Abstract
Background
There are increased numbers of pulmonary CD8 lymphocytes in COPD. Calcium-release activation calcium (CRAC) channels play a central role in lymphocyte activation though the regulation of the transcription factor nuclear factor of activated T cells (NFAT).
Objective
We studied the expression of NFAT in COPD lungs compared to controls, and evaluated the effects of CRAC inhibition compared to corticosteroids on NFAT activation and cytokine production from COPD CD8 cells.
Methods
The effects of the corticosteroid dexamethasone, the calcineurin inhibitor cyclosporin and the CRAC inhibitor synta-66 were studied on cytokine production and NFAT activation using peripheral blood and isolated pulmonary CD8 cells. NFAT1 and CD8 co-expression in the lungs was compared in COPD and controls using combined immunohistochemistry and immunofluorescence.
Results
NFAT inhibition with either cyclosporin or synta-66 resulted in significantly greater maximal inhibition of cytokines than dexamethasone in both peripheral blood and pulmonary CD8 cells (e.g. > 95% inhibition of IFNγ production from pulmonary CD8 cells using cyclosporin and synta-66 compared to <50% using dexamethasone). The absolute number of pulmonary CD8 cells co-expressing NFAT1 was significantly raised in COPD lung compared to controls, but the percentage of CD8 cells co-expressing NFAT1 was similar between COPD and controls (80.7 % vs 78.5 % respectively, p=0.3).
Conclusions
Inhibition of NFAT using the CRAC inhibitor synta-66 produces greater anti-inflammatory effects on COPD CD8 cells than corticosteroids. NFAT is expressed in a high proportion of COPD pulmonary CD8 cells.
Keywords: Chronic Obstructive Pulmonary Disease, CD8 cell, Nuclear factor of Activated T cells, Calcium-release Activated Calcium channel, Orai1
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is characterised by poorly reversible airflow obstruction and increased levels of airway inflammation [1]. Corticosteroids are the mainstay of anti-inflammatory treatment for COPD, but have limited clinical benefits [2, 3]. Novel anti-inflammatory therapies are needed for COPD patients.
Pulmonary CD8 lymphocyte numbers are increased in COPD and are associated with disease severity [4, 5]. COPD pulmonary CD8 cells have increased effector functions such as cytotoxicity [6] and cytokine production [7]. Furthermore, there are increased numbers of tertiary pulmonary lymphoid follicles in severe COPD [8]. These follicles have a B cell core surrounded by T cells that co-ordinate the adaptive immune response [9]. These findings have led to the “auto-immune hypothesis” of COPD, where the abnormal adaptive immune response in COPD patients drives persistent inflammation [10]. The reason for persistent adaptive immune activation is unclear; possible explanations include the presentation of self antigens from tissue breakdown [11] or pathogens within the lungs [10, 12].
Nuclear factor of activated T cells (NFAT) is a family of transcription factors involved in T cell activation; NFAT functions primarily through binding to DNA in co-operation with other transcription factors such as activator protein-1 (AP1) and nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) inducing transcription of cytokines such as interleukin-2 (IL-2) and interferon-γ (IFNγ) [13]. Cyclosporin inhibits calcineurin activation of NFAT, and is used to treat auto-immune diseases and transplant rejection [14, 15]. Cyclosporin provides clinical benefits in severe asthma [16, 17], but the side effect profile limits widespread use [18]. Novel agents targeting NFAT activation with a more acceptable therapeutic index may be effective treatments in COPD patients, acting through the modulation of CD8 function.
T cell receptor (TCR) stimulation rapidly releases calcium from intracellular stores, which leads to opening of cell surface calcium release-activation calcium (CRAC) channels and the influx of extracellular calcium; this is called store operated calcium entry (SOCE). SOCE activates calcineurin which dephosphorylates cytoplasmic NFAT allowing nuclear translocation [19]. ORAI1 is the protein that constitutes the pore forming CRAC units [20]. ORAI1 deficient mice display reduced T cell cytokine production and are less prone to develop skin allograft rejection, suggesting a key role for CRAC channels in T cell mediated inflammation [21]. Small molecule inhibitors of CRAC have been developed that reduce SOCE in T cells and consequently are able to suppress cytokine production from healthy peripheral blood lymphocytes [22, 23]. Healthy peripheral blood lymphocytes may not reflect phenotypically different lymphocytes obtained from inflamed tissues. The effects of CRAC inhibitors on COPD lung lymphocytes have not been studied.
We hypothesized that inhibition of CRAC would suppress cytokine production from COPD pulmonary CD8 cells more effectively than corticosteroids. Isolated CD8 cells from the lungs and blood of COPD patients and controls were used to compare the anti-inflammatory effects of the CRAC inhibitor synta-66 [24] with corticosteroids. Cyclosporin was also used in order to compare the effectiveness of synta-66 with a well known NFAT inhibitor. Additionally, we investigated whether the expression of NFAT was altered within COPD lungs compared to controls.
METHODS
Subjects
Patients undergoing surgical resection for known or suspected lung cancer were recruited to obtain lung tissue for immunohistochemistry or to isolate lung CD8 cells; Table 1 shows the demography of participants. COPD was diagnosed in accordance with the GOLD guidelines [1]. Patients with a previous smoking history and normal lung function were also recruited (S). Immunohistochemistry was performed on samples from ex-smoking COPD patients and S only, defined as subjects who had not smoked for at least 1 year. A separate group of COPD patients, S and healthy non-smokers (HNS) were recruited to obtain blood CD8 cells, while a further different group of COPD patients were recruited for experiments on peripheral blood mononuclear cells (PBMCs); the demography of these patients is shown in supplemental table 1. All subjects gave written informed consent. The study was approved by the local research ethics committee (South Manchester Research Ethics Committee, reference: 03/SM/396).
Table 1.
Patient Demographics for immunohistochemistry (NFAT1 expression), and experiments using isolated pulmonary and blood CD8 cells.
| NFAT1 Expression | Pulmonary CD8 | Blood CD8 | |||||
|---|---|---|---|---|---|---|---|
| COPD (n=13) | S (n=7) | COPD (n=6) | S (n=5) | COPD (n=10) | S (n=6) | HNS (n=8) | |
| *Age (yrs) | 69 (53-80) | 74 (58-80) | 74 (54-77) | 68 (64-76) | 68 (49-74) | 49 (42-57) | 48 (43-69) |
| Male/Female | 9/4 | 2/5 | 3/3 | 2/3 | 8/2 | 3/3 | 2/6 |
| FEV1 (L) | 1.61 (0.41) | 2.30 (0.53) | 1.69 (0.97) | 2.75 (0.89) | 1.18 (0.36) | 3.46 (0.65) | 3.46 (0.6) |
| FEV1 % predicted | 54.9 (8.02) | 98.0 (12.46) | 66.9 (22.8) | 99.1 (9.4) | 39.8 (7.8) | 104.5 (10.0) | 97.2 (16.8) |
| FEV1:FVC (%) | 58.5 (11.3) | 77.0 (6.92) | 0.51 (0.08) | 0.73 (0.04) | 39.4 (8.72) | 79.6 (3.5) | 76.7 (6.6) |
| Current Smoker | 0 | 0 | 5 | 2 | 3 | 3 | 0 |
| *Smoking History (pkyr) | 55 (13-180) | 32 (10-47) | 55 (40-124) | 37 (4-68) | 51 (21-67) | 15.8 (7.2) | 0 |
| ICS | 6 | 0 | 2 | 0 | 10 | 0 | 0 |
Data are presented mean (SD) or * which denotes median (range). NFAT1: Nuclear Factor of Activated T cells 1; COPD: Chronic Obstructive Pulmonary Disease; S: Smoker with normal lung function; HNS: Healthy non-smoker; FEV1: Forced expiratory volume in 1 second; FVC: Forced vital capacity; pkyr: pack years; ICS: Inhaled corticosteroids
Isolation of Cells
PBMCs and CD8 cells were isolated from whole blood using positive selection CD8 microbeads (Miltenyi Biotec) as described in supplemental data.
CD8 cells were isolated from homogenised lung tissue by Percoll centrifugation followed by purification using positive selection CD8 microbeads (Miltenyi Biotec) as described in supplemental data.
Cell Culture
Peripheral blood CD8 cells were seeded in triplicate in 96-well plates at 100,000 cells/well. Pulmonary CD8 cells were seeded at 50,000 cells/well. They were pre-treated for 1 hour with the following conditions; Dexamethasone 10, 100 and 1000nM, Cyclosporin 10, 100, 250, 500, 1000nM and synta-66 1, 10, 100, 1000, 10000nM. Cells were then stimulated for 24 hours with phorbol 12-myristate 13-acetate (PMA) 10ng/ml and Ionomycin (Io) 500ng/ml. Separate experiments were performed to compare the effects of dexamethasone and synta-66 on COPD PBMCs (n=10) stimulated with anti-CD2/3/28 beads (T cell activation/expansion kit, Miltenyi); PBMCs were pre-treated with dexamethasone 10, 100 and 1000nM or synta-66 10, 100, 1000nM, followed by stimulation with anti-CD2/3/28 beads at a bead:cell ratio of 1:2. Supernatants were harvested and stored at −20°C until ELISA or luminex was performed. For the study of regulation of NFAT, enriched pulmonary lymphocytes were pre-treated with 1000nM of dexamethasone, cyclosporin or synta-66 for 1 hour and then stimulated with PMA (10ng/ml) and Io (500ng/ml) for 1 hour. Cytospins were then prepared for dual label immunofluorescence.
Cytokine measurement
Supernatant IL-2 was measured by ELISA, while IFNγ, tumour necrosis factor alpha (TNFα) and interleukin 17 (IL-17) were measured using luminex as described in supplemental data.
Cytotoxicity Assay
Cytotoxicity was measured using a Lactate Dehydrogenase (LDH) assay as described in supplemental data.
Immunohistochemistry
Lung tissue far distal from any tumour was formalin fixed and paraffin embedded. 4μm sections were cut and mounted on polysine-coated glass slides. Sections were stained for NFAT1 (#4389, Cell signalling) and detected with avidin-biotin peroxidase. Sections were also dual stained for CD8 (Dako C8/144B) detected with alexa-568 conjugated goat anti-mouse antibody and NFAT1 (#4389) detected with avidin-biotin peroxidase. Detailed methods are in supplemental data.
Dual label immunofluorescence
Cytospins were produced for each condition described above (see cell culture) and cells were fixed in 4% paraformaldehyde. Cells were dual stained with anti-NFAT1 (#4389), detected with alexa-488 conjugated goat anti-rabbit antibody (Vector), and anti-CD8 (C8/144B), detected with alexa-568 conjugated goat anti-mouse antibody (vector). Detailed methods are in supplemental data.
Image analysis
NFAT1 positive cells and NFAT1 positive CD8 cells were counted in the sub-epithelium of small airways using a Nikon eclipse 80i microscope (Nikon UK Ltd, Surrey, UK) with a QImaging digital camera (Media Cybernetics, UK) and Image ProPlus imaging software (Media Cybernetics). To study the nuclear translocation of NFAT in isolated pulmonary lymphocytes the sub-cellular location of NFAT1 was used as a marker of NFAT1 activation, with cytoplasmic NFAT1 being inactive and nuclear NFAT1 being activated. Accurate analysis of sub-cellular location of NFAT in tissue CD8 cells was not possible. However, immune-reactivity in a halo pattern surrounding the nucleus was considered cytoplasmic and central distribution was considered nuclear. Further details of image analysis are in supplemental data.
Data Analysis
NFAT1 and CD8 expression were not normally distributed and were compared between groups using Mann-Whitney U tests. Correlation co-efficients were calculated by Spearman Rank method. Cytokine data were normally distributed; ANOVA was performed to compare drug effects and if p<0.05 then pairwise comparisons were performed using bonferroni multiple comparisons test. Dose response curve fitting was performed by least squares nonlinear regression using Graphpad Prism 4 (Graphpad software inc, San Diego, USA) for cyclosporin and synta-66. Curves were not fitted for dexamethasone as it is not possible to do this accurately with 3 concentrations. All statistical analysis was carried out using Graphpad InStat 3 (Graphpad software inc, San Diego, USA).
RESULTS
Peripheral blood CD8 cell cytokine production
The purity of CD8 cells obtained from peripheral blood was assessed using both trypan blue staining and immunofluorescent staining. Trypan blue staining confirmed a lymphocyte purity of >97% for all samples. Immunofluorescent staining of 3 samples confirmed the purity of CD8 cells to be > 93%.
There was no statistically significant difference between secreted IL-2, IFNγ, TNFα and IL-17 levels from unstimulated peripheral blood CD8 cells from COPD patients (n=10), S (n=6) and HNS (n=8) (Table 2). Stimulation with PMA/Io significantly increased the levels of these cytokines (Table 2). Stimulated IL-2 levels from COPD and S cells were significantly higher compared to HNS (bonferroni multiple comparisons test p<0.05 and p<0.001 respectively) and there was a trend towards significance for higher TNFα in COPD patients (ANOVA p=0.06). IL-17 was higher in S compared to COPD and HNS (bonferroni multiple comparisons test p<0.05). There was no difference between groups for IFNγ. The results from all subjects showed no correlation between cytokine release and either % predicted FEV1 or FEV1:FVC.
Table 2.
Basal and stimulated cytokine levels from peripheral blood and pulmonary CD8 cells.
| Blood CD8 | Pulmonary CD8 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Basal | PMA/Io | ANOVA P value | Basal | PMA/Io | P value | |||||||
| COPD | S | HNS | COPD | S | HNS | COPD | S | COPD | S | |||
| IL-2 (pg/ml) | 0 | 0 | 0 | 12520* (6382) | 33109** (17385) | 4486 (2697) | 0.0001 | 0 | 0 | 2903 (2057) | 2953 (2099) | 0.91 |
| IFNγ (pg/ml) | 84.7 (122) | 10.4 (3.2) | 65 (61) | 16771 (1742) | 12532 (9451) | 5842 (4611) | 0.30 | 109.6 (77.3) | 39.4 (56.3) | 7039 (3729) | 4692 (1819) | 0.30 |
| TNFα (pg/ml) | 47.1 (51.5) | 4.8 (2.7) | 26.0 (16.7) | 3557 (2020) | 2185 (1147) | 1348 (932) | 0.06 | 15.9 (9.8) | 11.2 (5.4) | 890 (789) | 558 (185) | 0.68 |
| IL-17 (pg/ml) | 10.6 (12.7) | 14.6 (16.2) | 6.5 (4.2) | 205 (201) | 860*,† (576) | 200 (202) | 0.02 | nd | nd | nd | nd | |
Data are presented as mean (SD). one-way ANOVA was performed to compare stimulated COPD, S and HNS cytokine levels from blood CD8 cells. Bonferroni multiple comparisons test comparing COPD or S to HNS are indicated as significant as follows;
denotes p<0.05,
denotes p<0.01.
Bonferroni multiple comparisons test comparing COPD to S are indicated by † which denotes p<0.05.
For pulmonary CD8 cells, students t tests were used to compare stimulated cytokine levels between COPD and S. PMA/Io: Phorbol 12-myristate 13-acetate 10ng/ml & Ionomycin 500ng/ml; COPD: Chronic Obstructive Pulmonary Disease; HNS: Healthy non-smoker; S: Smoker with normal lung function; IL2: Interleukin 2; IFNγ: Interferon gamma; TNFα: Tumour necrosis factor alpha; IL17: Interleukin 17; nd: not detected
Dexamethasone, cyclosporin and synta-66 each caused significant inhibition of IL-2, IFNγ, TNFα and IL-17 secretion from peripheral blood CD8 cells in a concentration dependent manner (Figure 1). Both cyclosporin and synta-66 caused significantly greater maximum inhibition at a concentration of 1000nM of IL-2, IFNγ, TNFα and IL-17 compared to dexamethasone in both COPD patients and HNS (Figure 1). IC50 values for cyclosporin ranged from 5.5-49 nM and were similar between patient groups for each cytokine measured. For synta-66 IC50 values ranged from 1.3-213.8 nM with higher values for TNFα compared to other cytokines (supplemental table 2). There was no cytotoxic effect of dexamethasone, cyclosporin or synta-66 at any concentration examined (supplemental figure 1).
Figure 1. Inhibition of peripheral blood CD8 cell cytokine production by dexamethasone, cyclosporin and synta-66.
Percent inhibition of (A) interleukin 2 (IL-2), (B) Interferon gamma (IFNγ), (C) Tumour necrosis factor alpha (TNFα) and (D) Interleukin 17 (IL-17) from peripheral blood CD8 cells from COPD (white, n=10), smokers with normal lung function (grey, n=6) and healthy non-smokers (black, n=8) pre-treated with either dexamethasone (dex), cyclosporin (CsA) or synta-66 and stimulated with PMA (10ng/ml)/ Io (500ng/ml). Data presented as mean ± SD. Statistically significant difference between maximal percent inhibition at a concentration of 1000M of dexamethasone and cyclosporin or synta-66 indicated by * p<0.05. COPD: Chronic Obstructive Pulmonary Disease; HNS: Healthy non smoker; Io: Ionomycin; PMA: Phorbol 12-myristate 13-acetate .
Anti-CD2/3/28 stimulation induced a significant increase in release of IL-2 from COPD PBMCs (n=10; 6.8 pg/ml vs. 4045.8 pg/ml, p<0.001). Synta-66 caused significantly greater maximal inhibition of IL-2 release at a concentration of 1000nM compared to dexamethasone (mean maximal inhibition 98.7% vs. 79.0% respectively, p<0.0001; supplemental figure 2).
Pulmonary CD8 cell cytokine production
The mean CD8 cell yield /gram of lung tissue was 0.17×106 for COPD and 0.11 ×106 for S (p=0.4). Using trypan blue staining, the mean purity of lymphocytes obtained from pulmonary samples was 92.6%. Immunofluorescent staining of 3 samples confirmed the purity of CD8 cells to be >90%.
Under resting conditions pulmonary CD8 cells from COPD patients (n=6) and S (n=5) secreted IL-2, IFNγ, and TNFα at similar levels, while IL-17 was undetectable (Table 2). PMA/Io stimulation caused a significant increase in IL-2, IFNγ and TNFα levels, while IL-17 remained undetectable. Stimulated levels of IL-2, IFNγ and TNFα from pulmonary CD8 cells were similar between COPD patients and S (Table 2). The data from all the subjects showed no correlation between cytokine release and either % predicted FEV1 or FEV1:FVC.
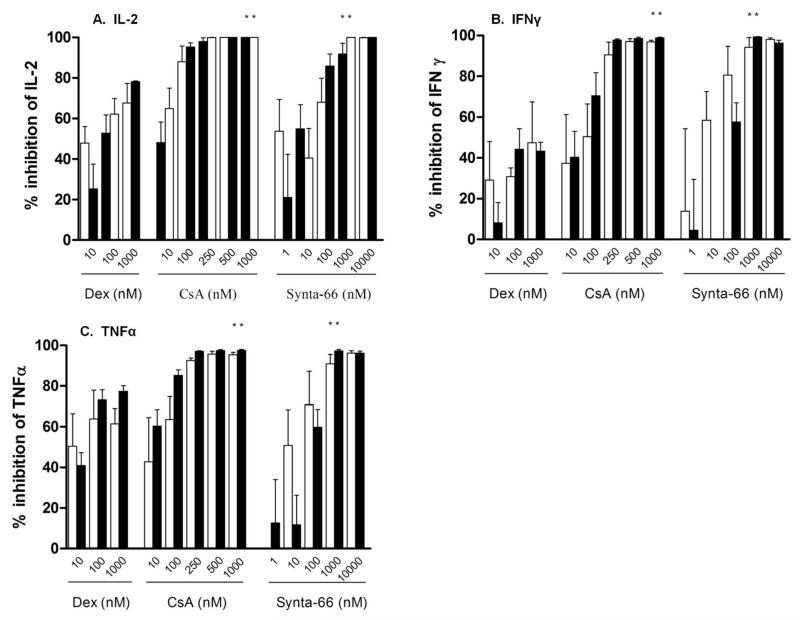
Dexamethasone, cyclosporin and synta-66 each caused significant inhibition of IL-2, IFNγ and TNFα secretion from pulmonary CD8 cells in a concentration dependent manner (Figure 2). Both cyclosporin and synta-66 caused significantly greater maximum inhibition at a concentration of 1000nM of IL-2, IFNγ and TNFα compared to dexamethasone in pulmonary CD8 cells in both COPD patients and S (Figure 2,); the inhibitory effects of cyclosporin and synta-66 were >90% for all cytokines, while <80% was observed for dexamethasone. IC50 values for cyclosporin ranged from 4.1-275.4 nM. For synta-66 IC50 values ranged from 8.3-195.0 nM (supplemental table 1).
Figure 2. Inhibition of pulmonary CD8 cell cytokine production by dexamethasone, cyclosporin and synta-66.
Percent inhibition of (A) interleukin 2 (IL-2), (B) Interferon gamma (IFNγ), (C) Tumour necrosis factor alpha TNFα from pulmonary CD8 cells from COPD (white, n=6) and smokers with normal lung function (black, n=5) pre-treated with either dexamethasone (dex), cyclosporin (CsA) or synta-66 and stimulated with PMA (10ng/ml) / Io (500ng/ml). Data presented as mean ± SD. Statistically significant difference between maximal percent inhibition at a concentration of 1000M of dexamethasone and cyclosporin or synta-66 indicated by * p<0.05. COPD: Chronic Obstructive Pulmonary Disease Io: Ionomycin; PMA: Phorbol 12-myristate 13-acetate.
Regulation of NFAT1 in pulmonary CD8 cells
Dual staining immunofluorescence of enriched COPD pulmonary lymphocytes (n=3) showed NFAT1 located in the cytoplasm of unstimulated CD8 cells (figure 3a). PMA/Io stimulation for 1 hour caused NFAT1 nuclear translocation (figure 3b); this was not inhibited by dexamethasone (figure 3c). Both cyclosporin and synta-66 inhibited nuclear translocation of NFAT1 in pulmonary CD8 cells (figure 3 d&e).
Figure 3. The regulation of NFAT in pulmonary CD8 cells by dexamethasone, cyclosporin and synta-66.
Representative images of enriched pulmonary lymphocytes labelled for NFAT1 (green), CD8 (red), and DAPI (blue). Panel A = basal, panel B= PMA/Io stimulated, panel C= Dexamethasone pre-treated, panel D= cyclosporin pre-treated and panel E = synta-66 pre-treated. Stimulation results in nuclear translocation of NFAT1 which is inhibited by CsA and synta-66 but not by dexamethasone. Io: Ionomycin; NFAT: nuclear factor of activated T cells; PMA: Phorbol 12-myristate 13-acetate.
Small Airway Expression of CD8 and NFAT1
NFAT1 immunoreactivity was present in the nuclei and in a halo-like distribution surrounding the nucleus, suggesting the presence of cells with both activated nuclear NFAT and inactive NFAT (Figure 4a-c show representative images). The absolute number of CD8+, NFAT1+ and double positive CD8+NFAT1+ cells were significantly increased in the small airway sub-epithelium of COPD patients (n=13) compared with S (n=7) (Figure 4d). However, there was no difference between groups in the proportion of CD8 cells which co-expressed NFAT1 (medians COPD 80.7 % vs. S 78.5 %, p=0.3).
Figure 4. The expression of NFAT1 and CD8 cells in human lung small airway sub-epithelium.
A. Representative image of the immunohistochemical analysis of NFAT1 expression. B. Increased magnification of the highlighted area from (A) demonstrating immunoreactivity for NFAT1 in both a halo like cytoplasmic (arrow) and central nuclear (arrowhead) distribution. C. Representative image of combined immunohistochemical and immunofluorescence of NFAT1 and CD8 demonstrating NFAT1+ CD8 cells with both cytoplasmic (arrow) and nuclear (arrowhead) distribution of NFAT1. D. Individual counts for NFAT1+ cells, CD8 + cells and NFAT1+CD8+ cells in the small airway sub-epithelium of COPD and Smoker controls (S). Horizontal bar represents median. COPD: Chronic Obstructive Pulmonary Disease; NFAT1: Nuclear Factor of Activated T cells 1.
There was a significant negative correlation between the expression of CD8+NFAT1+ cells in small airway sub-epithelium and FEV1 % predicted (r= −0.60, p=0.048, figure 5), and a similar correlation for CD8 expression and FEV1 % predicted (r= −0.59, p=0.006, figure 5). There was also a significant negative correlation between FEV1:FVC and both CD8+NFAT1+ (r= −0.68, p=0.001) and CD8 cell expression (r= −0.67, p= 0.001).
Figure 5. Relationship between CD8+ and NFAT1+CD8+ cells in small airway sub-epithelium and % predicted FEV1.
4μm sections of explanted human lung were dual stained for CD8 and NFAT1. The number of CD8+ (panel A) and dual-CD8+NFAT1+ cells (panel B) were counted in the subepithelium of the small airways. Cell counts were correlated individually with % predicted forced expiratory volume in 1 minute (FEV1) using the spearman rank method. The correlation coefficient (R) is stated on each graph with statistical significance indicated when * p<0.05 and ** p<0.001. NFAT1: nuclear factor of activated T cells 1.
DISCUSSION
We have shown that targeting NFAT by inhibition of either CRAC or calcineurin effectively suppresses PMA/Io induced cytokine production from COPD CD8 cells. Importantly, the effects of synta-66 and cyclosporin on cytokine production were greater than dexamethasone. Furthermore, the PMA/Io induced activation of isolated COPD pulmonary CD8 cells was associated with NFAT nuclear translocation that was inhibited by the CRAC inhibitor synta-66 and cyclosporin but not dexamethasone. CRAC inhibition is a novel means of suppressing corticosteroid insensitive NFAT activation in COPD CD8 cells. A major strength of this study is the use of CD8 cells isolated from the lungs and blood of COPD patients. Obtaining isolated CD8 cells from COPD lungs is practically and technically difficult; we are unaware of any studies that have investigated the effects of anti-inflammatory drugs on such cells. Our results provide a realistic insight into the limitations of using corticosteroids to suppress the activity of these cells, and the potential advantages of alternative anti-inflammatory approaches that specifically target NFAT.
NFAT is a key signalling transcription factor in the activation of T cells [13]. TCR activation leads to production of inositol triphosphate which drives the release of stored calcium from the endoplasmic reticulum. The depletion of stored calcium results in opening of the transmembrane CRAC channels thus allowing a greater and prolonged increase in intracellular calcium. This activates calcineurin which dephosphorylates NFAT allowing it to translocate to the nucleus [19]. NFAT increases the transcription of pro-inflammatory cytokines, predominantly through co-operation with other transcription factors such as AP-1 [13]. Synta-66 works by inhibiting CRAC channel function; this prevents the influx of calcium and thus the activation of NFAT [24]. This has the effect of suppressing the release of key inflammatory cytokines such as IL-2 and IFNγ. The studies presented here suggest that synta-66 has at least equivalent anti-inflammatory effect on COPD CD8 cells as corticosteroids, which are the most widely used anti-inflammatory therapy for COPD.
Corticosteroids repress the activity of the transcription factors AP-1 [25] and NFκB [26], both of which are involved in T cell activation. AP-1 co-operates with NFAT to promote the transcription of T cell cytokines [27]. We observed that dexamethasone had no effect on the nuclear translocation of NFAT. Furthermore, high dexamethasone concentrations inhibited pulmonary CD8 cytokine production by <80%. It appears that corticosteroids partially inhibit cytokine production from CD8 cells through repression of NFκB and AP-1, but cannot achieve complete suppression of cytokine production due to the lack of inhibition of NFAT activation. The corticosteroid results contrast with those of Synta-66, where >90% maximal inhibition of cytokine production was observed in all experiments using pulmonary CD8 cells.
Some of the COPD patients were using inhaled corticosteroids. It is unlikely that this affected the in vitro results with dexamethasone, as the effect of dexamethasone was not different in COPD patients and S who were not taking corticosteroids.
Synta-66 inhibited the production of IFNγ, IL-2 and IL-17 by CD8 cells from COPD patients; there was almost complete suppression of cytokine production at 1μM. This effect is greater than the effect of this compound used at the same and higher concentrations on cytokine production by isolated gut T cells from patients with inflammatory bowel disease [24]. This may be due to an increased sensitivity of isolated CD8 cells to CRAC inhibition compared to a mixed T cell population, or reflect differences between the characteristics of lymphocytes from different organs. Synta-66 has also been shown to potently inhibit effector function of pulmonary mast cells [28]. Although the role of the mast cell in COPD is far from clear [29], this is another potential mechanism through which CRAC inhibitors could modulate pulmonary inflammation.
We used PMA/Io as a stimulant due to the fact that it robustly activates NFAT by increasing intracellular calcium levels. This could be criticised as a non-physiological method for activation of T cells as it does not involve the TCR. However, similar effects of synta-66 on the inhibition of cytokine release from TCR stimulated PBMCs were observed. We observed increased numbers of CD8 cells in COPD small airways, in agreement with previous studies [4, 5]. The expression of total NFAT1 positive cells and NFAT1 expressing CD8 cells were increased in COPD small airways. However, the proportion of CD8 cells that were positive for NFAT1 in COPD compared to S was similar, indicating that the increased presence of NFAT1 in the COPD small airways is due to increased CD8 cell numbers. The expression levels of NFAT1 were high; approximately 80% of pulmonary CD8 cells positively expressed NFAT1. Furthermore, NFAT inhibition resulted in almost complete suppression of cytokine production. These results demonstrate a major role for NFAT signalling in the inflammatory activity of CD8 cells, which are increased in number in the lungs of COPD patients.
There is no antibody currently available to stain for the activated form of NFAT1, so we could not make accurate inferences about activation status within tissue cells. We observed both nuclear and cytoplasmic NFAT1 expression in pulmonary CD8 cells, suggesting that this transcription factor is in a dynamic state between active and inactive forms within the lungs.
Stimulation of peripheral blood CD8 cells caused significantly greater production of IL-2 with a trend towards higher levels of TNFα in COPD patients compared to S and HNS. This agrees with a previous report [30] and suggests that circulating COPD CD8 cells react more potently upon activation. This disease specific phenomenon was not observed in pulmonary CD8 cells. It should be highlighted that there was no HNS control group in the pulmonary CD8 study. It is difficult to recruit lifelong non-smokers from patients undergoing lung surgery for suspected or confirmed lung cancer as such patients are a minority. Furthermore, the nature of primary lung samples is that there can be relatively large variability between patients [31,32], which we also observed. Our conclusions about the cytokine levels from COPD pulmonary CD8 cells compared to controls are restricted by these limitations.
We have previously reported that airway lymphocytes from COPD patients show decreased sensitivity to dexamethasone [31]. We did not observe this phenomenon in the current study. There are important methodological differences between these studies; the current paper used isolated CD8 cells in contrast to a mixed culture of lymphocytes and the stimulus used to activate cells also differed. It is possible that the previously reported corticosteroid insensitivity was driven by CD4 cells, and it is of interest to investigate the effects of CRAC inhibition on COPD CD4 cells.
Monoclonal antibodies directed against IL-2 [32] and CD4 [33] and the calcineurin inhibitor cyclosporin [17] are selective inhibitors of lymphocyte function which have clinical benefits in patients with asthma. However, the side effects of these drugs have prevented their widespread use. We have demonstrated similar anti-inflammatory effects of cyclosporin and Synta-66 on COPD CD8 cells. However, cyclosporin is not a well tolerated drug, so has not been developed for the treatment of COPD. Inhibitors of CRAC activation may provide an alternative approach in COPD patients, and our data provides pharmacological proof of the efficacy of the CRAC inhibitor Synta-66.
We provide here unequivocal in vitro evidence that CRAC inhibition has a greater effect on the suppression of COPD CD8 cell inflammatory cytokine release than corticosteroids. Novel approaches to treating inflammation in COPD are required. The degree of clinical benefit that can be achieved through selective T cell inhibition in COPD patients is not known, as clinical trials using such drugs have not been reported. Clinical trials of CRAC inhibitors may provide insights into this important issue.
Supplementary Material
CLINICAL PERSPECTIVE.
Currently available anti-inflammatory therapies have limited efficacy in COPD. There is a need for novel anti-inflammatory treatments. We show that targeting COPD CD8 cells with a CRAC inhibitor produces greater anti-inflammatory effect than corticosteroids. CRAC inhibitors may have important anti-inflammatory effects in COPD patients.
Acknowledgments
Dr Grundy has received lecture fees and support for conference attendance from pharmaceutical companies including GSK, Novartis, Boehringer Ingelheim and AstraZeneca. Prof Singh has received lectures fees, support for conference attendance, advisory board fees, and research grants from a range of pharmaceutical companies, including GlaxoSmithKline, Chiesi Pharmaceuticals, AstraZeneca, CIPLA, Novartis, Forest, MSD, Boehringer Ingelheim, and Allmiral. Drs Hall, House and Begg are employees of GlaxoSmithKline.
Funding
This work was supported by the Wellcome Trust [Grant number WT092281MA]
Abbreviations
- AP-1
Activator Protein 1
- COPD
Chronic Obstructive Pulmonary Disease
- CRAC
Calcium Release-Activated Calcium
- DAPI
4′,6-diamidino-2-phenylindole
- ELISA
Enzyme-linked immunosorbant assay
- FEV1
Forced expiratory volume in 1 second
- FVC
Forced vital capacity
- HNS
Healthy non smoker
- IL
Interleukin
- IFN
interferon
- Io
Ionomycin
- LDH
Lactate dehydrogenase
- NFAT
Nuclear Factor of Activated T cells
- NFκB
nuclear factor-κB
- PBMC
Peripheral blood mononuclear cell
- PMA
Phorbol 12-myristate 13-acetate
- S
Smoker with normal lung function
- SOCE
Store Operated Calcium Entry
- TCR
T cell receptor
- TNF
Tumour Necrosis Factor
Footnotes
Drs Kaur, Plumb, Reynolds and Prof Ray have no conflicts of interest to declare.
Contributor Information
Seamus Grundy, University of Manchester, NIHR Translational Research Facility, Manchester Academic Health Science Centre, University Hospital of South Manchester Foundation Trust, Southmoor Road, Manchester M23 9LT, UK.
Manminder Kaur, University of Manchester, NIHR Translational Research Facility, Manchester Academic Health Science Centre, University Hospital of South Manchester Foundation Trust, Southmoor Road, Manchester M23 9LT, UK.
Jonathan Plumb, University of Manchester, NIHR Translational Research Facility, Manchester Academic Health Science Centre, University Hospital of South Manchester Foundation Trust, Southmoor Road, Manchester M23 9LT, UK.
Sophie Reynolds, University of Manchester, NIHR Translational Research Facility, Manchester Academic Health Science Centre, University Hospital of South Manchester Foundation Trust, Southmoor Road, Manchester M23 9LT, UK.
Simon Hall, GlaxosmithKline, Stevenage, UK.
David House, GlaxosmithKline, Stevenage, UK.
Malcolm Begg, GlaxosmithKline, Stevenage, UK.
David Ray, School of Medicine and Manchester Academic Health Science Centre, University of Manchester, Oxford Road, Manchester, M13 9PT.
Dave Singh, University of Manchester, NIHR Translational Research Facility, Manchester Academic Health Science Centre, University Hospital of South Manchester Foundation Trust, Southmoor Road, Manchester M23 9LT, UK.
REFERENCES
- 1.GOLD Global Strategy for the Diagnosis, Management and Prevention of COPD. 2011 cited 2011; Available from: http://www.goldcopd.org/
- 2.Alsaeedi A, Sin DD, McAlister FA. The effects of inhaled corticosteroids in chronic obstructive pulmonary disease: a systematic review of randomized placebo-controlled trials. Am J Med. 2002;113(1):59–65. doi: 10.1016/s0002-9343(02)01143-9. [DOI] [PubMed] [Google Scholar]
- 3.Yang IA, Clarke MS, Sim EH, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;7:CD002991. doi: 10.1002/14651858.CD002991.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Shaughnessy TC, Ansari TW, Barnes NC, Jeffery PK. Inflammation in bronchial biopsies of subjects with chronic bronchitis: inverse relationship of CD8+ T lymphocytes with FEV1. Am J Respir Crit Care Med. 1997;155(3):852–7. doi: 10.1164/ajrccm.155.3.9117016. [DOI] [PubMed] [Google Scholar]
- 5.Saetta M, Di Stefano A, Turato G, Facchini FM, Corbino L, Mapp CE, Maestrelli P, Ciaccia A, Fabbri LM. CD8+ T-lymphocytes in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(3 Pt 1):822–6. doi: 10.1164/ajrccm.157.3.9709027. [DOI] [PubMed] [Google Scholar]
- 6.Freeman CM, Han MK, Martinez FJ, Murray S, Liu LX, Chensue SW, Polak TJ, Sonstein J, Todt JC, Ames TM, Arenberg DA, Meldrum CA, Getty C, McCloskey L, Curtis JL. Cytotoxic potential of lung CD8(+) T cells increases with chronic obstructive pulmonary disease severity and with in vitro stimulation by IL-18 or IL-15. J Immunol. 2010;184(11):6504–13. doi: 10.4049/jimmunol.1000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gadgil A, Zhu X, Sciurba FC, Duncan SR. Altered T-cell phenotypes in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3(6):487–8. doi: 10.1513/pats.200603-064MS. [DOI] [PubMed] [Google Scholar]
- 8.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Pare PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645–53. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 9.Brusselle GG, Demoor T, Bracke KR, Brandsma CA, Timens W. Lymphoid follicles in (very) severe COPD: beneficial or harmful? Eur Respir J. 2009;34(1):219–30. doi: 10.1183/09031936.00150208. [DOI] [PubMed] [Google Scholar]
- 10.Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med. 2009;360(23):2445–54. doi: 10.1056/NEJMra0804752. [DOI] [PubMed] [Google Scholar]
- 11.Lee SH, Goswami S, Grudo A, Song LZ, Bandi V, Goodnight-White S, Green L, Hacken-Bitar J, Huh J, Bakaeen F, Coxson HO, Cogswell S, Storness-Bliss C, Corry DB, Kheradmand F. Antielastin autoimmunity in tobacco smoking-induced emphysema. Nat Med. 2007;13(5):567–9. doi: 10.1038/nm1583. [DOI] [PubMed] [Google Scholar]
- 12.Retamales I, Elliott WM, Meshi B, Coxson HO, Pare PD, Sciurba FC, Rogers RM, Hayashi S, Hogg JC. Amplification of inflammation in emphysema and its association with latent adenoviral infection. Am J Respir Crit Care Med. 2001;164(3):469–73. doi: 10.1164/ajrccm.164.3.2007149. [DOI] [PubMed] [Google Scholar]
- 13.Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5(6):472–84. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 14.Ferraccioli GF, Tomietto P, De Santis M. Rationale for T cell inhibition by cyclosporin A in major autoimmune diseases. Ann N Y Acad Sci. 2005;1051:658–65. doi: 10.1196/annals.1361.110. [DOI] [PubMed] [Google Scholar]
- 15.Snell GI, Westall GP. Immunosuppression for lung transplantation: evidence to date. Drugs. 2007;67(11):1531–9. doi: 10.2165/00003495-200767110-00002. [DOI] [PubMed] [Google Scholar]
- 16.Fukuda T, Asakawa J, Motojima S, Makino S. Cyclosporine A reduces T lymphocyte activity and improves airway hyperresponsiveness in corticosteroid-dependent chronic severe asthma. Ann Allergy Asthma Immunol. 1995;75(1):65–72. [PubMed] [Google Scholar]
- 17.Lock SH, Kay AB, Barnes NC. Double-blind, placebo-controlled study of cyclosporin A as a corticosteroid-sparing agent in corticosteroid-dependent asthma. Am J Respir Crit Care Med. 1996;153(2):509–14. doi: 10.1164/ajrccm.153.2.8564089. [DOI] [PubMed] [Google Scholar]
- 18.Pallet N, Legendre C, Gaston RS. Deciphering calcineurin inhibitor nephrotoxicity: a pharmacological approach Chronic calcineurin inhibitor nephrotoxicity: reflections on an evolving paradigm. Pharmacogenomics. 2009;11(10):1491–501. doi: 10.2217/pgs.10.137. [DOI] [PubMed] [Google Scholar]
- 19.Gwack Y, Feske S, Srikanth S, Hogan PG, Rao A. Signalling to transcription: store-operated Ca2+ entry and NFAT activation in lymphocytes. Cell Calcium. 2007;42(2):145–56. doi: 10.1016/j.ceca.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Qu B, Al-Ansary D, Kummerow C, Hoth M, Schwarz EC. ORAI-mediated calcium influx in T cell proliferation, apoptosis and tolerance. Cell Calcium. 2011;50(3):261–9. doi: 10.1016/j.ceca.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 21.McCarl CA, Khalil S, Ma J, Oh-hora M, Yamashita M, Roether J, Kawasaki T, Jairaman A, Sasaki Y, Prakriya M, Feske S. Store-operated Ca2+ entry through ORAI1 is critical for T cell-mediated autoimmunity and allograft rejection. J Immunol. 2010;185(10):5845–58. doi: 10.4049/jimmunol.1001796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sweeney ZK, Minatti A, Button DC, Patrick S. Small-molecule inhibitors of store-operated calcium entry. ChemMedChem. 2009;4(5):706–18. doi: 10.1002/cmdc.200800452. [DOI] [PubMed] [Google Scholar]
- 23.Chen G, Panicker S, Lau KY, Apparsundaram S, Patel VA, Chen SL, Soto R, Jung JK, Ravindran P, Okuhara D, Bohnert G, Che Q, Rao PE, Allard JD, Badi L, Bitter HM, Nunn PA, Narula SK, Demartino JA. Characterization of a novel CRAC inhibitor that potently blocks human T cell activation and effector functions. Mol Immunol. 2013;54(3-4):355–367. doi: 10.1016/j.molimm.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Di Sabatino A, Rovedatti L, Kaur R, Spencer JP, Brown JT, Morisset VD, Biancheri P, Leakey NA, Wilde JI, Scott L, Corazza GR, Lee K, Sengupta N, Knowles CH, Gunthorpe MJ, McLean PG, MacDonald TT, Kruidenier L. Targeting gut T cell Ca2+ release-activated Ca2+ channels inhibits T cell cytokine production and T-box transcription factor T-bet in inflammatory bowel disease. J Immunol. 2009;183(5):3454–62. doi: 10.4049/jimmunol.0802887. [DOI] [PubMed] [Google Scholar]
- 25.De Bosscher K, Vanden Berghe W, Haegeman G. The interplay between the glucocorticoid receptor and nuclear factor-kappaB or activator protein-1: molecular mechanisms for gene repression. Endocr Rev. 2003;24(4):488–522. doi: 10.1210/er.2002-0006. [DOI] [PubMed] [Google Scholar]
- 26.Ray A, Prefontaine KE. Physical association and functional antagonism between the p65 subunit of transcription factor NF-kappa B and the glucocorticoid receptor. Proc Natl Acad Sci U S A. 91(2):752–6. doi: 10.1073/pnas.91.2.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–47. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 28.Ashmole I, Duffy SM, Leyland ML, Morrison VS, Begg M, Bradding P. CRACM/Orai ion channel expression and function in human lung mast cells. J Allergy Clin Immunol. 2012;129(6):1628–35. doi: 10.1016/j.jaci.2012.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mortaz E, Folkerts G, Redegeld F. Mast cells and COPD. Pulm Pharmacol Ther. 2011;24(4):367–72. doi: 10.1016/j.pupt.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Zhu X, Gadgil AS, Givelber R, George MP, Stoner MW, Sciurba FC, Duncan SR. Peripheral T cell functions correlate with the severity of chronic obstructive pulmonary disease. J Immunol. 2009;182(5):3270–7. doi: 10.4049/jimmunol.0802622. [DOI] [PubMed] [Google Scholar]
- 31.Kaur M, Smyth LJ, Cadden P, Grundy S, Ray D, Plumb J, Singh D. T lymphocyte insensitivity to corticosteroids in chronic obstructive pulmonary disease. Respir Res. 2012;13:20. doi: 10.1186/1465-9921-13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freeman CM, Curtis JL, Chensue SW. CC chemokine receptor 5 and CXC chemokine receptor 6 expression by lung CD8+ cells correlates with chronic obstructive pulmonary disease severity. Am J Pathol. 2007;171(3):767–76. doi: 10.2353/ajpath.2007.061177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Busse WW, Israel E, Nelson HS, Baker JW, Charous BL, Young DY, Vexler V, Shames RS. Daclizumab improves asthma control in patients with moderate to severe persistent asthma: a randomized, controlled trial. Am J Respir Crit Care Med. 2008;178(10):1002–8. doi: 10.1164/rccm.200708-1200OC. [DOI] [PubMed] [Google Scholar]
- 34.Kon OM, Sihra BS, Loh LC, Barkans J, Compton CH, Barnes NC, Larche M, Kay AB. The effects of an anti-CD4 monoclonal antibody, keliximab, on peripheral blood CD4+ T-cells in asthma. Eur Respir J. 2001;18(1):45–52. doi: 10.1183/09031936.01.00064101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.