Abstract
Background
Epidemiology studies have demonstrated inconsistent associations between type 2 diabetes mellitus and the risk of malignant melanoma. To this end, the aim was to perform a meta-analysis of cohort studies.
Method
Medline, PubMed, Embase and the Cochrane Library were searched up to February 2014. Cohort studies addressing the relative risk of type 2 diabetes mellitus on malignant melanoma were included in this meta-analysis. The Newcastle-Ottawa Scale was applied for quality evaluation. The pooled relative risks with the corresponding 95% confidence intervals (95% CIs) were calculated by using random-effects or random-effects model. Heterogeneity and publication bias were evaluated by I2 and funnel plot analysis, respectively. Data was analyzed using STATA 11.0.
Results
A total of 9 independent cohorts from 8 manuscripts were entered this meta-analysis. Type 2 diabetes mellitus was slightly associated with an increased risk of malignant melanoma, and the pooled relative risk was 1.15 (95% CI, 1.00-1.32) in diabetes compared with non-diabetes with significant evidence of heterogeneity among these studies (P=0.016, I2 =57.6%). For the studies adjusted for age, gender and obesity, the relative risks were 1.21 (95% CI, 1.03-1.42), 1.17 (95% CI, 1.01-1.35) and 1.11 (95% CI, 1.00-1.24), respectively. For the population-based studies in which case cohort established, the relative risk was 1.85 (95% CI, 1.31-2.62).
Conclusion
Type 2 diabetes might be an independent risk factor for malignant melanoma. Further studies are needed to specifically test the effect, and fully elucidate the underlying pathophysiologic mechanisms.
Keywords: Diabetes mellitus, Malignant melanoma, Meta-analysis
Introduction
During the last decades, the incidence of malignant melanoma (hereafter called melanoma) has increased faster than any other solid tumor, and has developed from a very rare disease entity into a cancer with growing importance medically. Global incidence is about 160 000 new cases per year, with 48 000 deaths (1). New Zealand and Australia have the highest incidence and mortality rates from melanoma in the world (2). In USA (3) and Europe (4, 5), incidence rates are similarly increasing. The contemporary incidence rate exceeding 125 cases per 100,000 men aged 65 and older in USA, making malignant melanoma the fifth most common cancer in older white men following prostate, lung, colorectal, and bladder cancer (6). Solar radiation has been identified as a principal factor for the causation of melanoma (7), other factors, such as obesity (8), oral contraceptive (9), decreased physical exercise (10), smoking and alcohol (11) might also contribute to the increasing incidence of malignancy.
Type 2 diabetes mellitus (T2DM) is serious and becoming increasingly common in many countries (12, 13), which accounts for 90-95% of all diagnosed cases of diabetes. Evidence-derived from case control studies, cohort studies, and meta-analyses, suggests that diabetes could increases the risks of site-specific cancers of breast (14), kidney (15), colorectal (16), gastric (17), liver (18), endometrial (19), thyroid (20), lung (21) and pancreatic (22), but decreases the risk of prostate cancer (23). Recently, a large population-based survey in USA indicated that the prevalence of melanoma in persons with diabetes was significantly higher than those without diabetes, and clinical studies demonstrated that melanoma was common among people with diabetes. Lifestyle risk factors for developing diabetes (24, 25), such as physical inactivity and obesity, have also been linked to an increased risk of melanoma (8, 10).
Thus, it has been hypothesized that diabetes itself may be a risk factor for melanoma. To present, epidemiology studies of the relationship between diabetes and melanoma have shown inconsistence results. To this end, we aimed to perform a meta-analysis of cohort studies to evaluate the association between T2DM and melanoma.
Methods
Search strategy and selection criteria
Literature retrieval was conducted by searching Medline, PubMed, Embase and the Cochrane Library, up to February 2014 by two independent investigators (Qi Li and Qi Xiaoling). The search strategy included the following keywords: diabetes, diabetes mellitus, NIDDM, neoplasm(s), cancer, carcinoma, skin cancer and melanoma. The reference lists of pertinent articles were also inspected manually. We contacted the authors of included studies for additional eligible studies.
Inclusion and exclusion criteria
Studies were eligible for the meta-analysis if they met the following inclusion criteria 1) a cohort design; 2) one of the exposure interests was T2DM; 3) one of the outcome of interests was melanoma; 4) rate ratio, hazard ratio or standardized incidence rates (SIR) with their 95% confidence intervals (CIs) (or data to calculate them) were reported. Studies were excluded if they 1) provided only an effect estimate with no means to calculate a CIs or 2) were derived from non-peer-reviewed sources or 3) only contained patients with type 1 diabetes or (4) only provided standardized mortality rate without standardized incidence rate (because mortality rate could be confounded by survival related factors easily). When multiple publications on a same cohort were identified or when study populations overlapped, only the study of the most recent report or the publication with the most control for confounders was included, unless the reported outcomes were mutually exclusive.
Study selection and Data extraction
Data extracted from eligible studies included publication data (the first author’s last name, year of publication, and country of population studied), year of the study conducted, case resource, methods of ascertainment of diabetes and outcome, the follow-up period, risk estimates with their corresponding confidence intervals, and variables adjusted for in the analysis. When studies provided more than one RRs according to the duration of diabetes before melanoma was diagnosed, we extracted and combined the RRs for individuals diagnosed with diabetes more than 1 year prior to the diagnosis of melanoma.
Quality evaluation
The Newcastle-Ottawa Scale (NOS) (26) was applied to evaluate the qualities of the included studies. A ‘star system’ was used to judge data quality based on three broad perspectives for these studies: the selection, the comparability and the outcome or exposure of interest. The scores ranged from 0 to 9 scores. Studies with scores of 7 stars or greater were considered to be of high quality. The scores were added up to compare the study quality in a quantitative fashion. Two reviewers independently evaluated and crosschecked the quality of the included studies, as well as assessed the bias of the studies. Disagreements between the reviewers were resolved by open discussion.
Statistical analysis
All statistical tests were performed using STATA, version 11.0 (STATA, College Station, TX, USA). We included in this meta-analysis reporting different measures of relative risks (RRs): rate ratio and standardized incidence rate (SIR). In practice, both of them yield similar estimates of RR because the absolute risk of melanoma is low.
The variance of the logRR from each study was calculated by converting the 95% CI to its natural logarithm by taking the width of the CI and dividing by 3.92. The pooled RR estimates with corresponding 95% CIs was derived with the method of DerSimonian and Laird (27, 28) using the assumptions of a random-effects model, which accounts for heterogeneity among studies (I2 ≥50%); otherwise, a fixed effects model was applied.
We conducted subgroup analyses stratified by different participants and study characteristics. Only the studies based on rate ratio or SIR were included for subgroup analysis. To assess heterogeneity among studies, we used the Cochran Q and I2 statistics. This was used to test whether the differences obtained between studies was due to chance. For the Q statistic, a P value < 0.10 was considered statistically significant for heterogeneity; for I2, a value >50% was considered as a measure of severe heterogeneity. Publication bias was evaluated using the funnel plot and Begg’s and Egger’s test, a P value of less than 0.10 was considered to be statistically significant.
Results
Description of the eligible studies
The publication search process is shown in Fig. 1. First, a total of 2052 potentially relevant publications up to February 2014 were systematically identified through the previously listed databases. Of them, 1727 articles were excluded because they either did not meet the inclusion criteria or failed to provide adequate information to determine whether the criteria were satisfied in the first round screening after reading titles or abstracts. Among the remaining 325 studies, 306 articles were excluded because they did not contain data about melanoma or skin cancer. The remaining 19 articles were subjected to full-text reviews. And then, 11 articles were excluded because they were case reports, cross-sectional survey, case-control study, or only provided relationship of type 1 diabetes and melanoma, other type of skin cancer, or SMR of diabetes and melanoma. Finally, 9 cohort studies from 8 literatures (29-36) were eligible and included in the meta-analysis.
Fig. 1.
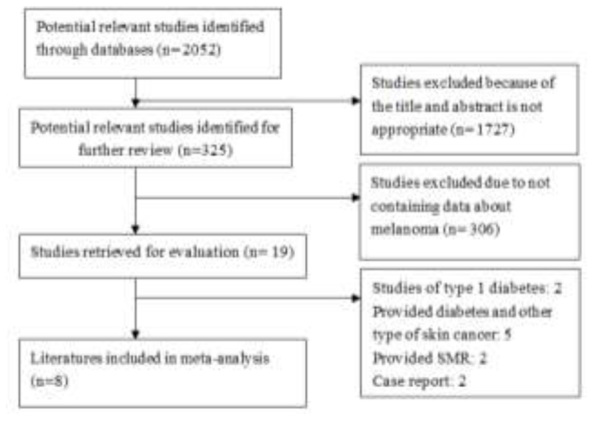
Flow chart of the literature search strategy to identify cohort studies on melanoma and T2DM
We established a database according to the information extracted from each study. Detailed characteristics of the 9 studies are listed in Table 1. The countries in which the studies were conducted were as follows: USA (n = 3); Sweden (n = 3); UK (n=2); Denmark (n=1). All the cohorts included in this Meta-analysis included both men and women, expect one cohort consisted entirely of men veterans.
Table 1.
Characteristics of 9 cohort studies of T2DM and melanoma based on rate ratio and standardized incidence rate
| Year | First author | Country | Study population | Diagnosis of DM | Study period | Age range (yr) | Mean follow-up(year) | Gender | SIR/RR(95%CI) | Adjustment factors |
|---|---|---|---|---|---|---|---|---|---|---|
| 2010(31) | Kari Hemminki | Sweden | National wide hospital discharge database | Hospital disease record | 1964-2007 | 39-- | 15 | Both | 1.03 (0.88-1.20)* | Age, sex, period, socioeconomic status, obesity and region |
| 2007(33) | Par Stattin | Sweden | Northern Sweden health and disease cohort | Fasting glucose level | 1985-2013 | 29-61 | 8.25 | Both | 2.16(1.14-4.35) | Age, calendar year and smoking |
| 2011(30) | Elizabeth A.Atchison | USA | U.S. veterans men | Hospital disease record | 1969-1996 | 18-100 | 10.5 | Male | 1.13(1.03-1.24) | Age, time, latency, race, obesity and number of visits |
| 2011(29) | C.J.Wotton | UK | Hospital admissions and deaths data | Hospital disease record | 1963-1998 | ≥ 30 | NA | Both | 1.15(0.68-1.82) | sex, age in 5-year bands, time period in single calendar years and districts |
| 2011(29) | C.J.Wotton | UK | Hospital admissions and deaths data | Hospital disease record | 1999-2008 | ≥ 30 | NA | Both | 0.93(0.42-1.79) | sex, age in 5-year bands, time period in single calendar years and districts |
| 2009(32) | Marianne Ulcickas Yood | USA | National population-based database | Hospital disease record | 2000-2004 | 18- | 3.6 | Both | 1.63(1.21-2.19) | Age, gender |
| 1997(34) | Wideroff | Denmark | Hospital discharge database | Hospital disease record | 1977-1989 | mean(male) : 64 ; mean(female):69 | 5.7 | Both | 1.0(0.84-1.20) | NA |
| 1991(35) | Hans-Olov Adami | Sweden | Hospital inpatient register database | Hospital disease record | 1965-1984 | 20- | 5.2 | Both | 0.92(0.71-1.34) | NA |
| 1982(36) | Mark Ragozzino | USA | Hospital medical reports | Hospital medical report | 1945-1969 | NA | 8.7 | Both | 4.3(0.9-12.5) | NA |
*Refer to standardized incidence rate (SIR)
A total of 1,071,257 persons (range: 7,771-594,815) were included in the 9 cohort studies, with 1,876 cases of melanoma were reported. Among the 9 studies, potential confounders were controlled in 6 studies (at least for age), 5 of them adjusted by gender, 2 of them adjusted by obesity, 3 of them were population-based cohort, whereas the other 6 were hospital-based cohorts. Quality assessment of studies was performed using the NOS method. The results ranged from a star rating of 5 to 9 (with a mean star rating of 7), with a higher value indicating the better methodology (Table 2).
Table 2.
Quality indicators from Newcastle-Ottawa scale
| Study | Selection | Comparability | Exposure/Outcome | Score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5a | 5b | 6 | 7 | 8 | ||
| Hemminki 2010 | yes | yes | yes | yes | yes | Yes | Yes | Yes | yes | 9 |
| Stattin 2007 | yes | yes | yes | yes | yes | yes | no | yes | yes | 8 |
| Atchison 2011 | yes | yes | yes | yes | yes | yes | yes | yes | yes | 9 |
| Wotton 2011(a) | yes | yes | yes | yes | yes | no | yes | yes | no | 7 |
| Wotton 2011(b) | yes | yes | yes | yes | yes | no | yes | yes | yes | 8 |
| Yood 2009 | yes | yes | yes | yes | yes | no | yes | no | yes | 7 |
| Wideroff 1997 | yes | no | yes | yes | no | no | yes | yes | no | 5 |
| Adami 1991 | yes | no | yes | yes | no | no | yes | yes | yes | 6 |
| Ragozzino 1980 | yes | no | yes | yes | no | no | yes | yes | yes | 6 |
Footnote: 1, indicates exposed cohort truly representative; 2, the non exposed cohort drawn from the same community; 3, ascertainment of exposure by secure record or structured interview; 4, outcome of interest was not present at start of study; 5A, cohorts comparable on basis of sex and age; 5B, cohorts comparable on other factor(s); 6, quality of outcome assessment; 7, follow-up long enough for outcomes to occur; and 8, complete follow up
Diabetes and melanoma risk
As shown in Fig. 2, the summary RR with 95% CI was 1.15 (95% CI, 1.00-1.32) in a random-effects model for individuals with T2DM compared with those without T2DM (Q =18.85, P=0.016, I2 =57.6%).
Fig. 2.
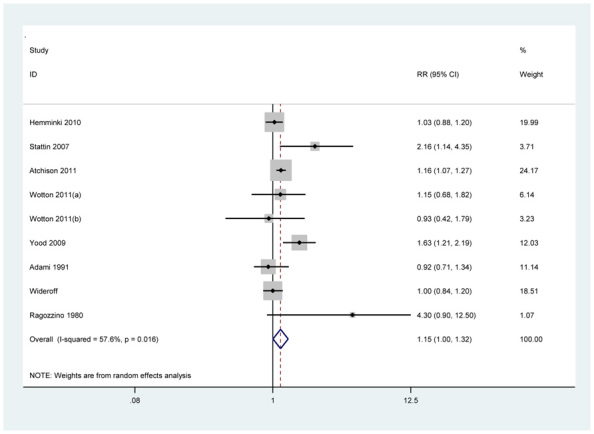
Analysis of studies were examined the association between T2DM and melanoma. RRs and 95% CI for all studies and the overall effect estimate are reported in the log scale. Among diabetics, the overall RR for melanoma is statistically significant at 1.15 (95% CI, 1.00-1.32)
We then conducted subgroup meta-analyses on the basis of case source and geographical area (Table 3). The association between T2DM and melanoma was somewhat stronger in population-based studies (pooled RR 1.82, 95% CI, 1.31-2.62; test for heterogeneity Q =2.38, P = 0.303,
Table 3.
Stratified analysis of relative risks for the association between T2DM and melanoma
| Subgroup | Reference | P value for overall effect | Pooled RR (95% CI) | Tests for heterogeneity | ||
|---|---|---|---|---|---|---|
| Chi2 | P | I2 (%) | ||||
| Adjustment for age | ||||||
| Yes | 29-33 | 0.019 | 1.21(1.03-1.42) | 10.98 | 0.052 | 54.5 |
| No | 34-36 | 0.753 | 1.06(0.75-1.49) | 4.99 | 0.083 | 59.9 |
| Adjustment for gender | ||||||
| Yes | 29-32 | 0.031 | 1.17(1.01-1.35) | 7.62 | 0.107 | 47.5 |
| No | 33-36 | 0.253 | 1.25(0.85-1.85) | 9.81 | 0.02 | 69.4 |
| Adjustment for obesity | ||||||
| Yes | 30,31 | 0.058 | 1.11(1.00-1.24) | 1.73 | 0.189 | 42.2 |
| No | 29,32-36 | 0.100 | 1.25(0.96-1.64) | 18.11 | 0.009 | 64.9 |
| Diabetes cohort resource | ||||||
| Population-based | 32,33,36 | 0 | 1.85(1.31-2.62) | 2.38 | 0.303 | 16.1 |
| Hospital-based | 29-31,34,35 | 0.006 | 1.10(1.03-1.17) | 4.71 | 0.452 | 0 |
| Country | ||||||
| USA | 30,32,36 | 0.053 | 1.47(1.00-2.17) | 8.31 | 0.016 | 58.1 |
| Sweden | 31,33,34 | 0.455 | 1.08(0.88-1.34) | 4.78 | 0.092 | 75.9 |
| UK | 29 | 0.726 | 1.08(0.72-1.62) | 0.23 | 0.635 | 0 |
| Study quality | ||||||
| 7 stars or greater | 29-33 | 0.019 | 1.21(1.03-1.42) | 10.98 | 0.052 | 54.9 |
| Below 7 stars | 34-36 | 0.753 | 1.06(0.75-1.49) | 4.99 | 0.083 | 59.9 |
I2 =16.1%) than in hospital-based studies (pooled RR 1.10, 95% CI, 1.03-1.17; test for heterogeneity Q =4.71, P= 0.452, I2 =0%). Subgroup analysis based on geographical area revealed that the pooled RR was statistically significant in USA (pooled RR =1.47; 95 % CI=1.00-2.17; test for heterogeneity Q =8.31, P =0.016, I2 =58.1%), but no significant positive association was found in Sweden, UK and Denmark.
The impact of confounding factors such as age, gender and obesity on the assessment of the relative risks between T2DM and melanoma were further investigated. Five studies provided data on melanoma risk adjusted for age. In a stratified analysis, diabetes were at an increased risk of developing melanoma in the studies adjusted for age (pooled RR =1.21, 95% CI=1.03-1.42; test for heterogeneity Q =10.98, P =0.052, I2 =54.5%), but the overall slope-association of studies without adjusted by age was null, the pooled RR was 1.06(95%CI, 0.75-1.49).
Similarly, when we restricted the meta-analysis to studies adjusted for gender, we found a positive association between T2DM and melanoma, the pooled RR was 1.17(95%CI, 1.01-1.35; test for heterogeneity Q =7.62, P =0.107, I2 =47.5%), but the overall slope-association of studies without adjusted by gender was null, the pooled RR was 1.24(95%CI, 0.85-1.85).
When we restricted the meta-analysis to the studies adjusted for obesity, the positive association between T2DM and melanoma risk remained, the pooled RR was 1.11(95%CI, 1.00–1.24; test for heterogeneity Q =1.73, P =0.189, I2 =42.2%).
In addition, the summary estimate from studies whose quality was considered high (with a start rating of 7 stars or greater by assessment using NOS method) was significantly higher than from others: 1.21 (95% CI 1.03–1.42) vs. 1.06(0.75-1.49). In the study consisted of entirely veteran men, men with diabetes had a higher risk of melanoma than those without diabetes, with a RR and 95% CI of 1.13(1.03–1.24).
Publication bias of the studies
There was no funnel plot asymmetry for the association between diabetes and melanoma risk (Fig.3). P values for Begg’s adjusted rank correlation test and Egger’s regression asymmetry test were 0.404 and 0.392, respectively, indicating a low probability of publication bias.
Fig. 3.
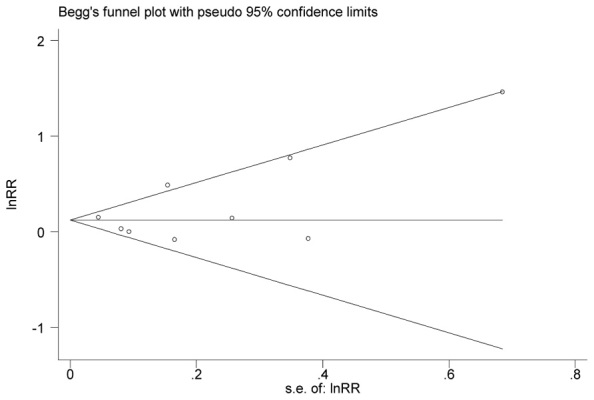
Funnel plot of log RR of developing melanoma, according to the SEs of all studies in analysis. Y-axis, RRs on the logarithmic scale; X-axis, SE. The horizontal line is drawn at the pooled log RR. Begg-Mazumdar test (P = 0.404) and Egger test (P = 0.392)
Discussion
Findings from this meta-analysis indicate that individuals with T2DM may slightly increase relative risk of developing melanoma compared with non-T2DM individuals. T2DM and melanoma share some similar risk factors, such as aging and obesity (8). Thus, the observed increased risk of melanoma associated with a history of diabetes may reflect confounding by these risk factors. However, a statistically significant association was still found when the analysis was limited to studies that adjusted for age and obesity, the pooled RR and 95% CI were 1.21(1.03 to 1.42) and 1.11(1.00 to 1.24), respectively. These results indicated that diabetes might be an independent risk factor of melanoma.
Several mechanisms have been proposed to explain the biological plausibility of T2DM increasing the risk of melanoma. First, T2DM is usually associated with insulin resistance for long periods both before and after disease onset (37), which has indeed recently been acknowledged as an independent risk factor for melanoma (38). Insulin resistance and secondary chronic hyperinsulinemia may stimulate tumor growth by increasing bioavailable Insulin-like Growth Factors- I (IGF-I), well-known to promote tumor cell proliferation and metastases (39) in T2DM. Second, recently, studies suggest that vitamin D deficiency correlates with diabetes (40), and polymorphisms in the vitamin D receptor (VDR) gene are implicated in susceptibility to diabetes (41), interestingly, those genes such as FokI, BsmI, and TaqI had also been reported to be affecting the risk of developing melanoma (42-45). Besides, other mechanisms like leptin and serum adiponectin were both regarded as risk factors of diabetes (46) and the development of melanoma (47, 48).
To some extent, some limitations may affect the objectivity of the conclusions and should be considered when interpreting the results. Firstly, the cohorts of diabetes in several studies were hospital-based, the comparison group (i.e. the general population) includes individuals with diabetes, this would tend to attenuate any true association between T2DM and melanoma risk. Our results have shown that the association between T2DM and melanoma was stronger in population-based studies than in hospital-based studies. Secondly, the baseline information of several studies was so meager that we cannot recognize the comparability between the cohort and the controls. Unadjusted effect estimates have been published in 3 studies of lower quality, and the overall slope-association of these studies was null 1.06(0.75-1.49). Thirdly, as in all meta-analysis, the possibility of publication bias is of concern. However, a formal statistical test did not provide evidence for such bias. Finally, most of the studies met our inclusion criteria conducted in European and North American, which might influence the generalizability of the findings to other types of geographic areas.
Conclusion
Despite the limitations, this meta-analysis supports the hypothesis that T2DM might be an independent risk factor for melanoma. It should be noted that this meta-analysis may have important health implication. T2DM is a serious and rapidly growing health problem, thus it is important to note that the increased severity and prevalence of T2DM could contribute significantly to the incidence of melanoma, even if the effect is small. Hence, it is urgent to implement effective intervention strategies for T2DM in context of efforts for enhanced melanoma prevention and control. Clinician caring for patients with T2DM should remain alert to melanoma and minimize the number of missed opportunities for its treatment.
Further large-scale prospective studies are needed to test specifically the effect of T2DM on melanoma risk, and fully elucidate the underlying pathophysiologic mechanisms.
Ethical considerations
This study was approved by Ethical Committee of Research in Chongqing Municipal Center for Disease Control and Prevention.
Ethical issues (Including plagiarism, Informed Consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc) have been completely observed by the authors.
Acknowledgements
This study was funded by Chongqing Health Bureau (Topic No: 2012-2-233). The authors would like to thank all consultants who have contributed in this study. The authors declare that there are no conflicts of interest.
References
- Valero M A, Darce NA, Panova M, Mas-Coma S (2001). Relationships between host species and morphometric patterns in Fasciola hepatica adults and eggs from the Northern Bolivian Altiplano hyper endemic region. Vety Parasitol, 102. [DOI] [PubMed] [Google Scholar]
- Sneyd MJ, Cox B (2013). A comparison of trends in melanoma mortality in New Zealand and Australia: the two countries with the highest melanoma incidence and mortality in the world. BMC Cancer, 13: 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Saraiya M, Patel P, Cherala SS, Barnholtz-Sloan J, Kim J, Wiggins CL, Wingo PA (2011). Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992–2006. J Am Acad Dermatol, 65: S17–25 e1-3. [DOI] [PubMed] [Google Scholar]
- de Vries E, Bray FI, Coebergh JW, Parkin DM (2003). Changing epidemiology of malignant cutaneous melanoma in Europe 1953-1997: rising trends in incidence and mortality but recent stabilizations in western Europe and decreases in Scandinavia. Int J Cancer, 107: 119–26. [DOI] [PubMed] [Google Scholar]
- Forsea AM, Del Marmol V, de Vries E, Bailey EE, Geller AC (2012). Melanoma incidence and mortality in Europe: new estimates, persistent disparities. Br J Dermatol, 167: 1124–30. [DOI] [PubMed] [Google Scholar]
- Linos E, Swetter SM, Cockburn MG, Colditz GA, Clarke CA (2009). Increasing burden of melanoma in the United States. J Invest Dermatol, 129: 1666–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull AP (1983). Melanoma and solar radiation. Hosp Pract (Off Ed), 18: 17–8. [PubMed] [Google Scholar]
- Sergentanis TN, Antoniadis AG, Gogas HJ, Antonopoulos CN, Adami HO, Ekbom A, Petridou ET (2013). Obesity and risk of malignant melanoma: a meta-analysis of cohort and case-control studies. Eur J Cancer, 49: 642–57. [DOI] [PubMed] [Google Scholar]
- Karagas MR, Stukel TA, Dykes J, Miglionico J, Greene MA, Carey M, Armstrong B, Elwood JM, Gallagher RP, Green A, Holly EA, Kirkpatrick CS, Mack T, Osterlind A, Rosso S, Swerdlow AJ (2002). A pooled analysis of 10 case-control studies of melanoma and oral contraceptive use. Br J Cancer, 86: 1085–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TK, MacArthur AC, Gallagher RP, Elwood MJ (2009). Occupational physical activity and risk of malignant melanoma: the Western Canada Melanoma Study. Melanoma Res, 19: 260–6. [DOI] [PubMed] [Google Scholar]
- Freedman DM, Sigurdson A, Doody MM, Rao RS, Linet MS (2003). Risk of melanoma in relation to smoking, alcohol intake, and other factors in a large occupational cohort. Cancer Causes Control, 14: 847–57. [DOI] [PubMed] [Google Scholar]
- Whiting DR, Guariguata L, Weil C, Shaw J (2011). IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract, 94: 311–21. [DOI] [PubMed] [Google Scholar]
- Shaw JE, Sicree RA, Zimmet PZ (2010). Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract, 87: 4–14. [DOI] [PubMed] [Google Scholar]
- Boyle P, Boniol M, Koechlin A, Robertson C, Valentini F, Coppens K, Fairley LL, Zheng T, Zhang Y, Pasterk M, Smans M, Curado MP, Mullie P, Gandini S, Bota M, Bolli GB, Rosenstock J, Autier P (2012). Diabetes and breast cancer risk: a meta-analysis. Br J Cancer, 107: 1608–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib SL, Prihoda TJ, Luna M, Werner SA (2012). Diabetes and risk of renal cell carcinoma. J Cancer, 3: 42–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer HU, Schottker B, Raum E, Brenner H (2012). Type 2 diabetes mellitus and colorectal cancer: meta-analysis on sex-specific differences. Eur J Cancer, 48: 1269–82. [DOI] [PubMed] [Google Scholar]
- Shimoyama S (2013). Diabetes mellitus carries a risk of gastric cancer: a meta-analysis. World J Gastroenterol, 19: 6902–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Yang WS, Gao J, Wang J, Xiang YB (2010). [A meta-analysis of cohort studies on the association between diabetes and the risk of primary liver cancer]. Zhonghua Yu Fang Yi Xue Za Zhi, 44: 711–6. [PubMed] [Google Scholar]
- Zhang ZH, Su PY, Hao JH, Sun YH (2013). The role of preexisting diabetes mellitus on incidence and mortality of endometrial cancer: a meta-analysis of prospective cohort studies. Int J Gynecol Cancer, 23: 294–303. [DOI] [PubMed] [Google Scholar]
- Schmid D, Behrens G, Jochem C, Keimling M, Leitzmann M (2013). Physical activity, diabetes, and risk of thyroid cancer: a systematic review and meta-analysis. Eur J Epidemiol, 28: 945–58. [DOI] [PubMed] [Google Scholar]
- Lee JY, Jeon I, Lee JM, Yoon JM, Park SM (2013). Diabetes mellitus as an independent risk factor for lung cancer: a meta-analysis of observational studies. Eur J Cancer, 49: 2411–23. [DOI] [PubMed] [Google Scholar]
- Ben Q, Xu M, Ning X, Liu J, Hong S, Huang W, Zhang H, Li Z (2011). Diabetes mellitus and risk of pancreatic cancer: A meta-analysis of cohort studies. Eur J Cancer, 47: 1928–37. [DOI] [PubMed] [Google Scholar]
- Xu H, Jiang HW, Ding GX, Zhang H, Zhang LM, Mao SH, Ding Q (2013). Diabetes mellitus and prostate cancer risk of different grade or stage: a systematic review and meta-analysis. Diabetes Res Clin Pract, 99: 241–9. [DOI] [PubMed] [Google Scholar]
- Admiraal WM, van Valkengoed IG, JS LdM, Stronks K, Hoekstra JB, Holleman F (2011). The association of physical inactivity with Type 2 diabetes among different ethnic groups. Diabet Med, 28: 668–72. [DOI] [PubMed] [Google Scholar]
- Garg SK, Maurer H, Reed K, Selagamsetty R (2013). Diabetes and cancer: two diseases with obesity as a common risk factor. Diabetes Obes Metab, 2013May13. doi: 10.1111/dom.12124. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stang A (2010). Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol, 25: 603–5. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N (1986). Meta-analysis in clinical trials. Control Clin Trials, 7: 177–88. [DOI] [PubMed] [Google Scholar]
- Mantel N, Haenszel W (1959). Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst, 22: 719–48. [PubMed] [Google Scholar]
- Wotton CJ, Yeates DG, Goldacre MJ (2011). Cancer in patients admitted to hospital with diabetes mellitus aged 30 years and over: record linkage studies. Diabetologia, 54: 527–34. [DOI] [PubMed] [Google Scholar]
- Atchison EA, Gridley G, Carreon JD, Leitzmann MF, McGlynn KA (2011). Risk of cancer in a large cohort of U.S. veterans with diabetes. Int J Cancer, 128: 635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemminki K, Li X, Sundquist J, Sundquist K (2010). Risk of cancer following hospitalization for type 2 diabetes. Oncologist, 15: 548–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulcickas Yood M, Oliveria SA, Campbell UB, Koro CE (2009). Incidence of cancer in a population-based cohort of patients with type 2 diabetes. Diabetes & Metabolic Syndrome: Clinical Research & Reviews, 3: 12–16. [Google Scholar]
- Stattin P, Bjor O, Ferrari P, Lukanova A, Lenner P, Lindahl B, Hallmans G, Kaaks R (2007). Prospective study of hyperglycemia and cancer risk. Diabetes Care, 30: 561–7. [DOI] [PubMed] [Google Scholar]
- Wideroff L, Gridley G, Mellemkjaer L, Chow WH, Linet M, Keehn S, Borch-Johnsen K, Olsen JH (1997). Cancer incidence in a population-based cohort of patients hospitalized with diabetes mellitus in Denmark. J Natl Cancer Inst, 89: 1360–5. [DOI] [PubMed] [Google Scholar]
- Adami HO, McLaughlin J, Ekbom A, Berne C, Silverman D, Hacker D, Persson I (1991). Cancer risk in patients with diabetes mellitus. Cancer Causes Control, 2: 307–14. [DOI] [PubMed] [Google Scholar]
- Ragozzino M, Melton LJ, Chu CP, Palumbo PJ (1982). Subsequent cancer risk in the incidence cohort of Rochester, Minnesota, residents with diabetes mellitus. J Chronic Dis, 35: 13–9. [DOI] [PubMed] [Google Scholar]
- Barclay AW, Petocz P, McMillan-Price J, Flood VM, Prvan T, Mitchell P, Brand-Miller JC (2008). Glycemic index, glycemic load, and chronic disease risk—a meta-analysis of observational studies. Am J Clin Nutr, 87: 627–37. [DOI] [PubMed] [Google Scholar]
- Antoniadis AG, Petridou ET, Antonopoulos CN, Dessypris N, Panagopoulou P, Chamberland JP, Adami HO, Gogas H, Mantzoros CS (2011). Insulin resistance in relation to melanoma risk. Melanoma Res, 21: 541–6. [DOI] [PubMed] [Google Scholar]
- Khandwala HM, McCutcheon IE, Flyvbjerg A, Friend KE (2000). The effects of insulin-like growth factors on tumorigenesis and neoplastic growth. Endocr Rev, 21: 215–44. [DOI] [PubMed] [Google Scholar]
- Grineva EN, Karonova T, Micheeva E, Belyaeva O, Nikitina IL (2013). Vitamin D deficiency is a risk factor for obesity and diabetes type 2 in women at late reproductive age. Aging (Albany NY), 5: 575–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Wu B, Liu JY, Yang LB (2013). Vitamin D receptor gene polymorphisms and type 2 diabetes: a meta-analysis. Arch Med Res, 44: 235–41. [DOI] [PubMed] [Google Scholar]
- Gapska P, Scott RJ, Serrano-Fernandez P, Mirecka A, Rassoud I, Gorski B, Cybulski C, Huzarski T, Byrski T, Nagay L, Maleszka R, Sulikowski M, Lubinski J, Debniak T (2009). Vitamin D receptor variants and the malignant melanoma risk: a population-based study. Cancer Epidemiol, 33: 103–7. [DOI] [PubMed] [Google Scholar]
- Mocellin S, Nitti D (2008). Vitamin D receptor polymorphisms and the risk of cutaneous melanoma: a systematic review and meta-analysis. Cancer, 113: 2398–407. [DOI] [PubMed] [Google Scholar]
- Mandelcorn-Monson R, Marrett L, Kricker Aet al. (2011). Sun exposure, vitamin D receptor polymorphisms FokI and BsmI and risk of multiple primary melanoma. Cancer Epidemiol, 35: e105-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlow I, Roy P, Reiner ASet al. (2012). Vitamin D receptor polymorphisms in patients with cutaneous melanoma. Int J Cancer, 130: 405–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana JS, Li TY, Manson JE, Hu FB (2007). Adiposity compared with physical inactivity and risk of type 2 diabetes in women. Diabetes Care, 30: 53–8. [DOI] [PubMed] [Google Scholar]
- Mantzoros CS, Trakatelli M, Gogas H, Dessypris N, Stratigos A, Chrousos GP, Petridou ET (2007). Circulating adiponectin levels in relation to melanoma: a case-control study. Eur J Cancer, 43: 1430–6. [DOI] [PubMed] [Google Scholar]
- Gogas H, Trakatelli M, Dessypris N, Terzidis A, Katsambas A, Chrousos GP, Petridou ET (2008). Melanoma risk in association with serum leptin levels and lifestyle parameters: a case-control study. Ann Oncol, 19: 384–9. [DOI] [PubMed] [Google Scholar]


