr Opin Pharmacol. 2007;7:381–91. doi: 10.1016/j.coph.2007.06.003. [DOI] [PubMed] [Google Scholar]
- [97].Biswas S, Chida AS, Rahman I. Redox modifications of protein-thiols: emerging roles in cell signaling. Biochem Pharmacol. 2006;71:551–64. doi: 10.1016/j.bcp.2005.10.044. [DOI] [PubMed] [Google Scholar]
- [98].Chen CA, Lin CH, Druhan LJ, Wang TY, Chen YR, Zweier JL. Superoxide induces endothelial nitric-oxide synthase protein thiyl radical formation, a novel mechanism regulating eNOS function and coupling. J Biol Chem. 2011;286:29098–107. doi: 10.1074/jbc.M111.240127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Zweier JL, Chen CA, Druhan LJ. S-glutathionylation reshapes our understanding of endothelial nitric oxide synthase uncoupling and nitric oxide/reactive oxygen species-mediated signaling. Antioxid Redox Signal. 2011;14:1769–75. doi: 10.1089/ars.2011.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Ungvari Z, Bagi Z, Feher A, et al. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol. 2010;299:H18–24. doi: 10.1152/ajpheart.00260.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Tu B, Wallin A, Moldeus P, Cotgreave I. The cytoprotective roles of ascorbate and glutathione against nitrogen dioxide toxicity in human endothelial cells. Toxicology. 1995;98:125–36. doi: 10.1016/0300-483x(94)02977-3. [DOI] [PubMed] [Google Scholar]
- [102].Heiss EH, Schachner D, Werner ER, Dirsch VM. Active NF-E2-related factor (Nrf2) contributes to keep endothelial NO synthase (eNOS) in the coupled state: role of reactive oxygen species (ROS), eNOS, and heme oxygenase (HO-1) levels. J Biol Chem. 2009;284:31579–86. doi: 10.1074/jbc.M109.009175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Knorr M, Hausding M, Kroller-Schuhmacher S, et al. Nitroglycerin-induced endothelial dysfunction and tolerance involve adverse phosphorylation and S-Glutathionylation of endothelial nitric oxide synthase: beneficial effects of therapy with the ATI receptor blocker telmisartan. Arterioscler Thromb Vase Biol. 2011;31:2223–31. doi: 10.1161/ATVBAHA.111.232058. [DOI] [PubMed] [Google Scholar]
- [104].Srinivasan V, Tatu U, Mohan V, Balasubramanyam M. Molecular convergence of hexosamine biosynthetic pathway and ER stress leading to insulin resistance in L6 skeletal muscle cells. Mol Cell Biochem. 2009;328:217–24. doi: 10.1007/s11010-009-0092-7. [DOI] [PubMed] [Google Scholar]
- [105].Einstein FH, Fishman S, Bauman J, et al. Enhanced activation of a “nutrient-sensing” pathway with age contributes to insulin resistance. FASEB J. 2008;22:3450–7. doi: 10.1096/fj.08-109041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Brunori M, Giuffre A, Forte E, Mastronicola D, Barone MC, Sarti P. Control of cytochrome c oxidase activity by nitric oxide. Biochim Biophys Acta. 2004;1655:365–71. doi: 10.1016/j.bbabio.2003.06.008. [DOI] [PubMed] [Google Scholar]
- [107].Sarti P, Forte E, Mastronicola D, Giuffre A, Arese M. Cytochrome c oxidase and nitric oxide in action: molecular mechanisms and pathophysiological implications. Biochim Biophys Acta. 2012;1817:610–9. doi: 10.1016/j.bbabio.2011.09.002. [DOI] [PubMed] [Google Scholar]